Immune activation throughout a boosted darunavir monotherapy simplification strategy
Abstract
Our aim was to assess the evolution and the impact that blips, intermittent low-level viraemia and virological failure (VF) episodes have on patients’ immune activation (IA) profiles during ritonavir-boosted darunavir monotherapy (mtDRV/rtv). A prospective cohort of human immunodeficiency virus-1-infected patients who switched to mtDRV/rtv was followed for 2 years. Cellular IA was assessed according to HLA-DR and CD38 expression in CD4+ and CD8+ T-cells and their naïve, effector memory and central memory subpopulations, and systemic IA was evaluated according to sCD14 and D-dimer levels. Seventy-five patients from the MonDAR cohort were selected for this substudy, and classified according to viral outcome as having continuous undetectable viraemia (n = 19), blips (n = 19), intermittent viraemia (n = 21), and VF (n = 16). The IA profile was closely linked to viral behaviour. Patients on viral suppression for 24 months showed a significant decrease in CD4+ and CD8+ T-cell activation and sCD14 and D-dimer levels. Patients with transient low-level viraemia episodes (blips and intermittent viraemia) showed cellular and systemic IA similar to baseline values. In contrast, significant increases in T-cell activation and sCD14 and D-dimer levels were observed in patients with VF. Baseline levels of HLA-DR+CD38+CD8+ T-cells of >6.4% were independently associated with the emergence of VF. Therefore, mtDRV/rtv might be considered as a safe simplification strategy, on the basis of the IA results, whenever viral replication is under medium-term and long-term control. Transient low-level viraemia episodes do not affect patients’ IA status. Moreover, HLA-DR+CD38+CD8+ T-cell baseline levels should be considered when patients are switched to mtDRV/rtv.
Introduction
Persistent immune activation (IA) is a hallmark of chronic human immunodeficiency virus (HIV) infection, even under long-term suppressive combined antiretroviral therapy. Higher levels of IA are associated with impaired CD4+ T-cell reconstitution 1, 2, higher mortality rates 3, 4, and AIDS and non-AIDS-defining illnesses 5, 6. On the other hand, an increasing number of studies have demonstrated that ritonavir-boosted protease inhibitor monotherapy (mtPI/rtv), mainly based on lopinavir and darunavir, might be considered as an alternative strategy for treatment simplification in long-term virologically suppressed HIV-1-infected patients, with similar efficacy rates as triple therapy 7-17. Nevertheless, important concerns regarding the safety of this therapeutic option remain, such as the increased incidence of blips and persistent viraemia episodes 18. This residual viraemia might potentially induce a substantial increase in IA 19; however, no studies of mtPI/rtv have addressed this issue to date. In this study, we assessed IA evolution throughout a period of darunavir/ritonavir monotherapy (mtDRV/rtv), and particularly the influence that blip, intermittent viraemia and virological failure (VF) episodes have on the IA profile of HIV-1-infected patients.
Materials and Methods
Patients and study design
The MonDAR study (Clinical Trials.gov identifier: NCT01606722) prospectively enrolled all HIV-1-infected patients who were started on an mtDRV/rtv (800/100 mg once daily) simplification strategy at our outpatient clinic from January 2010 to April 2011 if they had: (i) a plasma HIV RNA level that was consistently <50 copies/mL for at least 6 months; and (ii) no resistance mutations that would confer decreased susceptibility to darunavir according to the International AIDS Society 20. The study was conducted after informed consent had been obtained, according to the principles of the Declaration of Helsinki, and was approved by the Committee for Ethics in Biomedical Research of Andalucía and the Spanish Agency for Medicines.
