Human serum albumin stabilizes streptolysin S activity secreted in the extracellular milieu by streptolysin S-producing streptococci
Abstract
Anginosus group streptococci (AGS) are opportunistic pathogens of the human oral cavity; however, their pathogenicity has not been discussed in detail. Oral streptococci live in the gingival sulcus, from where they can easily translocate into the bloodstream due to periodontal diseases and dental treatment and cause hazardous effects on the host through their virulence factors. Streptolysin S (SLS), a pathogenic factor produced by β-hemolytic species/strains belonging to AGS, plays an important role in damaging host cells. Therefore, we investigated the SLS-dependent cytotoxicity of β-hemolytic Streptococcus anginosus subsp. anginosus (SAA), focusing on different growth conditions such as in the bloodstream. Consequently, SLS-dependent hemolytic activity/cytotoxicity in the culture supernatant of β-hemolytic SAA was stabilized by blood components, particularly human serum albumin (HSA). The present study suggests that the secreted SLS, not only from β-hemolytic SAA, but also from other SLS-producing streptococci, is stabilized by HSA. As HSA is the most abundant protein in human plasma, the results of this study provide new insights into the risk of SLS-producing streptococci which can translocate into the bloodstream.
Abbreviations
-
- AGS
-
- Anginosus group streptococci
-
- BSA
-
- bovine serum albumin
-
- CcpA
-
- catabolite control protein A
-
- CCR
-
- carbon catabolite repression
-
- CDCs
-
- cholesterol-dependent cytolysins
-
- FBS
-
- fetal bovine serum
-
- GCF
-
- gingival crevicular fluid
-
- HSA
-
- human serum albumin
-
- PBS
-
- phosphate-buffered saline
-
- PI
-
- propidium iodide
-
- rHSA
-
- recombinant HSA
-
- RT-qPCR
-
- quantitative reverse transcription polymerase chain reaction
-
- SAA
-
- Streptococcus anginosus subsp. anginosus
-
- SLO
-
- streptolysin O
-
- SLS
-
- streptolysin S
INTRODUCTION
Anginosus group streptococci (AGS) are opportunistic pathogens that are present in the oral cavity of humans. Contrary to the well-known human pathogenic streptococcal species, such as Streptococcus pyogenes, Streptococcus pneumoniae, and Streptococcus agalactiae, the clinical importance of the species belonging to AGS has been regarded as low because of the commonly accepted recognition that AGS are opportunistic pathogens in humans. However, reports on the hemolysin/cytolysin produced by β-hemolytic AGS subgroups have been increasing – in particular, on intermedilysin, a proteinaceous pore-forming toxin that is a member of the cholesterol-dependent cytolysins (CDCs) with human-cell-specific activity produced by Streptococcus intermedius,1 as well as on the peptide hemolysin, generally known as streptolysin S (SLS), produced by the β-hemolytic strains of Streptococcus anginosus subsp. anginosus (SAA)2, 3 and Streptococcus constellatus subsp. constellatus.4 These β-hemolysins produced by β-hemolytic species/strains of AGS indicated not only hemolytic activity but also cytotoxicity;4-6 they are gaining interest for their contribution to pathogenicity in humans.
Numerous microbes inhabit the human oral cavity and pose high risks, owing to their easy translocation into the bloodstream in cases of periodontal diseases and dental treatment. Blood contains many soluble components, such as serum proteins, saccharides, lipids, and minerals, some of which support the growth of translocated microbes in the bloodstream. Among the soluble blood components, serum albumin is the most abundant protein in the plasma. Human serum albumin (HSA) serves multiple functions, including as a carrier protein of many drugs and chemicals.7-9 Moreover, HSA interacts with microbial toxins such as mycotoxins10-15 and bacterial toxins.16, 17 A recent study reported that HSA binds to streptolysin O (SLO), the CDC secreted by S. pyogenes, and inhibits its cytotoxic and hemolytic effects.18 Further, the serum and serum components of animal origin (bovine, horse, and human) were reported to stabilize SLS over half a century ago, focused on the preparations of SLS-containing fractions from the bacterial cells of S. pyogenes as toxic extracts,19 hemolysins extracted by serum,20-22 hemolysins extracted by albumin,23, 24 and “streptolysin D”.25 However, only a few studies have addressed the contribution of blood components to the SLS secreted into the extracellular milieu from the growing SLS-producing streptococci, such as a study on horse serum for the preparation of serum streptolysin.26
