OX40L enhances the immunogenicity of dendritic cells and inhibits tumor metastasis in mice
Lin Zhao and Wenjie Zhang contributed equally to this work.
Abstract
A well preserved immune system is a powerful tool to prevent foreign invasion or to suppress internal mutation, which must be tightly controlled by co-stimulatory molecules in different pathophysiological conditions. One such critical molecule is OX40L expressed on activated antigen-presenting cells (APCs). Consistently, its abnormality is associated with various immunological disorders such as autoinflammatory diseases and allergy. However, a comprehensive analysis of the immune-moderating role of OX40L in dendritic cells (DCs), the most powerful APCs to initiate immune responses in vivo, and investigation of its anti-tumor efficacy in the disease setting have not been performed properly. In this study, genetic approaches for both gain-of-function and reduction-of-function were employed to reveal that OX40L was required for the efficient presentation, but not uptake, of antigens by DCs to stimulate CD4+, as well as CD8+ T cells in vivo. As a result, CD4+ T cells were promoted towards Th1, but inhibited on Treg differentiation, by the LPS-induced OX40L on DCs, which was supported by their altered expression of co-inhibitory receptor, PD-L1. CD8+ T cells, on the other hand, also enhanced their cytotoxicity towards target cells in response to OX40L expression on the DCs transferred in vivo. Finally, in a DC-mediated tumor immunity model, the strong immunogenic roles of OX40L on DCs led to better metastasis inhibition in vivo. Collectively, our results demonstrate that OX40L could serve as a potential target in the DC-based vaccine for enhanced anti-tumor efficacy in vivo.
Abbreviations
-
- APCs
-
- antigen presenting cells
-
- CFSE
-
- carboxyfluorescein succinimidyl ester
-
- CTL
-
- cytotoxic T lymphocyte
-
- DCs
-
- dendritic cells
-
- EAE
-
- experimental autoimmune encephalomyelitis
-
- ERK
-
- extracellular regulated protein kinases
-
- GM-CSF
-
- granulocyte-macrophage colony-stimulating factor
-
- FOXP3
-
- forkhead box P3
-
- ITIM
-
- immunoreceptor tyrosine-based inhibitory motif
-
- ITSM
-
- immunoreceptor tyrosine-based switch motif
-
- mTOR
-
- molecular target of rapamycin
-
- NK
-
- natural killer
-
- OX40L
-
- OX40 ligand
-
- OVA
-
- ovalbumin
-
- PD-1
-
- programmed death-1
-
- RA
-
- rheumatoid arthritis
-
- TGF
-
- transforming growth factor
-
- Th cells
-
- T helper cells
-
- TNSF
-
- tumor necrosis factor superfamily
-
- Tregs
-
- regulatory T cells
INTRODUCTION
OX40L, a member of the tumor necrosis factor superfamily (TNSF), can activate its cognate receptor OX40 to cause a series of immune responses. Whereas OX40 is found on the activated T cells, regulatory T cells (Tregs), natural killer (NK) cells, NKT cells, and neutrophils, OX40L is an inducible surface molecule expressed mainly on antigen presenting cells (APCs), such as B cells, macrophages, and dendritic cells (DCs), although it can also be found to be expressed on other cell types such as Langerhans cells, endothelial cells, smooth muscle cells, mast cells, and NK cells under certain circumstances.1-3 Many studies demonstrated that OX40L on the APCs is closely related to T cell-mediated immune responses. For example, it has been found that the interaction between OX40L and OX40 enhances the proliferation, differentiation, and survival of T cells and prevents the development of Tregs.4, 5 In terms of proinflammatory T cell responses, further studies proved that OX40–OX40L interactions can enhance the Th1-mediated immune response, and promote the generation and maintenance of Th2 cells.4, 6-8
Consistent with the proinflammatory effects of OX40L on immune responses, several studies have demonstrated that OX40–OX40L interaction plays a crucial role in a variety of immunological diseases, including experimental inflammatory bowel disease, experimental autoimmune encephalomyelitis (EAE), rheumatoid arthritis (RA), colitis, and graft-versus-host disease.9-13 In addition, OX40L has also been confirmed to be associated with the pathogenesis of atherosclerosis.14, 15 As a result, blocking the OX40–OX40L interaction significantly improved these specific immune diseases, which suggests that interruption of the OX40–OX40L interaction may be a novel therapeutic strategy to treat many diseases.16, 17
Within the APCs, DCs are the most potent that can efficiently absorb, process, and present antigens to activate naïve T cells. Immature DCs are endowed with a strong migratory ability, which allows them to pass antigenic information from the periphery immediately to lymphoid organs where an army of adaptive T cells could be quickly activated. Other APCs, on the contrary, although they might be better at phagocytosing antigens, are mostly immobile following capturing the antigens. In addition, DCs become mature along the migratory pathway, and up-regulate co-stimulated molecules on their surface to effectively activate T cells for the initiation, regulation, and maintenance of the immune responses. Among these co-stimulatory molecules, OX40L plays a key role in the survival and homeostasis of effector and memory T cells. Therefore, it is important to investigate the auxiliary function of this key costimulatory molecule on DCs, the most potent and efficient APCs in vivo, in the host immunity.