Patients with available samples at months 0, 6, 12, 18 and 24 were included in this IA substudy, and were classified into four non-overlapping groups according to the following viral outcome on mtDRV/rtv based on a median of 10 viral load determinations per subject (interquartile range (IQR) 8–10): (i) continuous undetectable viraemia (CUV), for patients on viral suppression (<20 copies/mL) during the 24 months of the follow-up; (ii) blips, defined as transitory episodes of HIV RNA viral loads of >50 copies/mL, preceded and followed by a plasma viral load of <50 copies/mL without changes in the antiretroviral treatment; (iii) VF, defined as two consecutive viral load measurements of >200 copies/mL; and (iv) intermittent viraemia (IV), defined as episodes of detectable plasma HIV RNA during the follow-up without meeting the blip or VF criteria. Adherence was assessed by personal interview at each patient visit, and by analysis of the hospital pharmacy records.
Follow-up and laboratory procedures
Patient assessments were performed at baseline, after the first month on treatment, and every 3 months thereafter, including biochemical and haematological profiles, CD4+ T-cell counts, and plasma HIV-1 viraemia (COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, version 2.0; Roche Diagnostic Systems, Branchburg, NJ, USA), with a limit of detection of 20 copies/mL.
Blood samples were collected in Vacutainer cell preparation tubes (BD Biosciences, Madrid, Spain) at baseline and at each clinical visit during the 24 months of follow-up. We analysed samples from the CUV, IV and VF groups drawn at months 0, 6, 12, 18 and 24 of mtDRV/rtv treatment. Moreover, the IA profile changes in patients with blips and VF episodes were intensively analysed before each episode, at the moment of the viral rebound, and after each episode.
Peripheral blood mononuclear cell samples were stained with the following antibodies: αCD3-V450 (clone UCHT1), αCD4-APC-H7 (clone RPA-T4), αCD8-V500 (clone RPA-T8), αCD45RA-FITC (clone HI-100), αCD45RO-PE (cloneUCHL1), αCCR7-PerCP-Cy5.5 (clone 150503), αCD38-APC (clone HIT-2), and αHLA-DR-PE-Cy7 (clone G46.6). Unstained controls and the following control antibodies were used: αCD4-APC-H7, αCD38-APC, αCD45RO-PE, and αHLA-DR-PE-Cy7. All of the antibodies were from BD Biosciences. Briefly, peripheral blood mononuclear cell samples were lysed with 100 μL of FACS lysing solution (BD Biosciences) to remove any remaining red blood cells. Then, the cells were washed with phosphate-buffered saline, stained for 30 min in the dark, washed again with phosphate-buffered saline, and resuspended in 1% paraformaldehyde. The cells were acquired with an LSR Fortessa flow cytometer (BD Biosciences), and at least 30 000 CD3+ T-cells were analysed with FACSDiva software (BD Biosciences). IA was studied according to the single and double expression of HLA-DR and CD38 in T-cell subsets, grouped as naïve (CD45RA+CD45RO−), effector memory (CD45RA−CD45RO+CCR7+) and central memory (TCM; CD45RA−CD45RO+CCR7−), as previously described 21, 22.
Moreover, sCD14 levels (Human sCD14 Quantikine ELISA; R&D Systems, Abingdon, UK) and plasma D-dimer levels (Human D-Dimer ELISA Kit; CUSABIO, Wuhan, China) were quantified by the use of ELISA-based assays, following the manufacturer's recommendations, at baseline and after 24 months of mtDRV/rtv.
Statistical analysis
Results were expressed as median and IQR for continuous variables, and percentages for categorical variables. The Mann–Whitney U-test and Kruskal–Wallis H-test were used to compare the medians between the different groups, and the Wilcoxon and Freidman tests were used to compare patients within the same group. Correlations between age, time on highly active antiretroviral therapy or duration of viral suppression and IA markers (HLA-DR/CD38 expression in both CD4+ and CD8+ T-cells) were assessed with the Pearson correlation test. Receiver operating characteristic curves were constructed to discriminate between the different cut-off values of the IA markers at baseline, and their ability to predict VF on mtDRV/rtv, and areas under the curve of >0.65 were considered for further analysis. The cumulative incidence of VF during mtDRV/rtv treatment by month 24 as a function of the baseline levels of the IA markers was assessed with Kaplan–Meier analysis and long-rank tests. A Cox regression analysis was performed to identify potential predictive factors for VF during mtDRV/rtv treatment. The variables associated with VF in the bivariate analysis were introduced into the model, and hazard ratios (HRs) were obtained. Potential time-dependent interactions were studied for each variable before their introduction into the model. The differences were considered to be statistically significant at p <0.05. The statistical analyses were performed with SPSS software (v. 19.0; SPSS, Chicago, IL, USA), and the graphs were generated with GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA).