Previously, we reported that SLS-dependent cytotoxicity against the human culture cell line HSC-2 was caused by the secreted SLS from β-hemolytic SAA strain NCTC10713T.6 This result indicated that the secreted SLS will be one of the potential pathogenic factors of β-hemolytic SAA against human cells. In the present study, focusing on the risk of translocation of oral streptococci into the bloodstream, the effects of human blood components, particularly HSA, on SLS-dependent hemolytic and cytotoxic activities were investigated. To reveal the exact mechanism for the interaction between SLS and human blood components including HSA, the purified SLS is thought to be suitable for the assay. Some investigations on the purification of SLS using the SLS-containing fraction prepared from bacterial cells and the culture supernatant were conducted previously for S. pyogenes.27, 28 The investigations on the production of recombinant SLS were also conducted using the Escherichia coli expression system,29-31 however, no purification of the mature form of recombinant SLS had been reported. As for our study, we could not investigate the interaction between purified SLS and human blood components including HSA because the preparation of purified SLS had not been achieved despite cumulative trials, probably because of the original nature of the mature SLS and/or the requirement of the complicated production system of mature SLS encoded by the gene cluster for SLS production called the sag operon. By the way, the β-hemolytic SAA strain NCTC10713T is thought to be suitable for evaluating SLS-dependent hemolytic activity and cytotoxicity because SLS is the sole β-hemolytic and cytotoxic factor produced by this strain.2, 6 Interestingly, the β-hemolytic SAA, including the strain NCTC10713T, possesses two sagA genes (sagA1 and sagA2) in the sag operon.2 Based on the information about hemolytic activity/cytotoxicity of SAA strain NCTC10713T described above, we thought that the SLS-dependence for the hemolytic activity/cytotoxicity of the strain could be adequately and effectively evaluated and discussed using the sagA1 and sagA2 double-deletion mutant (ΔsagAs) for comparison. For the reasons described above, this study was conducted using the culture supernatant of the β-hemolytic SAA strain NCTC10713T and the mutant strains of SLS-encoding gene(s).
In addition, the translational products from sagA1 and sagA2 genes of β-hemolytic SAA strain NCTC10713T have different amino acid sequences, especially in their C-terminal region.2 Each of the amino acid sequences of these two SLSs produced from the strain NCTC10713T is also different from the SLS of S. pyogenes at the C-terminus.2 Based on the information, we investigated further the effect of HSA on SLS-dependent hemolysis of other SLS-producing streptococci to confirm whether the effect of HSA observed in SAA is species specific.
MATERIALS AND METHODS
Bacterial strains and culture conditions
The SAA strain NCTC10713T, exhibiting SLS-dependent β-hemolysis,2 was primarily used in the current study. In addition, some genetic mutants derived from the SAA strain NCTC10713T2 and streptococcal species/strains described in Table 2 were used. All tested strains were pre-cultured overnight in brain-heart infusion (BHI) broth (Becton Dickinson and Co. Ltd) at 37°C in a 5% CO2 atmosphere.
Preparation of culture supernatants
Heat-inactivated (incubated at 56°C for 30 min) animal serum (fetal bovine serum [FBS], horse serum, and human serum), HSA fraction V (product No. 01282, Nacalai Tesque, Inc.), human plasma γ-globulin (product No. 075-06691, Fujifilm Wako Pure Chemical Corp.), α1-acid glycoprotein (product No. G9885, Sigma-Aldrich Co. LLC), recombinant HSA (rHSA) (product No. 19597, Nacalai Tesque, Inc.), purified HSA (product No. A3782, Sigma-Aldrich Co. LLC), or purified bovine serum albumin (BSA) (product No. A0281; Sigma-Aldrich Co. LLC) was added to the culture medium for the serum component. Human serum was prepared from the blood of healthy volunteers with written informed consent in accordance with Protocol No. 4092 approved by the Ethics Committee of Tokushima University Hospital. The tested strain was inoculated at the optical density of 600 nm (OD600) to 0.01 into the co-cultivation medium composed of Eagle's Minimal Essential Medium (EMEM) (Fujifilm Wako Pure Chemical Corp.) containing 25 mM 2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (pH 7.4), 10% (v/v) BHI broth, and the serum component [10% (v/v) of heat-inactivated animal serum, 1.0% (w/v) of albumin, 1.0% (w/v) of γ-globulin, or 0.1% (w/v) of α1-acid glycoprotein] and incubated at 37°C in 5% CO2 atmosphere. The culture supernatants were obtained by centrifugation (13,000 × g, 5 min), after 4 h incubation to obtain log-phase cultures. If needed, supernatants were prepared from the stationary-phase culture that was incubated overnight.