Although a number of studies have been conducted in the past to address this issue,18-21 different experimental conditions gave rise to different outcomes, some of which were even contradictory to the others.20, 21 In the current study, we hereby set up to examine comprehensively the impact of OX40L on the immunological functions of purified bona fide DCs in vivo by the adoptive transfer of stable DC lines with either ox40l gene knocked-down or overexpressed. Furthermore, the antitumor efficacy of OX40L on DCs as a potential target were also evaluated in an antigen specific tumor model to provide a novel therapeutic idea for tumor treatment.
MATERIALS AND METHODS
Mice and cells
Specific pathogen-free (SPF) C57BL/6, and Kunming mice, aged 8–11 weeks, were purchased from the Model Animal Research Center of Anhui Normal University (ANU) Animal Facilities. All procedures conducted on mice were in accordance with the conditions specified and approved by the ANU Animal Experimentation Ethics Committee.
DC2.4 cells, an immature DC cell line established from the culture of C57BL/6 mice bone marrow progenitors in the presence of GM-CSF,22 were kindly provided by Professor Yongjun Dong from Tsinghua University, China. B16 melanoma cells overexpressing OVA antigen (B16-OVA) were a kind gift from Professor Xuefeng Wang, Suzhou University, China.
Cell cultures
The cells were cultured using standard methods as described before.23 Briefly, B16-OVA cells, spleen cells separated from Kunming mice, the immature murine DC2.4, and its transduced derivatives were all cultured in RPMI1640 medium, supplemented with 100 U/mL penicillin, 100 mg/L streptomycin, 2 mmol/L L-glutamine, 50 µM 2-ME, and 10% FBS.
Antigen cross-presentation
The antigen cross-presentation assay was performed using standard methods as described before.24 Briefly, vectorctrl DCs and OX40Lkd DCs were stimulated with LPS (1 μg/mL) and pulsed with OVA (0.5 mg/mL), before the cells were harvested and stained with SIINFFKL-APC for FACS analysis.
Proliferation assay in vitro
The proliferation of T cells in vitro was performed using standard methods as described before.25 Briefly, CD4+T from Kunming mice were sorted by FACS before they were CFSE-labeled to co-culture with LPS pre-activated gene-modified DCs for 3 days in different proportions (T cells: DCs = 1:5, 1:10, 1:20, 1:40) in 24-well plates. The proliferated cells were calculated by the number of acquired CFSElow CD4+ T cells × added BD calibrated APC beads/acquired bead number.
CD4+ T cell differentiation assays in vivo
The differentiation of T cells in vivo was performed using standard methods as described before.26 Briefly, C57BL/6 mice were immunized with 1 × 106 OVA-pulsed vectorctrl DCs and OX40Lkd DCs three times with 1 week between before the spleen cells were obtained and perforated. The CD4+ T cells were stained intracellularly with IFN-γ, IL-17A, and IL-4 or the cells were fixed, perforated by the Foxp3 buffer set, and then stained intracellularly for Foxp3 for FACS analysis.
Analysis of antigen uptake ability of DCs
The phagocytosis assay was performed using standard methods as described before.27 Briefly, 0.2 × 106 gene modified DCs were incubated at 37°C for 4 hr with fluorescent chicken ovalbumin (OVA-FITC) (50 µg/mL) in 500 mL complete RPMI-1640 medium. After washing with phosphate-buffered saline, the cells were analyzed immediately by flow cytometry. Cells incubated with OVA-FITC at 4°C were used as a negative control.