Results
Seventy-five subjects from a total of 150 Caucasian patients enrolled in the MonDAR study were selected on the basis of sample availability, and categorized as having CUV (n = 19), blips (n = 19), IV (n = 21), and VF (n = 16). The baseline characteristics were similar among the different groups, especially for factors that may potentially influence IA status, such as age, duration of viral suppression, or hepatitis C virus co-infection (Table 1). The mean adherence throughout the follow-up period was 100% for subjects with CUV (IQR 96–100%), 100% for those with blips (IQR 93.5–100%), 100% for those with IV (IQR 96.5–100%), and 93% for those with VF (IQR 80–100%, p 0.033). No correlations were found between age or type of previous regimen and any of the IA markers studied at baseline. Only a weak correlation between time on highly active antiretroviral therapy and baseline levels of HLA-DR+CD38+CD8+ T-cells was found (r2 = 0.138, p 0.008).
| CUV (n = 19) | B (n = 19) | IV (n = 21) | VF (n = 16) | p-valuea | |
|---|---|---|---|---|---|
| Age (years), median (IQR) | 47 (42–50) | 47 (39–50) | 44 (39–50) | 46 (42–49) | 0.852 |
| Sex (male), n (%) | 14 (73.7) | 13 (68.4) | 19 (90.5) | 10 (62.5) | 0.147 |
| Chronic hepatitis C, n (%) | 4 (21.1) | 6 (31.6) | 4 (19.0) | 4 (25.0) | 0.286 |
| Time on HAART (months), median (IQR) | 117.0 (86.0–160.0) | 82.0 (42.0–136.0) | 100.0 (66.0–130.0) | 83.5 (47.3–121.8) | 0.432 |
| Time on viral suppression (months), median (IQR) | 68.0 (40.0–97.0) | 39.0 (32.0–97.0) | 51.0 (34.5–84.5) | 42.5 (16.0–67.8) | 0.507 |
| CD4+ T-cell counts (cell/μL), median (IQR) | 545 (388–713) | 592 (502–856) | 560 (327–897) | 598 (435–784) | 0.597 |
| Nadir CD4+ T-cell counts (cell/μL), median (IQR) | 163 (32–224) | 143 (67–214) | 177 (94–264) | 125 (37–222) | 0.310 |
| Zenit VL (log copies/mL), median (IQR) | 5.1 (4.7–5.7) | 5.2 (4.9–5.9) | 5.2 (4.8–5.4) | 5.2 (5.0–5.5) | 0.574 |
| Blips in the previous 12 months, n (%) | 2 (10.5) | 6 (31.6) | 8 (38.1) | 5 (31.3) | 0.127 |
| CD4+ T-cells | |||||
| HLA-DR+ (%), median (IQR) | 4.4 (3.5–5.1) | 2.2 (1.4–5.3) | 2.8 (2.2–3.9) | 2.8 (2.0–3.6) | 0.185 |
| CD38+ (%), median (IQR) | 31.1 (22.4–49.0) | 27.4 (18.9–55.0) | 34.6 (29.2–44.4) | 38.6 (30.2–55.2) | 0.456 |
| HLA-DR+CD38+ (%), median (IQR) | 4.6 (3.9–7.0) | 3.2 (2.3–4.9) | 5.5 (2.7–7.0) | 6.1 (4.5–7.2) | 0.389 |
| CD8+ T-cells | |||||
| HLA-DR+ (%), median (IQR) | 6.7 (3.1–4.7) | 3.2 (2.5–5.2) | 3.0 (1.5–4.9) | 3.5 (1.5–5.1) | 0.605 |
| CD38+ (%), median (IQR) | 15.7 (9.7–28.9) | 12.5 (7.9–18.9) | 16.8 (11.3–23.5) | 21.4 (13.4–35.2) | 0.329 |
| HLA-DR+CD38+ (%), median (IQR) | 5.0 (4.2–8.8) | 4.6 (2.0–6.1) | 5.6 (4.0–7.8) | 7.4 (6.3–10.6) | 0.090 |
- B, blips; CUV, continuous undetectable viraemia; HAART, highly active antiretroviral therapy; IQR, interquartile range; IV, intermittent viraemia; VF, virological failure; VL, viral load.