Preparation of the bacterial cell-associated SLS
The bacteria were cultured in the co-cultivation medium containing 1.0% (w/v) serum albumin for 4 h at 37°C in a 5% CO2 atmosphere. The bacterial cells were collected by centrifugation (5,800 × g, 5 min) and washed once with sterilized phosphate-buffered saline (PBS). The washed bacterial cells were resuspended in PBS containing 1.0% (w/v) serum albumin and incubated for 20 min at 37°C in a 5% CO2 atmosphere. After centrifugation (13,000 × g, 5 min), the centrifuged supernatant containing cell-associated SLS was used to measure hemolytic activity.
Measurement of hemolytic activity
Preserved horse blood was purchased from Japan Bio Serum Co., Ltd, and the erythrocyte suspension was prepared by washing several times with sterilized PBS. The prepared erythrocyte suspension was added to a reaction mixture containing 1/10 volume of the sample (i.e., the prepared culture supernatant or the fraction containing cell-associated SLS) in sterilized PBS to adjust the final concentration to 0.5% (v/v) and incubated at 37°C for 1 h. In order to measure the hemolytic activity, the diluted culture supernatants equivalent to the supernatant obtained from the bacterial culture containing the same number of bacteria with OD600 = 0.1 were prepared by dilution with sterilized PBS and used for the measurement. After incubation, the reaction mixture was centrifuged (750 × g, 5 min), and the absorbance of each supernatant was measured at 540 nm. Hemolytic activity was calculated as the percentage of hemolysis. Complete hemolysis (100%) and nonhemolysis (0%) controls were prepared using sterilized pure water and sterilized PBS, respectively, instead of the reaction mixture containing the samples.
To evaluate the stability of the secreted SLS into the extracellular milieu of bacteria, antibiotics (200 units/mL penicillin G potassium and 200 μg/mL streptomycin sulfate, Fujifilm Wako Pure Chemical Corp.) were added to the prepared culture supernatant to inhibit bacterial growth and then incubated for the indicated time (2–8 h) at 37°C in 5% CO2 atmosphere. The stability of the secreted SLS is indicated as the percentage of retained hemolytic activity of the sample incubated at 37°C compared with the hemolytic activity of the same sample preserved in an ice bath.
Cytotoxicity assay
The cytotoxicity of the culture supernatant of the SAA strain NCTC10713T on the human acute monocytic leukemia cell line THP-1 (Riken, RCB1189) was evaluated using propidium iodide (PI) staining. THP-1 cells were cultivated at 37°C in a 5% CO2 atmosphere in the cell culture medium [Roswell Park Memorial Institute (RPMI) 1640 medium (Fujifilm Wako Pure Chemical Corp.) containing 10% (v/v) heat-inactivated FBS and antibiotics (100 units/mL penicillin G potassium and 100 μg/mL streptomycin sulfate; Meiji Seika Pharma Co., Ltd, Chuo-ku, Tokyo, Japan)]. Subsequently, the THP-1 cells were washed once by cell culture medium without phenol red, suspended in the same medium and dispensed into a 96-well plate at 1.0 × 105 cells/50 μL/well. An equal amount of the prepared bacterial culture supernatant containing antibiotics (Fujifilm Wako Pure Chemical Corp.) was mixed and incubated overnight at 37°C in a 5% CO2 atmosphere. For the control experiment, THP-1 cells incubated in cell culture medium were also prepared. After incubation, PI was added to the cell suspension at a final concentration of 1 μg/mL and incubated at 37 °C for 15 min in a 5% CO2 atmosphere. Cytotoxicity was evaluated according to the fluorescence intensity of PI-stained cells using a fluorescent plate reader (Infinite® M200, TECAN) at excitation and emission wavelengths of 530 and 620 nm, respectively.