Cytotoxic T lymphocyte (CTL) in vivo
OVA pulsed DCs stimulated with 1 µg/mL LPS were used to immunize mice once a week for two consecutive weeks.28 The mice injected with PBS only were used as non-immunized controls. In the third week, splenocytes from C57BL/6 mice were differentially labeled with either a high concentration of CFSE (10 μM) and loaded with OVA protein or a low concentration of CFSE (2 μM) but not loaded with OVA protein as CFSEhi and CSFElow target cells, respectively, and a total of 10 × 106 target cells per mouse were injected, consisting of a mixture of the CFSEhi and CSFElow cells in a 1:1 ratio. In the other case, a similar experiment was conducted except DCs were pulsed with 1 mg/mL B16 lysates instead of OVA protein, whereas CFSEhi and CSFElow target cells were pulsed with B16 lysates and spleen cell lysates, respectively. 24 hr later, the percentages of residual CFSEhigh and CFSElow target cells remaining in the recipients’ spleens were analyzed by flow cytometry. The percentage of specific lysis was calculated using the following equation: % specific lysis = 100% – (%CFSEhigh – %CFSElow)/(%CFSEhigh in PBS Ctrl – %CFSElow in PBS Ctrl).
Tumor experiments in vivo
The establishment of the tumor model was performed using standard methods as described before.24 Briefly, on day 0, C57BL/6 mice were immunized s.c. with 1 × 106 gene-modified DC pulsed with 100 μg/mL OVA protein or B16 lysates and stimulated with 1 μg/mL LPS. On day 4, 9 × 105 B16-OVA cells or B16 cells were injected i.v. into mice. On day 18, the lungs were harvested and counted for tumor nodules until a limit of 250 nodules per lung. In another experiment, C57BL/6 mice were immunized s.c. in the left side of the back with 1 × 106 gene-modified DCs pulsed with 100 μg/mL B16 lysate and stimulated with 1 μg/mL LPS weekly. Two weeks later, 1 × 106 B16 melanoma cells were injected s.c. in the right side of the back of the mice, and the growth of the tumors was monitored by measuring their size at the indicated time.
RNA isolation and RT-qPCR analysis
RNA extraction and RT-qPCR analysis were performed using standard methods. Briefly, RNA isolation and cDNA transcription were performed using a kit according to the manufacturer's instructions. Real-time qPCR with SYBR Green was performed using a CFX96 real-time qPCR detection system. Analysis was performed using CFX Manager Software (Bio-Rad). All data were expressed relative to actin as an internal control. The primers used are listed in Table 1.
| Gene product | Sequence (5′→3′) |
|---|---|
| qOX40L-forward | ACG CTA AGG CTG GTG GTC TCT G |
| qOX40L-reverse | TCC TCA CAT CTG GTA ACT GCT CCT C |
| β-Actin-forward | TCA TCA CTA TTG GCA ACG AGC |
| β-Actin -reverse | AAC AGT CCG CCT AGA AGC AC |
Statistical analysis
All experiments were repeated at least three times with similar results. Statistical differences between the groups were determined by using an unpaired t-test.
RESULTS
Successful construction of gene-modified DCs
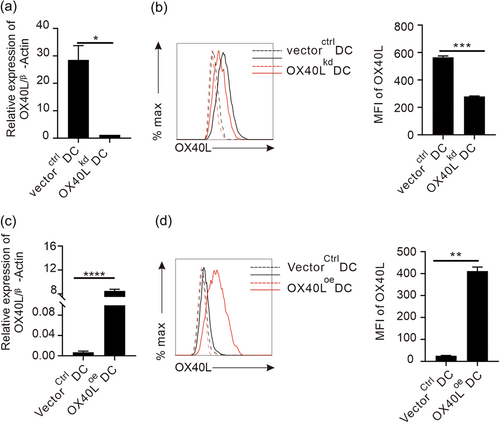
To genetically modify OX40L on DCs, the CDs region of ox40l gene was amplified from mouse splenic cells containing the ox40l gene sequence, and cloned into an overexpressing lentiviral vector pLJM-EGFP (Supporting Information: Figure 1) by restriction enzyme AgeI digestion and T (4) DNA ligase ligation. In the same way, a short hairpin knocking-down construct for OX40L was made by synthesizing shox40l and cloning into a lentiviral vector pLKO.1 (Supporting Information: Figure 2). After transformation into active E. coli cells, these two recombinant constructs were amplified in the bacteria, and positive clones were identified by PCR (Supporting Information: Figure 5a), before the correct DNA constructs were confirmed again by sequencing (Supporting Information: Figure 4). Furthermore, the confirmed ox40l-modifying constructs and two packaging plasmids (Supporting Information: Figure 3) were co-transfected into human embryonic