- a p-value between groups: Kruskal–Wallis H-test.
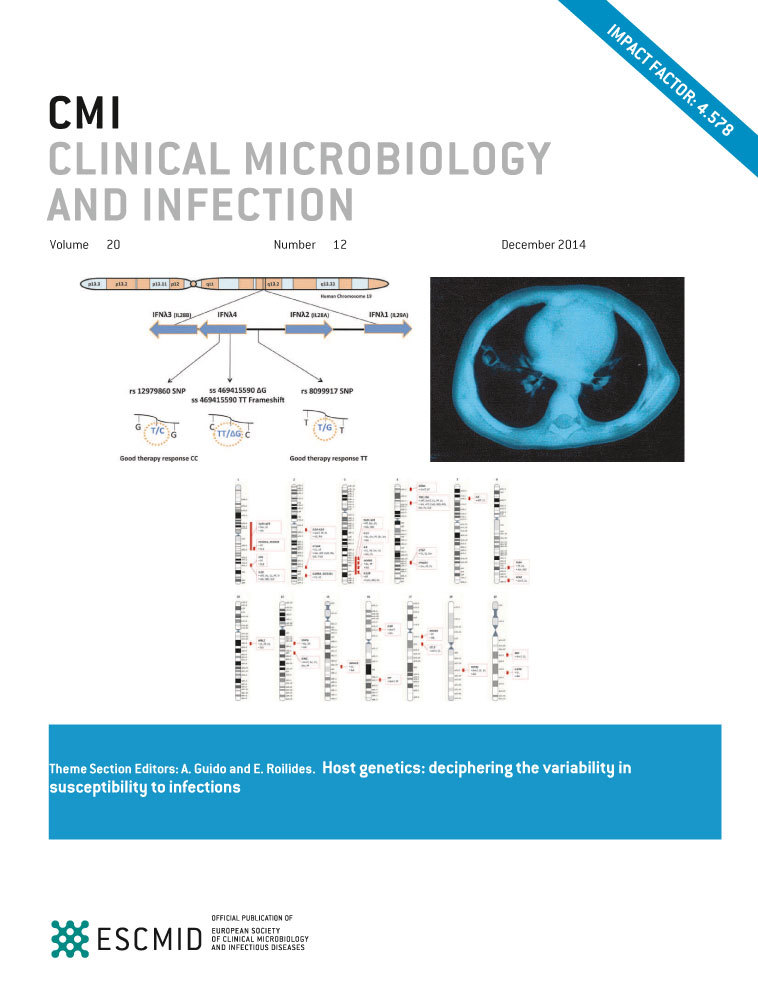
CD4+ and CD8+ T-cell activation during 24 months on mtDRV/rtv
Although no significant differences were found in the expression of HLA-DR+, CD38+ or HLA-DR+/CD38+ on CD4+ and CD8+ T-cells at baseline among the different groups (Table 1), their evolution was heterogeneous. Thus, CUV patients showed a decrease in cellular IA markers after 24 months on mtDRV/rtv in both CD4+ T-cells (HLA-DR+CD38+ baseline, 4.6% vs. month 24, 4.2%, p 0.022; Fig. 1a) and CD8+ T-cells (HLA-DR+CD38+, baseline, 5.0% vs. month 24, 4.4%, p 0.003; Fig. 1d, Table S1), whereas these values showed no significant changes in the IV group (Fig. 1b,e). By contrast, in the VF group, these IA markers showed a significant increase after 24 months of follow-up (CD4+HLA-DR+CD38+ baseline, 6.1% vs. month 24, 7.1%, p 0.022; Fig. 1c; CD8+HLA-DR+CD38+, baseline, 7.4% vs. month 24, 9.4%, p 0.068; Fig. 1f), even when all these patients had regained virological control at a median of 10 weeks previously (range: 3–15 months) through re-introduction of the previous nucleoside analogue backbone (n = 7) or continuation of mtDRV/rtv treatment after adherence counselling (n = 9) (Table 2). These changes were mostly attributable to the TCM subpopulations in both CD4+ and CD8+ T-cells (Tables S2 and S3).