Comparison of the expression level for the genes encoding SLS precursors
Total RNA was extracted from the 4 h (log-phase) culture of the SAA strain NCTC10713T in the presence or absence of 1.0% (w/v) rHSA. The bacterial cells collected by centrifugation (5,800 × g, 5 min) were resuspended in buffer LBP contained in the NucleoSpin® RNA Plus kit (Takara Bio Inc.) and then disrupted using a Zirco Prep Mini (NIPPON Genetics) using FastPrep FP100A (Thermo). The total RNA was obtained according to the protocol of the NucleoSpin® RNA Plus kit. To eliminate genomic DNA contaminating the prepared total RNA, additional DNase treatment was performed using recombinant DNase (Takara Bio Inc.). Subsequently, cDNA was synthesized from the total RNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer's instructions. Primers for the amplification of each SLS-precursor encoding gene (sagA1 and sagA2) and gyrB were originally designed for this study and were as follows: forward and reverse primers for sagA1, 5′-ATGTTAAAATTTTCTTCAAACG-3′ and 5′-TTATTTTGTAGGTGCTACGG-3′, respectively; sagA2, 5′-ATGCTTAAATTAGATTCACATATTATGG-3′ and 5′-TTAAGGTTTAATGTTTGTTGAACC-3′, respectively; gyrB, 5′-GTTTTCGTACAGCGCTCACA-3′ and 5′-CTTAGCTGCAATCCGAGCTT-3′, respectively. The primer set for gyrB was designed using GENETYX-MAC Network Version 20.0.4 (GENETYX Corp.). The quantitative reverse transcription polymerase chain reaction (RT-qPCR) was performed using TB Green® Premix Ex TaqTM II (Takara Bio Inc.) under the following reaction conditions: initial denaturation for 30 s at 95°C, followed by 40 cycles of the reaction for 5 s at 95°C and 30 s at 60°C before the dissociation step. Based on the results of RT-qPCR, the relative expression of the target genes was evaluated using the ΔΔCt method32 with gyrB as the internal control.
Statistics
Statistical evaluation was conducted using the R software for Mac OS X (version 3.6.1, https://cran.r-project.org/bin/macosx/).
RESULTS AND DISCUSSION
Effect of blood components on SLS-dependent hemolytic activity
The SLS-dependent cytotoxicity to the human oral squamous cell carcinoma cell line HSC-2 and human acute monocytic leukemia cell line THP-1 were observed after co-cultivation with the SAA strain NCTC10713T.6 In the present study, focusing on the risk of translocation of oral streptococci into the bloodstream, we investigated whether any blood components affected the SLS-dependent hemolytic activity and cytotoxicity of β-hemolytic AGS and other SLS-producing streptococci.
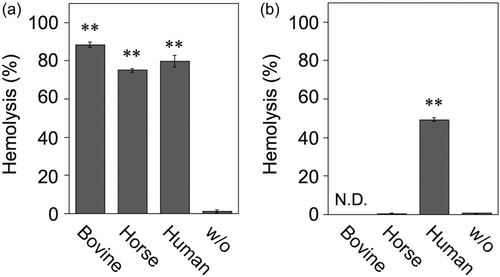
First, the supernatants of the SAA strain NCTC10713T culture in both the log and stationary phases in the presence of heat-inactivated animal serum (FBS, human serum, or horse serum) were obtained as described in the Materials and Methods section. Subsequently, the hemolytic activity of the culture supernatants was investigated. No hemolytic activity was observed in the culture supernatants obtained from the log-phase culture without animal serum (Figure 1a, sample “w/o”). In contrast, hemolysis was observed in the culture supernatants obtained from the log-phase culture containing the tested animal sera (Figure 1a). However, in the overnight culture, a significant decrease in hemolysis was observed when the culture was carried out with FBS and horse serum (Figure 1b). Notably, hemolytic activity was retained in the culture supernatant containing human serum, even in the overnight culture (Figure 1b). Subsequently, the retained hemolytic activity in the presence of human serum was further investigated by focusing on the components of human serum such as HSA (fraction V and recombinant protein), γ-globulin, and α1-acid glycoprotein. As depicted in the culture supernatant containing human serum (Figure 1), hemolytic activity was observed in the supernatants of cultures containing HSA (both fraction V and recombinant protein) or γ-globulin (Table 1). The highest hemolytic activity was observed in the culture supernatant containing rHSA (Table 1). No significant hemolysis was observed in the culture supernatant containing α1-acid glycoprotein (Table 1). Moreover, among the overnight culture supernatants containing human serum components, hemolytic activity was observed only in the culture supernatant containing rHSA (Table 1). As hypothesized, no hemolysis was observed in the culture supernatant of the non-hemolytic sagA gene-deletion mutant (ΔsagAs) prepared under all the culture conditions (Table 1). The cause of the difference in SLS stabilization by rHSA and fraction V of HSA is not clear; however, trace levels of contaminants of blood origin, such as fatty acids and other components, may be present in fraction V of HSA. This trace level of contaminants in fraction V of HSA may affect the interaction of HSA with SLS, resulting in impaired SLS stabilization.