kidney cell line 293FT cells in the presence of liposome transfection reagent to produce competent lentiviral particles for effective infection of an immobilized DC line, DC2.4.22 The transfection efficiency of the EGFP-containing construct was evaluated under a fluorescence microscope (Supporting Information: Figure 5b), and the expression of the OX40L gene and protein in transfected cells was detected by qPCR and flow cytometry, respectively. Following infection of the lentivirus containing recombinant pLKO.1-shOX40L plasmid that can knock-down ox40l gene expression in the target cells, the DCs with ox40l knocked-down (OX40Lkd DCs) had a significantly compromised expression of OX40L transcript than the DCs infected with virus containing control vector, vectorctrl DCs (Figure 1a). In addition, the successful knocking-down of gene expression in the OX40Lkd DCs was further verified in protein expression (Figure 1b). Likewise, an overexpression DC line for OX40L (OX40Loe DCs) was also successfully established by lentiviral infection of the DCs with recombinant pLJM-OX40L plasmid, expressing more OX40L transcripts and protein than that of mock-transduced DCs with same vector, VectorCtrl DCs (Figure 1c,d).
OX40L facilitates antigen presentation of DCs independent of antigen uptake
With valid modification of OX40L expression in DCs, we next examined its impact on the function of DCs. As professional antigen presenting cells, DCs primarily present antigenic information to stimulate T cells in vivo, triggering a series of immune reactions. Therefore, we first examined the T cell stimulation capacity of DCs affected by the OX40L. Purified CD4 + T cells in the spleen of Kunming mice were sorted by flow cytometry, stained with CFSE, and co-cultured with LPS-stimulated OX40Lkd DCs or vectorctrl DCs in 96-well plates for 72 hr before the proliferation of CD4 + T cells in the co-culture system was measured by CFSE dilution. As shown in Figure 2a, we found that with the increase of the DC proportion in the co-culture system, the proliferation of CD4 + T cells also increased, indicating a dose-dependent stimulation of T cells by DCs as expected. Compared with vectorctrl DCs, OX40Lkd DC-mediated CD4 + T cell proliferation, however, was significantly reduced, although the viability of the viral transfected DCs was the same (data not shown), suggesting an essential role of OX40L on DCs to stimulate CD4 + T cells.
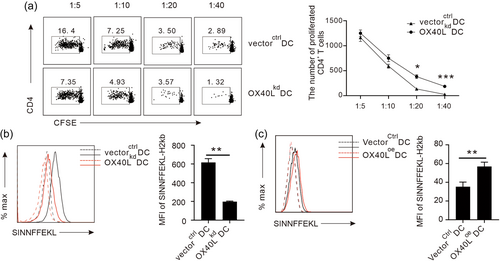
In addition to CD4 + T cells, DCs can also stimulate CD8 + T cells via mechanism of cross-presentation of exogenous antigen. After being pulsed with OVA antigen and stimulated by LPS for 24 hr, the cross-presentation capacity of the DCs with OX40L knocked-down was examined by their display of a fragment of specific OVA peptide bound to MHC-I molecule on the cell surface for CD8 + T cell recognition. Consistent with direct presentation data to stimulate CD4 + T cells in the MLR experiment, the cross-presentation capacity of DCs to stimulate CD8 + T cells was also greatly reduced when OX40L expression was suppressed, as OX40Lkd DCs expressed significantly lower amounts of SIINFFEKL-H2kb than vectorctrl DCs (Figure 2b). To further prove the direct positive regulation of cross-presentation of DCs by OX40L, a reverse approach was conducted in which OX40L was overexpressed on the OX40Loe DCs. Interestingly, in contrast to their knock-down counterparts, the OX40Loe DCs demonstrated a significantly higher capacity to present SIINFFEKL-H2kb compound on their surface than the controls (Figure 2c). To rule out the possibility of differential antigen intake affected by the OX40L variation, we further examined the uptake of OVA antigen by the gene modified DCs and found OX40L did not affect the OVA uptake in the cultures (Supporting Information: Figure 6). Collectively, these data suggest that OX40L promotes the antigen presentation of the DCs to stimulate both CD4 + and CD8 + T cells in vivo.
