Real-world safety and effectiveness of adalimumab in patients with hidradenitis suppurativa: A 52-week analysis of a postmarketing surveillance study in Japan
Abstract
Adalimumab is a human monoclonal antibody against tumor necrosis factor-α that was approved in Japan for the treatment of hidradenitis suppurativa (HS), a chronic recurrent inflammatory skin disease. We report the results of the final analysis of the postmarketing surveillance (PMS) study (ClinicalTrials.gov: NCT03894956), which evaluated the 52-week safety and efficacy of adalimumab for HS treatment in real-world clinical practice in Japan. This multicenter, prospective, open-label, observational study (March 2019 to May 2021) included patients with HS treated with subcutaneous adalimumab at doses following the package insert. The primary endpoint was safety, and the secondary endpoints were effectiveness, including HS clinical response (HiSCR), C-reactive protein (CRP), skin pain, and Dermatology Life Quality Index (DLQI). Of the 84 patients registered at 65 sites, 83 patients were included in the analyses. Adverse drug reactions (ADRs) were reported by 10 (12.0%) patients; two patients reported a serious ADR, including one patient with serious infection. Other safety events of special interest reported were liver disorder and dermatitis psoriasiform (one patient each). Almost all patients with ADRs were recovering or had recovered, except for one patient who experienced a serious ADR of liver disorder and died. At 12 weeks, 55.4% of patients achieved HiSCR; this increased to 60.5% and 62.8% at 24 and 52 weeks of adalimumab treatment, respectively. Significant reductions from baseline in CRP (P < 0.05), skin pain (P < 0.0001), and DLQI (P < 0.0001) were observed at all time points. The results from this PMS study demonstrated that long-term adalimumab treatment is well tolerated and effective in patients with HS in real-world clinical practice in Japan.
1 INTRODUCTION
Hidradenitis suppurativa (HS) is a chronic recurrent inflammatory disease that typically affects the apocrine gland-bearing skin, including axillae, groin, and buttocks.1 The global prevalence of HS, which is estimated to be 0.00033%–4.1%,2 varies markedly among geographic regions and ethnicity.3 HS is associated with painful inflammatory nodules, abscesses, sinus tract formations, and scarring, which can have a substantial impact on the patient's mental health, social functioning, and quality of life (QOL).4-8 The pathogenesis of HS is not fully understood, making disease management challenging. However, it is thought to be multifactorial, with genetic, immunological, behavioral, and endocrine factors interplaying in the development of HS.9 In particular, pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), are elevated in patients with HS10, 11 and may be involved in the excessive inflammatory responses that are observed in HS lesions.12
Adalimumab is a human monoclonal antibody against TNF-α that is approved for the treatment of HS in Europe, the USA, Canada, and Japan. Adalimumab has demonstrated efficacy and safety in patients with moderate-to-severe HS in several phase III clinical trials13, 14 and real-world observational studies.15, 16 In the PIONEER I and II studies,13 significantly higher clinical response rates were observed at 12 weeks in patients receiving adalimumab compared with those receiving placebo; the incidence of adverse events (AEs) was similar between the treatment groups. A phase III, open-label, single-arm study of adalimumab in Japanese patients with moderate-to-severe HS also showed sustained efficacy, with 66.7% of patients achieving clinical responses at 52 weeks, with no severe or new AEs reported.14 Furthermore, recent real-world observational studies conducted in Europe and Canada have demonstrated the efficacy of adalimumab, reporting sustained clinical responses through 52 weeks in patients with HS.15, 16 However, there are no long-term data on the effectiveness and safety of adalimumab in Japanese patients with HS in a real-world clinical setting.
A postmarketing surveillance (PMS) study of adalimumab in Japanese patients with HS was therefore conducted.17 In the 12-week interim analysis, 57.4% of patients had achieved a clinical response at 12 weeks of adalimumab treatment, and skin pain, C-reactive protein (CRP), and QOL, assessed by the Dermatology Life Quality Index (DLQI), had significantly improved from baseline; no serious infections or new safety concerns were reported. Here we report the final analysis of the PMS study and evaluate the 52-week safety and effectiveness results of adalimumab treatment in patients with HS in real-world clinical practice in Japan.
2 METHODS
2.1 Study design
This was a multicenter, prospective, open-label observational study conducted at 65 centers in Japan between March 11, 2019 and May 14, 2021. The study design has been described previously in the 12-week interim report.17 Briefly, patient enrollment started in April 2019 and ended in February 2020. Patients were registered using a central registration method, and their data were collected using a case report form, which was completed by the physician in charge of the study and submitted via an internet-based electronic data capture system. The study was conducted in accordance with the GPSP Ordinance (Good Post-marketing Study Practice; Ministerial Ordinance No. 171 of the Ministry of Health, Labour and Welfare dated December 20, 2004) and was registered at ClinicalTrials.gov (identifier: NCT03894956). All patients provided informed consent prior to study participation.