| Hemolysis (% ± SD)† | ||||
|---|---|---|---|---|
| NCTC10713T | ΔsagAs | |||
| Log-phase | Overnight | Log-phase | Overnight | |
| HSA (fraction V) | 47.9 ± 0.2** | N.D.‡ | N.D.‡ | N.D.‡ |
| γ-Globulin | 21.6 ± 2.4** | 0.1 ± 0.1 | N.D.‡ | 0.2 ± 0.4 |
| α1-Acid glycoprotein | 0.8 ± 0.4 | 0.8 ± 0.4 | 1.8 ± 1.4 | 0.3 ± 0.6 |
| HSA (recombinant) | 83.4 ± 2.9** | 24.7 ± 1.5** | N.D.‡ | N.D.‡ |
| Without blood components | 0.6 ± 0.3 | 0.7 ± 0.6 | 1.0 ± 0.5 | 0.2 ± 0.3 |
- Note: The significance of the increase in hemolysis in the presence of blood components was evaluated against the sample without blood components using Welch's or Student's t-test (**P < 0.01). For the significance test, the hemolytic activity of the sample without hemolysis was used as “0%”.
- Abbreviations: HSA, human serum albumin; N.D., not determined.
- † Hemolytic activity was shown as means % with standard deviation (SD) (n = 3).
- ‡ Not detected (without hemolysis).
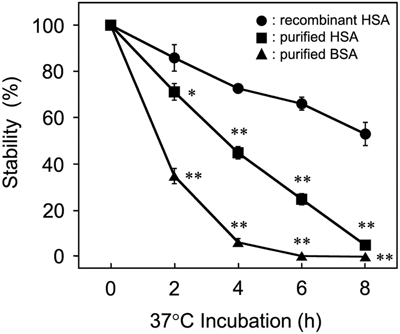
Stabilization of the hemolytic activity of secreted SLS was further investigated using highly purified albumin. Consequently, we found that HSAs were more effective in stabilizing secreted SLS than BSA under the conditions generally used for cell culture (37°C in a 5% CO2 atmosphere) (Figure 2). Regarding the HSAs used in this assay, rHSA was superior to purified HSA in stabilizing the hemolytic activity of the secreted SLS in the culture supernatant of β-hemolytic SAA. In addition, rHSA was more effective than purified HSA for the preparation of the fraction containing cell-associated SLS from PBS-washed bacterial cells (Supporting Information: Table S1). The reason for the difference in the effect on the stabilization of the hemolytic activity of SLS by rHSA and purified HSA is not clear now; however, this difference may be attributed to their origins – the purified natural protein or recombinant protein. Regarding the highly purified albumins, though the effect of SLS extraction from PBS-washed bacterial cells was not so different (Supporting Information: Table S1), purified HSA was superior to purified BSA in stabilizing the secreted SLS-dependent hemolytic activity in the culture supernatant of β-hemolytic SAA (Figure 2). We hypothesized that the difference in the effect of stabilization would depend on the difference in amino acid sequences between HSA and BSA. The identity and similarity of the amino acid sequences between HSA and BSA were 75% and 96%, respectively. Some of the regions with different amino acid sequences were located near the drug-binding site and surface of the molecule. One of the reasons of the difference in the stabilizing effect of these serum albumins on SLS is supposed to be that the secreted SLS can interact more effectively with the specific region(s) characteristic of HSA. Further investigation in vitro and in silico is necessary to clarify the mechanisms of the stabilizing effect of serum albumin on SLS depending on origin of serum albumin.
To further investigate the HSA-dependent stabilization of the hemolytic activity of SLSs secreted by β-hemolytic SAA, additional experiments were conducted using the mutant strains ΔsagA1 and ΔsagA2 derived from the SAA strain NCTC10713T and trans-complement strains of sagA1 and/or sagA2.2 Consequently, from the results depicted in Figure 3, HSA-dependent stabilization of SLS was observed in the tested SLS-producing strains, regardless of the difference in the amino acid sequence in their C-terminal region. A time-course assay for the HSA-dependent stabilization of the hemolytic activity of the strains (except for the trans-complement strains) was also conducted. The HSA-dependent stabilization of hemolytic activity for SLS was observed at every time point of the assay, and the hemolytic activity reached a maximum at approximately 4 h of cultivation and was maintained for at least 12 h (Figure 4). This HSA-dependent stabilization of hemolytic activity was also observed in the culture supernatant obtained from prolonged culture (approximately 24 h) (Supporting Information: Figure S1).