OX40L on DCs enhances proinflammatory CD4 + T cell differentiation in vivo
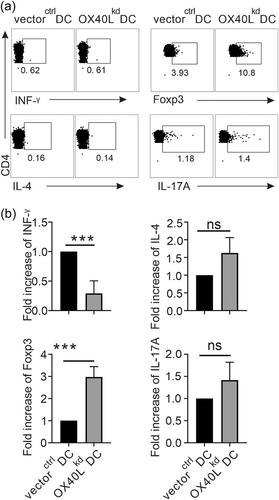
In response to the stimulating antigen presented by the DCs, CD4 + T cells will proliferate several cycles and differentiate into various effector cells in the context of different environment. To investigate whether OX40L on DCs affects T cell differentiation in vivo following activation, the DCs with OX40L knocked-down or not, were pulsed with OVA and stimulated with LPS overnight before injection into C57BL/6 mice. The spleen cells from the immunized mice were collected, and cultured in the presence of PMA, BFA, and ionomycin. After 4–6 hr, their intracellular expressions of INF-γ for Th1, IL-4 for Th2, IL-17A for Th17, and Foxp3 for Treg differentiation were detected by flow cytometry. Compared with vectorctrl DCs, OX40Lkd DCs had better potential to promote the differentiation of antigen-specific CD4 + T cells into Treg but inhibit Th1 immunity in vivo, whereas the Th17 differentiation were similar in the mice injected by these two types of DCs (Figure 3). Interestingly, Th2 differentiation in vivo, indicated by IL-4 secretion, did not seem to be affected by OX40L on the DCs injected, a result different from others.7, 29-31 Furthermore, the T cell differentiation profiles affected by the DCs with suppressed OX40L expression were reversely proven by the experiments in which OX40L overexpressed DCs were transferred in vivo (Supporting Information: Figure 7). Overall, the differential effects of OX40L-modified DCs on T cell differentiation in vivo suggest that OX40L specifically promote the DC-mediated pro-inflammatory immunity, at least in part by suppressing Treg differentiation.
Up-regulated PD-L1 on the DCs with suppressed OX40L expression correlates with their ability to promote Treg differentiation in vivo
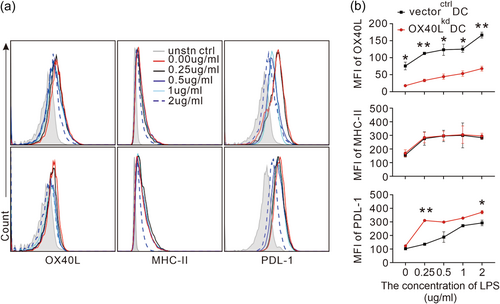
The proinflammatory effect of OX40L from DCs on T cell differentiation, especially the inhibitive effect on Treg, prompted us to investigate the mechanisms by which this surface molecule works on the DCs. Since the T cell immunity assays in vivo were performed with adoptive transfer of DCs following LPS stimulation, and OX40L is an inducible co-stimulatory molecule that works with antigen presenting receptor and other co-inhibitory molecules, we performed an in vitro experiment to examine MHCII and PDL-1 expression on the OX40L-modified DCs in the presence of increasing concentrations of LPS stimulation. We found that although OX40L protein expression on the surface of OX40Lkd DCs could still be up-regulated following LPS stimulation, it failed to go as high as the mock-transduced DCs at every concentration tested (Figure 4a, Figure 4b upper left panel). Under the same stimulation condition when OX40L protein expression was compromised, the expression of antigen-presenting receptor MHCII was not affected (Figure 4a, Figure 4b upper right panel). PD-L1, a co-inhibitive receptor that is associated with Treg induction, however, was elevated (Figure 4a, Figure 4b lower left panel). Thus the specific up-regulation of PD-L1 in the OX40Lkd DCs could partly account for the OX40L-suppressed Treg differentiation in vivo.