2.2 Study population
Patients included in this study were those diagnosed with HS and were prescribed adalimumab for the first time by their treating physicians per routine practice. Patients who were previously treated with adalimumab and who did not consent to participate in the study were excluded.
2.3 Treatment protocol
Dosage and administration of adalimumab followed the description in the package insert. Adalimumab was administered subcutaneously at a dose of 160 mg as the first dose, 80 mg as the second dose (2 weeks after the initial dose), then 40 mg every week or 80 mg every 2 weeks as the third (4 weeks after the initial dose) and subsequent doses. Patients were observed for 52 weeks from the start of adalimumab treatment. If treatment was discontinued before 52 weeks, the occurrence of AEs was observed up to 70 days after treatment discontinuation.
2.4 Survey items
Survey items in this study have been described previously.17 Briefly, patient information collected was as follows: baseline characteristics, including age, sex, body weight, HS severity per the Hurley classification, HS family history, and affected site(s); medical history, including comorbidities and previous disease; adalimumab treatment status; previous medication and nonpharmacological therapy for HS; concomitant medication and nonpharmacological therapy for HS; occurrence of AEs, including name and seriousness of the AE; and effectiveness evaluation (described below).
2.5 Outcome measures
Details on the outcome measures were reported previously.17 The primary endpoint was safety. The incidence of serious infection, any adverse drug reactions (ADRs), any infection, and any safety event of special interest were assessed using the Medical Dictionary for Regulation Activities/Japanese edition (MedDRA/J) Version 24.0. Safety events of special interest were serious infection, reactivation of hepatitis B, tuberculosis, demyelinating disorders, lupus-like syndrome, serious allergic reaction, interstitial pneumonia, serious blood disorder, liver disorder (fulminant hepatitis, liver dysfunction, jaundice, liver failure), malignant tumor, dermatitis psoriasiform (exacerbation and new onset of psoriasis), exacerbation of sarcoidosis, and immunogenicity. The secondary endpoints assessed at 12, 24, and 52 weeks, and at adalimumab discontinuation were as follows: the proportion of patients who achieved HS clinical response (HiSCR), defined as ≥50% reduction in the number of abscesses and inflammatory nodules, and no increase in the number of abscesses and draining fistulas relative to baseline; total number of abscesses and inflammatory nodules (AN), in which AN was defined as the sum of abscess and inflammatory nodule count measured in the same examination period; the proportion of patients achieving an overall improvement of “improved”, which was subjectively assessed by the physicians as “improved”, “unchanged”, or “impossible to evaluate”; change from baseline in CRP to evaluate the extent of inflammation; change from baseline in the patient's global assessment of skin pain, evaluated using an 11-point numeric rating scale (NRS), with 0 indicating “no pain” and 10 indicating “worst skin pain imaginable”; the proportion of patients achieving NRS30, defined as a ≥30% reduction and ≥1-unit reduction from baseline in NRS in patients whose baseline NRS value was ≥3; and change from baseline in DLQI.18 The DLQI is a questionnaire that assesses the patient's health-related QOL; the total score is the sum of each question, with a higher score indicating greater impairment in QOL.
2.6 Statistical analysis
As described previously,17 the planned target sample size was 80 patients, which was based on the safety results of two phase III studies of adalimumab in patients with HS (PIONEER I and PIONEER II).13 The safety analysis population included all patients who received ≥1 dose of adalimumab with ≥1 follow-up safety evaluation. The effectiveness analysis population included all patients who received ≥1 dose of adalimumab with ≥1 follow-up effectiveness evaluation. Categorical data are reported as n (%), and continuous data are reported as mean (standard deviation [SD]) and median (minimum, maximum). The achievement rate of HiSCR was assessed in the effectiveness analysis population and by subgroup (post hoc analyses, i.e. patient baseline characteristics, use of concomitant nonpharmacological therapy, and use of concomitant antibacterial drugs), and was reported as a percentage (95% confidence interval [CI]). Changes from baseline in AN, NRS, CRP, and DLQI are summarized using descriptive statistics; a paired t-test was performed, and two-sided P < 0.05 was considered statistically significant. Multiplicity was not considered in the hypothesis test. Missing data were not imputed. Statistical analyses were performed using SAS 9.3 or higher (SAS Institute Inc.).