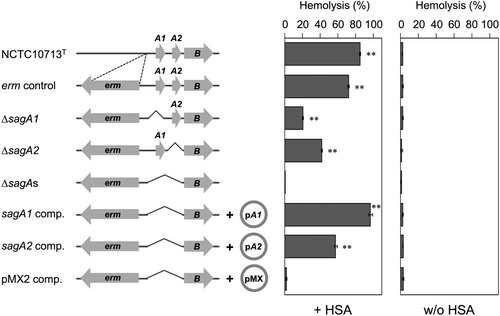
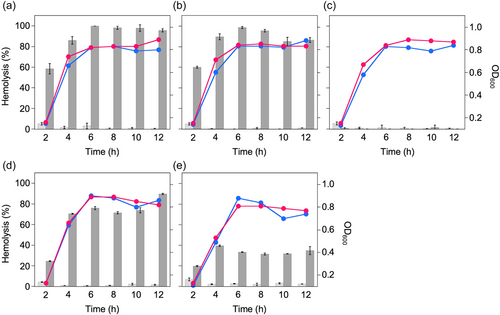
Regarding the role of serum albumin in the hemolytic activity of SLS, some findings have been reported for SLS produced by S. pyogenes so called “albumin hemolysin.”23 In addition, a review article on SLS describes that albumin is a carrier molecule of SLS and it stabilizes the hemolytic activity of SLS.33 In this study, the stabilization of SLS-dependent hemolytic activity of the SAA strain NCTC10713T was also observed in the presence of HSA. This result indicated that serum albumin, especially HSA, functioned as a stabilizer of SLS secreted from β-hemolytic SAA, regardless of the difference in amino acid sequence from the SLS of S. pyogenes.
Effect of HSA on the SLS-dependent cytotoxicity in human cells
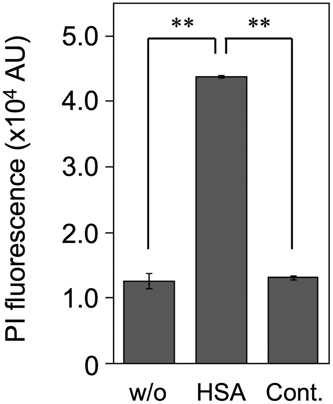
The effect of HSA on SLS-dependent cytotoxicity of β-hemolytic SAA was investigated using the human monocyte cell line THP-1. As depicted in Figure 5, the supernatant obtained from β-hemolytic SAA culture in the presence of 1.0% (w/v) rHSA indicated significantly higher cytotoxicity than that obtained from the culture in the absence of rHSA, indicating a low PI fluorescence intensity (Figure 5, “w/o” sample) similar to the result of THP-1 cells cultivated in the cell culture medium (Figure 5, “Cont.” sample). This result indicates that the cytotoxicity of the secreted SLS from β-hemolytic SAA is stabilized by rHSA.
Expression of genes encoding SLS-precursors in the presence or absence of HSA
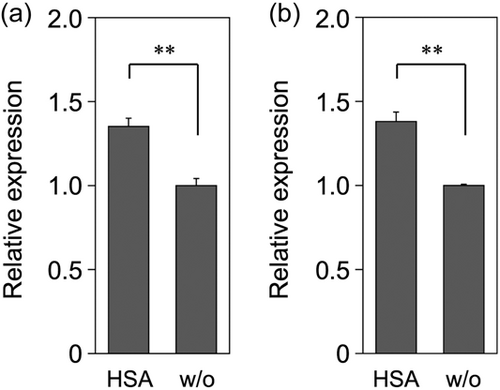
The expression of the genes encoding the SLS-precursors (sagA1 and sagA2) in the log-phase culture of the SAA strain NCTC10713T grown in the presence or absence of rHSA was investigated using relative RT-qPCR. As depicted in Figure 6, the expression of both sagA1 and sagA2 increased in the presence of rHSA compared with that in the absence of rHSA. Notably, although the expression of sagA1 and sagA2 was detected even in the absence of rHSA (Figure 6, “w/o” samples), no significant hemolytic activity was observed in the supernatants obtained from the SAA strain NCTC10713T culture grown in the absence of rHSA (Table 1, Table 2, Figure 3, and Supporting Information: Figure S2). Taken together, we suggest that rHSA in the culture medium mainly functions as a stabilizer of secreted SLS, whereas it does not play a significant role in the induction of sagA gene transcription in the SAA strain NCTC10713T.
 |
In S. anginosus, the catabolite control protein A (CcpA) functions in carbon catabolite repression (CCR) and directly regulates SLS expression.34 A similar regulatory mechanism was reported for S. pyogenes.35 Moreover, for pyogenic group streptococci, the contribution of the two-component system36-38 and quorum sensing39 to SLS production was reported. However, no information on the regulation of sagA transcription by serum albumin has been reported to date. Further investigation is needed to reveal the possible mechanism by which HSA induces sagA transcription.