OX40L promotes cytotoxicity of CD8 + T cells in vivo
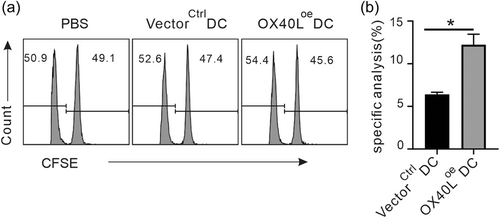
Following the inspection of CD4 + T cells, the function of CD8 + T cells under the influence of OX40L on DCs would also be examined in vivo. To this end, either OX40Loe DCs or OX40Lkd DCs with their mock-transduced counterparts, were pulsed by OVA and stimulated by LPS overnight, before they were injected into C57BL/6 mice for two consecutive weeks to prime OVA specific CD8 + T cells. Seven days later, OVA pulsed CFSE high and irrelevant-peptide pulsed CFSE low splenic cells from homologous mice as target cells were mixed in a 1:1 ratio and injected intravenously into the immunized mice. Because exogenous OVA-pulsed, CFSEhigh splenic cells can be killed via the cytotoxic response of the OVA specific CD8 + T cells sensitized earlier in vivo by the OVA-pulsed DCs, the changed proportion of CFSEhigh and CFSElow splenic cells can be used to judge the strength of cytotoxicity. Consistent with the results of antigen cross-presentation capacity of the genetically modified DCs in vitro (Figure 2b,c), the cytotoxicity of CD8 + T cells in vivo was significantly enhanced in the presence of OX40Loe DCs (Figure 5), but weaker in the presence of OX40Lkd DCs (Supporting Information: Figure 8), compared with their controls respectively.
Antigen specific anti-tumor efficacy is enhanced after adoptive transfer of OX40L-overexpressed DCs
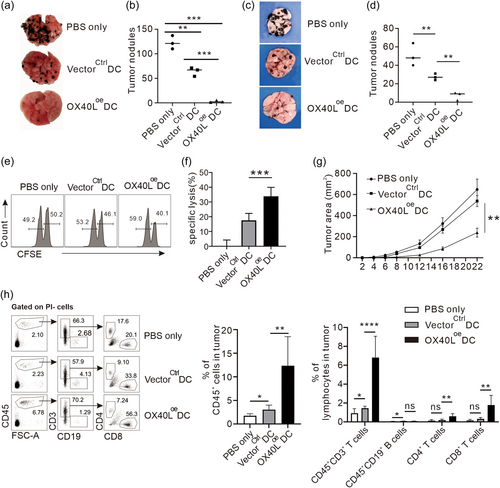
Identification of the positive roles of OX40L in DC-mediated immunology in vivo promotes us to verify them in an actual tumor disease setting, which is relatively less investigated in the field. To create an antigen specific model for the analysis of the role of DCs, we used B16 melanoma cells transduced to express OVA protein, against DCs pulsed to present the OVA antigen. Four days before intravenous injection of B16-OVA melanoma cells, OVA pulsed OX40Loe DCs or vectorctrl DCs were inoculated or not in the right heel and left heel to allow tumor metastasis. Fourteen days later, the lungs were collected and the tumor nodules were counted. B16-OVA tumor cells metastasized to the lungs and formed a large number of tumor nodules in the non-immunized animals (PBS-treated), indicating a successful establishment of a metastatic tumor model (Figure 6a). Noticeably, compared with the PBS-treated mice, inoculation of OVA-pulsed VectorCtrl DCs can greatly reduce the number of lung tumor nodules, indicating the presence of antigen-specific anti-tumor immunity by the DCs. Interestingly, this antigen specific tumor inhibition by the DCs was greatly enhanced by OX40Loe DCs, represented by a significant reduction of the tumor nodules (Figure 6b), indicating the immunostimulatory role of OX40L on DC-mediated immunity in vivo, which could be related to the enhanced activity of cytotoxic T cells and suppressed Treg differentiation identified above. To strengthen our anti-tumor regime in a closer preclinical setting, we used wild-type B16 melanoma cells against DCs directly pulsed with B16 tumor cell lysates instead of OVA to present tumor-associated antigens, and the derived results were consistent with that of OVA in that the overexpression of OX40L on DCs enhanced their antitumor efficacy (Figure 6c,d). Furthermore, we also evaluated the activities of antigen-specific CD8-T cells in the DC immunized mice pulsed with the tumor lysates by transferring splenocytes coated with the same tumor cell lysates or irrelevant cell lysates as readouts of tumor cell-specific cytotoxicity. In agreement with the better antitumor efficacy in Figure 6c, OX40Loe DCs had greater tumor cell-specific cytotoxicity than that of VectorCtrl DCs, which also had about 18% specific lysis in comparison with no tumor lysis in the PBS-immunized animals (Figure 6e,f). In addition to the metastatic model, the inhibitive effect of OX40L-modified DCs pulsed directly with the tumor lysates on the local growth of primary tumor were also examined. The results showed that although VectorCtrl DCs could slightly inhibit the growth of tumor, immunization with OX40Loe DCs led to a much smaller tumor size at each time point measured (Figure 6g). Consistently, in the same experimental system, we also detected a significant increase in the proportion of infiltrated CD45+ immune cells in the tumors of the mice immunized with DCs overexpressing OX40L by flow cytometry. Of which, many fewer B lymphocytes were observed than T lymphocytes that contained mostly cytotoxic CD8+ subset (Figure 6h). Collectively, these data indicate that OX40L on DCs can boost the prime APCs as a vaccine to enhance proinflammatory immune responses in vivo, which ultimately results in enhanced antitumor efficacy in disease settings.