| CUV | IV | VF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Basal | Month 24 | p-valuea | Basal | Month 24 | p-valuea | Basal | Month 24 | p-valuea | |
| CD4+ T-cells | |||||||||
| HLA-DR+ (%) | 4.4 (3.5–5.1) | 3.7 (1.9–6.0) | 0.600 | 2.8 (2.2–3.9) | 2.5 (1.9–3.1) | 0.683 | 2.8 (2.0–3.6) | 2.4 (0.9–4.7) | 0.959 |
| CD38+ (%) | 31.1 (22.4–49.0) | 28.8 (20.8–33.7) | 0.035 | 34.6 (29.2–44.4) | 42.4 (33.4–47.8) | 0.609 | 38.6 (30.2–55.2) | 51.1 (45.1–63.1) | 0.047 |
| HLA-DR+CD38+ (%) | 4.6 (3.9–7.0) | 4.2 (3.3–5.1) | 0.022 | 5.5 (2.7–7.0) | 4.0 (3.4–4.7) | 0.078 | 6.1 (4.5–7.2) | 7.1 (4.8–13.7) | 0.022 |
| CD8+ T-cells | |||||||||
| HLA-DR+ (%) | 6.7 (3.1–4.7) | 2.5 (1.2–4.5) | 0.008 | 3.0 (1.5–4.9) | 2.1 (1.0–2.7) | 0.147 | 3.5 (1.5–5.1) | 5.4 (3.0–9.6) | 0.008 |
| CD38+ (%) | 15.7 (9.7–28.9) | 11.8 (7.4–17.4) | 0.003 | 16.8 (11.3–23.5) | 23.1 (15.4–39.3) | 0.111 | 21.4 (13.4–35.2) | 35.7 (16.9–50.2) | 0.285 |
| HLA-DR+CD38+ (%) | 5.0 (4.2–8.8) | 4.4 (3.3–5.6) | 0.003 | 5.6 (4.0–7.8) | 4.4 (3.6–6.5) | 0.280 | 7.4 (6.3–10.6) | 9.4 (5.6–11.2) | 0.068 |
- CUV, continuous undetectable viraemia; IV, intermittent viraemia; VF, virological failure. Values are expressed as median and interquartile range.
- a p-value between groups: Wilcoxon test.
Impact of blips and VF on the T-cell activation profile
A total of 31 blip episodes from 19 patients on mtDRV/rtv were intensively analysed before, during and after the viraemic episodes. No major changes were observed in either CD4+ T-cells (prior to blip, 3.2% vs. blip, 2.9% vs. after blip, 3.3%, p 0.312; Table S4) or CD8+ T-cells (prior to blip, 4.6% vs. blip, 4.4% vs. after blip, 2.9%, p 0.368; Table S4) according to the expression of HLA-DR/CD38.