HSA-induced stabilization of secreted SLS-dependent hemolytic activity in other SLS-producing streptococci
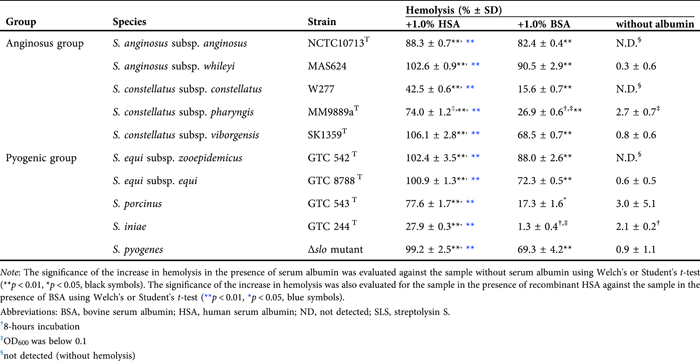
To investigate whether the concept found in β-hemolytic SAA strain NCTC10713T – HSA-induced stabilization of the secreted SLS-dependent hemolytic activity/cytotoxicity – is common to other SLS-producing streptococci, the HSA-induced stabilization of secreted SLS-dependent hemolytic activity of other SLS-producing streptococcal species (four species/subspecies belonging to AGS in addition to SAA strain NCTC10713T and five species belonging to the pyogenic group streptococci, including Δslo mutant of S. pyogenes) was also investigated. The species/subspecies used in this assay were selected based on a review article, which stated that SLS is the sole β-hemolytic factor of the species/subspecies.40 Consequently, stabilization of the hemolytic activity of the secreted SLS by serum albumin was also observed in other SLS-producing streptococcal species/subspecies, including the SLS-producing Δslo mutant of S. pyogenes, and higher hemolytic activity was observed in culture supernatants containing rHSA than in those containing BSA (Table 2). The marked stabilization of SLS-dependent hemolytic activity was observed in the samples in the presence of rHSA, and more than half of the samples prepared from the tested strains were retained even after incubation at 37°C (Supporting Information: Figure S3). The findings indicated that HSA-induced stabilization of the secreted SLS-dependent hemolytic activity and presumably secreted SLS-dependent cytotoxicity were observed not only in β-hemolytic SAA, but also in other SLS-producing streptococcal species/strains.
In this study, we investigated the HSA-dependent stabilization of hemolytic activity and cytotoxicity of SLS focused on the secreted SLS in the culture supernatant of β-hemolytic SAA and other SLS-producing streptococci. We could not investigate the interaction between purified SLS and HSA in this study because we had not prepared purified SLS, and the mechanism for the stabilization of SLS activity by HSA remains to be clarified. However, the results obtained from this study will be sufficiently informative to consider the pathogenic potential of SLS-producing streptococci in humans.
Pathogenic potential of SLS-producing streptococci in different environments
Many species and subspecies of opportunistic streptococci inhabit the oral cavity in humans. These streptococci are known to translocate easily into the bloodstream because of periodontal diseases and dental treatment, and the behavior of these oral streptococci in environments different from their natural residence is important for evaluating their pathogenic potential and risk of infection. However, sufficient information on their actual features in different environments, such as the bloodstream, has not yet been clarified.
Two types of representative β-hemolysins are pathogenic factors produced by human pathogenic and opportunistic streptococci: proteinaceous β-hemolysin, belonging to the CDC family, and peptide β-hemolysin SLS. Recently, the inhibitory effect of HSA on the cytotoxicity of SLO, the CDC produced by S. pyogenes, was reported.18 In contrast, in the present study, HSA stabilized the hemolytic activity/cytotoxicity of the secreted SLS not only from β-hemolytic SAA strain NCTC10713T but also from other SLS-producing streptococci. Thus, these different effects of HSA indicated that the SLS-dependent hemolytic activity/cytotoxicity from various streptococcal species/subspecies could be stabilized by HSA, whereas the SLO-dependent cytotoxic activity of S. pyogenes was inhibited. Moreover, in the presence of rHSA, SLS-dependent hemolytic activity was stabilized, and the growth of SLS-producing streptococci was enhanced compared with that of the culture conditions in the presence of BSA (Figure 7).