DISCUSSION
In this study, we have systematically studied the immunomodulatory effect of OX40L on DCs in vivo by the adoptive transfer of genetically modified myeloid DCs with either gain-of-function or reduction-of-function. The stable knockdown or overexpression of ox40l gene in DCs were obtained by lentiviral transfection of the modifying units into the genome DNA of target cells to explore the role of OX40L in DC-mediated T cell immunity, and its role in tumor immunity for potential therapeutic application. Consistent with the reported role of OX40L on APCs, this co-stimulatory molecule is found to be critical for enhancing the immunogenic effect of DCs to stimulate T cells in vivo, with unique features in skewing the differentiation of CD4+ T cell differentiation towards proinflammation direction by up-regulating Th1 but down-regulating Treg, and enhancing the cytotoxicity of CD8+ T cells to directly kill antigen specific target cells. Ultimately, the enhanced host immunity against foreign antigens, especially the cellular antigens induced by the OX40L on DCs, led to better antitumor efficacy with suppressed tumor metastasis. Of note, in the current study we found that the OX40L enhanced the T cell stimulation capacity of DCs by facilitating antigen presentation, rather increasing antigen uptake, and suppressed Treg differentiation by down-regulation of PD-L1 expression on the DCs.
It has been well documented that the OX40L–OX40 interaction promotes the binding of the MHC–antigen peptide complex on the APCs to the T cell surface receptors and provides essential signals for T cell activation.32-35 Among the professional APCs, DCs are the most efficient as they are the only cells that can present antigens to activate naive T cells at steady status. However, the detailed study for the roles of OX40L on DCs has not been systematically performed in vivo. With the generation of OX40L-deficient mice, it was found that the mutant animal suffered apparent suppression of the recall reaction of T cells, accompanied by a concomitant reduction of both Th1 and Th2 cytokines, and a significant reduction in humoral immune responses as well, in response to both protein antigens and alloantigens.1 However, since the OX40L was knocked-down in the whole animal, they concluded the compromised antigen specific T cell responses in vivo was caused by an impaired intrinsic APC function.1 To characterize the role of OX40L on DCs, the rare cell type but most potent APCs, one study turned to culture in vitro to generate large amounts of DC-like cells from myeloid leukemia cells (leukemia-DCs). They found that retroviral transduction of the DC-like cells with OX40L elicited a higher proliferative response of allogeneic CD4+ T cells, which were prone to differentiate into Th1 cells in the co-cultures.36 Consistently in the current study, OX40L on purified DCs enhanced CD4+ T cells proliferation and differentiation towards Th1 (Figure 3). In addition, we also found that this co-stimulatory molecule from the TNF family can increase cross-presentation of antigenic peptide on DCs to CD8+ T cells (Figure 2), which is not at the expense of increasing antigen uptake (Supporting Information: Figure 6). As a result, the killing capacity of cytotoxic T lymphocytes for the cellular antigen is augmented in vivo (Figure 5).