By contrast, VF episodes (n = 16) induced significant increases in the expression of CD38 (38.5% vs. 51.3%, p 0.039) and HLA-DR/CD38 (5.4% vs. 6.8%, p 0.045) on CD4+ T-cells as compared with the baseline and the median values during the periods with undetectable viral load. These changes were mainly attributable to the contribution of TCM CD4+ T-cells. Just at VF, similar trends were observed for CD8+ T-cells (HLA-DR, 4.4% vs. 6.1%, p 0.10; CD38, 15.9% vs. 19.8%, p 0.807; and HLA-DR/CD38, 7.3% vs. 8.5%, p 0.917), although without reaching statistical significance (Table S5), but being more evident in the later determinations at month 24.
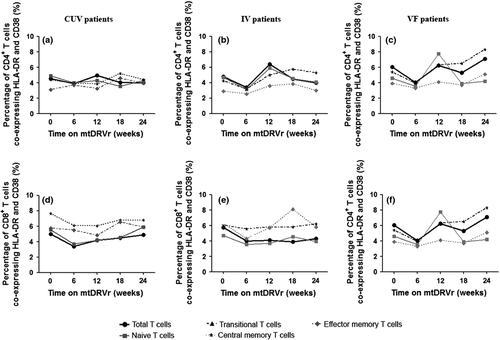
sCD14 and D-dimer levels during mtDRV/rtv treatment
No baseline differences were found in sCD14 and D-dimer levels among the groups with different virological outcomes. However, sCD14 levels decreased significantly in the CUV group (−22.6 μg/mL, IQR −67.7 to 1.0 μg/mL), remained stable in the blip (−2.1 μg/mL, IQR −24.9 to −8.1 μg/mL) and IV groups (1.5 μg/mL, IQR −17.9 to 15.6), but increased significantly in the VF group (31.5 μg/mL, IQR 7–69.7 μg/mL, p 0.027; Fig. 2a). Moreover, whereas D-dimer levels decreased in most of the groups after 24 months of treatment (CUV, −14.06 ng/mL, IQR −57.26 to −3.33 ng/mL; IV, −7.54 ng/mL, IQR −36.03 to 5.37 ng/mL; blips, −5.20 ng/mL, IQR −39.72 to 4.11 ng/mL), these levels increased significantly in the VF group (6.01 ng/mL, IQR −0.17 to 21.75 ng/mL, p 0.001; Fig. 2b).
Influence of IA on VF during mtDRV/rtv treatment
Comparison of the clinical and immunological baseline characteristics of patients with and without VF while on mtDRV/rtv showed that only the coexpression of HLA-DR and CD38 on CD8+ T-cells was significantly higher in patients who experienced VF while on mtDRV/rtv (7.4% vs. 5.6%, p 0.030; Table S6). After a receiver operating characteristic analysis, the best cut-off value for HLA-DR+CD38+CD8+ T-cells associated with VF during mtDRV/rtv treatment was 6.4% (AUC 0.699; 95% CI 0.531–0.876), with a sensitivity and specificity of 71.4% and 72.2%, respectively. In addition, positive and negative predictive values were calculated, and were 71.4% and 86.7%, respectively. The survival analysis indicated a cumulative probability of VF during mtDRV/rtv treatment by month 24 of 51.8% in patients with baseline levels of HLA-DR+CD38+CD8+ T-cells of >6.4%, whereas only 14% of patients with lower levels failed treatment (log-rank p 0.006; Fig. S1). A Cox regression model showed that the only variables independently associated with VF during mtDRV/rtv treatment were a baseline level of HLA-DR+CD38+CD8+ T-cells of >6.4% (HR 5.99; 95% CI 1.52–23.54; p 0.010) and adherence to treatment of <95% (HR 4.30; 95% CI 1.36–13.64; p 0.013; Table 3).
| Factor | HR | 95% CI | p-value |
|---|---|---|---|
| Adherence <95% | 4.309 | 1.361–13.642 | 0.013 |
| Nadir CD4+ T-cell count (cells/μL) | 0.996 | 0.990–1.002 | 0.157 |
| Time on viral suppression (months) | 0.997 | 0.980–1.015 | 0.755 |
| HLA-DR+CD38+CD8+ T-cells >6.4% | 5.993 | 1.525–23.548 | 0.010 |
- HR, hazard ratio.