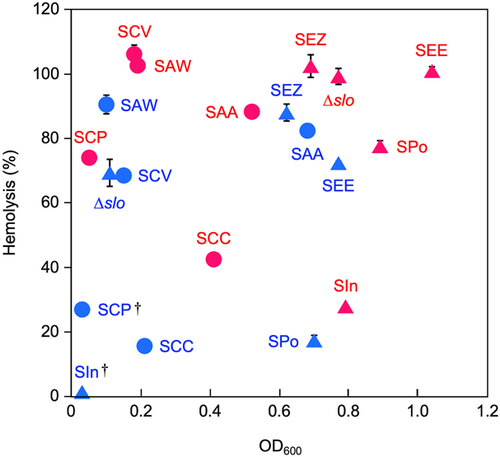
In the gingival crevicular fluid (GCF) from healthy humans, approximately 15 μg/μL of serum albumin is present.41 Our findings indicate that this concentration of serum albumin in GCF enables the stabilization of secreted SLS (Supporting Information: Figure S2), and SLS-dependent cytotoxicity against the host occurs in the case of SLS-producing β-hemolytic SAA is exposed to GCF. In addition, if the SLS-producing β-hemolytic SAA translocated into the bloodstream, the secreted SLS from the strain would be more effectively stabilized because of the higher concentration of serum albumin in the blood than in the oral cavity. Therefore, we strongly suggest that the pathogenic potential of SLS-producing human-opportunistic streptococci, including β-hemolytic SAA, is likely to be enhanced in the bloodstream in humans. This phenomenon facilitates understanding the pathogenicity of SLS-producing streptococci in human hosts.
The culture supernatant of S. anginosus is non-hemolytic upon addition of fetal calf serum.3 This result may depend on the difference in culture conditions between the previous and present studies; that is, the cultivation was conducted overnight in the previous study, whereas a shorter cultivation time of 4 h was chosen in this study. A longer cultivation period is likely to inactivate the hemolytic activity of the secreted SLS, even in the presence of albumin (Figure 1). Based on the results of the present study, we suggest that SLS produced by β-hemolytic SAA exhibits common characteristics of the well-known SLS produced by S. pyogenes. However, more detailed investigations are needed to characterize the SLS produced by β-hemolytic SAA and β-hemolytic AGS strains.
As for the bacterial β-hemolysin whose hemolytic activity is enhanced by HSA, only one report on the β-hemolysin produced from Vibrio has been published,42 except for the studies on the preparation of active SLS from bacterial cells of group A streptococci (S. pyogenes) to our knowledge. Regarding the interaction between HSA and other types of bacterial toxins, some studies reported the binding and autoproteolytic cleavage of Clostridium difficile toxins (TcdA and TcdB)16 and stabilization of Clostridium botulinum neurotoxin (BoNT/A).17 The former report is concerned with the host defense mechanism against C. difficile toxins and the latter is on therapeutic applications to protect against the effects of BoNT/A. Historical investigations of the interaction of SLS with serum and albumin of human origin were conducted focusing on the preparation of active SLS from the bacterial cells of group A streptococci.20, 21, 23, 25 In the present study, however, we revealed that HSA stabilizes the SLS secreted by AGS into the extracellular milieu, as well as that secreted by pyogenic group streptococci. These results are critically important for evaluating the pathogenic potential of SLS-producing streptococci, particularly those that are generally recognized as low- or nonpathogenic to humans. Since the mechanism of interaction between the secreted SLS and HSA have not yet been elucidated, we intended to characterize them as structurally and functionally complex. To reveal the true pathogenicity of SLS-producing streptococci, especially that of AGS, which is generally recognized as an opportunistic pathogen in humans, further investigations, both in vitro and in vivo, are necessary.
ACKNOWLEDGEMENTS
This work was supported by the JSPS KAKENHI Grant-in-Aid for Scientific Research (C) [grant numbers JP20K09919 and JP21K06655]. We are grateful to Dr Daisuke Takamatsu, Division of Bacterial and Parasitic Diseases, National Institute of Animal Health, National Agriculture and Food Research Organization, for gifting pMX2. We are also grateful to Dr Tomoko Sumitomo and Professor Shigetada Kawabata, Department of Oral and Molecular Microbiology, Osaka University Graduate School of Dentistry, for gifting the Δslo mutant of S. pyogenes strain NIH35 and Professor M. Kilian, Department of Biomedicine, Faculty of Health Sciences, Aarhus University, Denmark, for gifting S. constellatus subsp. viborgensis SK1359T. We would like to thank Editage (www.editage.jp) for English editing.
DISCLOSURE
The authors have no conflicts of interest to declare.
ETHICAL CONSIDERATION
Human serum was prepared from the blood of healthy volunteers under written informed consent in accordance with Protocol No. 4092 approved by the Ethics Committee of Tokushima University Hospital.
Open Research
DATA AVAILABILITY STATEMENT
The data in this manuscript are available within the article and supplemental materials.