In line with our enhanced stimulation of CD8+ T by OX40L on DCs, the proliferation and survival of OX40-deficient CD8+ T cells were compromised in systems with immunization of protein in adjuvant, delivered by adenoviral vector, or expressed on tumor cells.37-39 The mechanisms of CD8+ T cell activation in the current study could be multi-fold. First, OX40 is also expressed on CD8+ T cells although its expression is much more transient than on CD4+ T cells,38 therefore, direct activation is possible. Secondly, since CD4+ T cells express OX40 longer, indirect activation through augmenting the activity of CD4+ T helper cells could be also a likely reason as was demonstrated by injection of agonist antibodies to OX40 in virus systems.40 Finally, some primary CD8+ T cell responses to vaccinia virus were found to be strongly dependent on OX40, probably through the production of inflammatory cytokines, holding the Th1 cytokines induced in vivo in our system partly responsible.41
For the differentiation of CD4+ T cells, most studies revealed that OX40L on DCs promoted Th2 but inhibited Treg polarization.42 Interestingly, the results of our study in vivo disagreed with the former, but agree with the latter (Figure 3). Since it was believed that OX40L lost the ability to polarize Th2 cells in the presence of IL-12,30 we examined our LPS-stimulated DCs in the experiment and found decent amounts of IL-12 production (data not shown), suggesting this Th1 cytokine might be a possible reason for the failure of Th2 promotion in the current study. In addition to IL-12, another proinflammatory cytokine was also reported to interfere with OX40L mediated Th2 induction. For example, in an experiment where OX40 was stimulated with monoclonal antibody in combination with DCs pulsed with antigen, a biased Th1 immune response in vivo was found, although the anti-OX40 treatment enhanced the production of Th2 cytokines in IFN−γ−/− mice after immunization with Ag-pulsed DCs.43
Consistent with our Treg inhibition, OX40 signaling has been shown to suppress Foxp3 expression in naïve T cells, preventing their conversion to pTreg cells in the presence of TGF-β1.5, 44 Further down the track, we found that a co-inhibitory molecule PD-L1 is specifically up-regulated in the presence of diminished OX40L expression on DCs, when MHCII expression is intact on the same cells. As a member of the B7 family that can bind to PD-1 on activated T cells, PD-L1contains both ITIM and ITSM to recruit SHP-1 and SHP-245, 46 for the de-phosphorylation of Akt, mTOR, S6, and ERK2, the combination of which is required for efficient Treg differentiation and development in vivo.47, 48 Given its close relationship with Treg induction, we proposed that inhibition of PD-L1 expression in the presence of OX40L might be the additional mechanism to explain the suppressed immunological effect of DC-derived OX40L in vivo.
Consistent with approaches such as injection of agonist OX40L-Ig fusion proteins, OX40 antibodies, and OX40-binding RNA aptamers that have been proven effective in suppressing tumor growth in many animal models,49-51 adoptive transfer of overexpressed OX40L on DCs also leads to better inhibition of tumor metastasis, as significantly fewer pulmonary melanoma nodules were observed than the controls (Figure 6). This could be due to either a direct action on up-regulation of cytotoxic activity of CD8+ T cells, or promoting Th1 differentiation to help CD8+ T cells, or combination of the two. Furthermore, since tumor infiltrating Foxp3+ Treg express high levels of OX40, and intratumor triggering of OX40 suppressed the activity of these Treg,52 the inhibitive effect of Treg differentiation by OX40L on DCs should also contribute to the better anti-tumor efficacy to some extents.
Overall, the concomitant effect on promoting T cell immunity for both CD4+ and CD8+ T cells in vivo by OX40L on DCs makes this molecule a very attractive target to induce long-term protection against tumor growth. Since DCs are powerful stimulators of T cell responses and have long been used as vaccine to boost host immunity, our genetic upregulation of such strong immunogenic molecule on DCs will be both feasible and applicable in clinical trials to recover compromised anti-tumor immunity in cancer patients.
AUTHORS CONTRIBUTION
L.Z. and W.Z. performed the experiments, wrote the first draft of the manuscript; M.L., R.J., J.W., and F.W. helped in performing the experiments; Y.X. designed the study, supervised the work, and wrote the manuscript. All authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
This work is supported by National Natural Science Foundation of China Major Research Plan Project (91742101); Natural Science Foundation of Anhui Province, China (1608085MH160); Anhui International Science and Technology Collaborative Project, China (1604b0602017); Anhui Provincial Key Laboratory of Molecular Enzymology and Mechanism of Major Diseases, and Key Laboratory of Biomedicine in Gene Diseases and Health of Anhui Higher Education Institutes.
DISCLOSURE
The authors declare no conflict of interest.
ETHICS STATEMENT
All procedures conducted on mice were in accordance with the conditions specified and approved by the ANU Animal Experimentation Ethics Committee.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.