Discussion
Simplification therapies based on mtPI/rtv have been associated with higher rates of intermittent episodes of low-level viraemia than those seen with triple therapy 18. Nevertheless, the real consequences of these episodes need to be fully assessed, because those reported have only focused on the risk of subsequent VF 23-25.
Our results show that the IA profile was closely linked to viral outcome during mtDRV/rtv treatment. Thus, patients with undetectable viraemia throughout the 24 months of follow-up showed significant decreases both in the activation markers of CD4+ and CD8+ T-cells and in sCD14 and D-dimer levels. Likewise, the blip episodes had no effects on either CD4+ T-cells, CD8+ T-cells, or soluble markers of IA, most likely because of the transitory and low level of viraemia. In contrast, significant increases in both CD4+ and CD8+ T-cell activation, sCD14 levels and D-dimer levels were observed in those patients who experienced VF, these being evident after a median of 10 weeks after virological control had been regained. This is consistent with the theory originally proposed by Karlsson et al. 26, who suggested that increases in IA of T-cells would not be observed without a simultaneous increase in viral replication. Patients with transient low-level viraemia were halfway between the CUV and VF groups. This is particularly relevant, as previous studies have pointed out that these transient low-level viraemia episodes increase the IA profile of CD8+ T-cells 27; herein, our results showed that IA status was not modified in either CD4+ or CD8+ T-cells. Even though the cellular IA profile remained stable throughout the follow-up, it did not show the decrease seen in the CUV patients. Therefore, on the basis of the IA results, mtDRV/rtv is a safe simplification strategy, and blip episodes should not be a cause for alarm whenever viral replication is under control in the medium and long term. One limitation of our study might be the selection of the patients, as only those with available samples at all of the clinical visits were included. However, this criterion supposes blind selection of the patients, uninfluenced by any other parameter or baseline characteristic that would change the study results, as similar numbers of patients were included in each study group.
To the best of our knowledge, no previous study has focused on the value of baseline IA for predicting VF in patients on mtPI/rtv. With triple-therapy regimens, several studies have reported that IA markers could be considered as prognostic factors for high levels of HIV-1 viraemia 28 and disease progression 4, 29, 30. Here, we have also observed that coexpression of HLA-DR and CD38 by CD8+ T-cells is associated with VF during mtDRV/rtv treatment. This factor was independently associated with VF, together with adherence, whereas other factors, such as nadir CD4+ T-cell count or time on viral suppression, which are traditionally associated with worse treatment outcomes, had no influence on VF in our cohort. The cut-off value suggested for HLA-DR+CD38+CD8+ T-cells might be relevant for the enrolment of patients in future simplification strategies although larger cohort studies are needed to support this value.
To summarize, mtDRV/rtv is a safe simplification strategy according to the IA results, and blip episodes should not be cause for alarm whenever replication is under medium-term and long-term control. Higher baseline levels of HLA-DR+CD38+CD8+ T-cells were independently associated with the emergence of VF.
Acknowledgements
The authors are indebted to patients for their involvement in this study, and M. Rodríguez, F. Cano and R. Martin for their help with specimen processing.
Transparency Declaration
This work was supported by a grant from Dirección Gerencia del Servicio Andaluz de Salud, Consejería de Salud, Junta de Andalucía (exp. SAS 111237). L. F. Lopez-Cortes and P. Viciana have received unrestricted research funding, consultancy fees, and lecture fees from and have served on the advisory boards of Abbott, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, Roche España, and ViiV Healthcare. The other authors have nothing to disclose.