3 RESULTS
3.1 Demographic and baseline clinical characteristics
Of the 84 patients registered for the study, 83 patients were included in the safety analysis and the effectiveness analysis.17 One patient was excluded from the analyses due to consent withdrawal. Patient baseline demographic and disease characteristics have been described previously17 and are summarized in Table 1. Mean (SD) age was 42.0 (15.2) years and most patients were male (78.3%). Approximately 60% of patients had ≥1 comorbidity; almost 40% of patients had HS for ≥10 years, with 61.4% of patients with Hurley stage III disease.
| Characteristic | |
|---|---|
| Age, years | |
| <15 | 0 (0) |
| ≥15 to <65 | 75 (90.4) |
| ≥65 | 8 (9.6) |
| Mean (SD) | 42.0 (15.2) |
| Median (min, max) | 44.0 (15, 70) |
| Sex, male | 65 (78.3) |
| Body weight, kg | |
| n | 64 |
| Mean (SD) | 78.6 (23.2) |
| Median (min, max) | 74.6 (38.7, 140.0) |
| BMI, kg/m2 | |
| <25 | 24 (28.9) |
| ≥25 to <30 | 18 (21.7) |
| ≥30 | 19 (22.9) |
| Unknown | 22 (26.5) |
| n | 61 |
| Mean (SD) | 26.9 (6.8) |
| Median (min, max) | 25.9 (15.1, 46.8) |
| Smoking history | |
| No | 25 (30.1) |
| Yes | 42 (50.6) |
| Unknown | 16 (19.3) |
| Comorbidity | |
| No | 35 (42.2) |
| Yes | 48 (57.8) |
| Previous disease | |
| No | 58 (69.9) |
| Yes | 21 (25.3) |
| Unknown | 4 (4.8) |
| Duration of HS, years | |
| <2 | 7 (8.4) |
| ≥2 to <5 | 12 (14.5) |
| ≥5 to <10 | 14 (16.9) |
| ≥10 | 33 (39.8) |
| Unknown | 17 (20.5) |
| n | 66 |
| Mean (SD) | 12.8 (11.1) |
| Median (min, max) | 9.6 (0.4, 40.0) |
| HS severity (Hurley stage) | |
| I | 3 (3.6) |
| II | 27 (32.5) |
| III | 51 (61.4) |
| Unknown | 2 (2.4) |
| HS family history | |
| No | 60 (72.3) |
| Yes | 6 (7.2) |
| Unknown | 17 (20.5) |
| Affected sitea | |
| Armpit | 50 (60.2) |
| Breast | 11 (13.3) |
| Buttocks | 49 (59.0) |
| Inguinal and femoral regions | 39 (47.0) |
| Perianal region | 23 (27.7) |
| Perineum | 17 (20.5) |
| Otherb | 23 (27.7) |
| NRS, n | 52 |
| Mean (SD) | 5.3 (3.2) |
| Median (min, max) | 5.5 (0, 10) |
| DLQI, n | 39 |
| Mean (SD) | 8.5 (6.1) |
| Median (min, max) | 7.0 (1, 23) |
- Note: Data are n (%) unless otherwise stated.
- Abbreviations: BMI, body mass index; DLQI, Dermatology Life Quality Index; HS, hidradenitis suppurativa; max, maximum; min, minimum; NRS, numeric rating scale; SD, standard deviation.
- a Selection of multiple sites was possible.
- b “Other” included face area (5/83, 6.0%), head area (5/83, 6.0%), head and/or face and/or neck (4/83, 4.8%), neck (2/83, 2.4%), nuchal region (2/83, 2.4%), abdomen (2/83, 2.4%), back (2/83, 2.4%), and trunk (1/83, 1.2%).
3.2 Previous and concomitant treatments before and during adalimumab administration
Before starting adalimumab treatment, most patients (84.3%, 70/83 patients) were taking medications for HS (Supporting Information Table S1). Of these, 90.0% (63/70) of patients received systemic antibacterial drugs (orally [61/70 patients] and/or as an injection [3/70 patients]); of the three patients who received antibacterial injection, two patients had Hurley stage III disease. Systemic anti-inflammatory drugs were received by 11.4% (8/70) of patients. Previous nonpharmacological therapy was received by 65.1% (54/83) of patients, with 92.6% (50/54) of these patients undergoing surgical procedures including incision and drainage, and 46.3% (25/54) of these patients undergoing surgery, including local/wide excision and skin grafting. After starting adalimumab treatment, the proportion of patients receiving medications decreased, with 66.3% (55/83) of patients using concomitant medications for HS. Systemic (oral/injection) antibacterial drugs (all used for the treatment of HS) and anti-inflammatory drugs were received by 70.9% (39/55) and 14.5% (8/55) of these patients, respectively. Furthermore, the proportion of patients receiving nonpharmacological therapy also decreased to 22.9% (19/83 patients). During adalimumab treatment, 52.6% (10/19) and 63.2% (12/19) of patients who had nonpharmacological therapy underwent concomitant surgical procedures and surgery, respectively. Of the 12 patients who underwent concomitant surgery, 83.3% (10/12) had Hurley stage III disease. The types of antibacterial drugs used as previous and concomitant treatments were reported previously.17
3.3 Treatment status with adalimumab
More than half (56.6%) of patients received adalimumab for ≥52 weeks; mean (SD) duration of adalimumab treatment was 40.1 (16.1) weeks (Supporting Information Table S2). Almost all patients (96.4%) received adalimumab at a dose of 160 mg (first dose), 80 mg (second dose), and 40 mg every week (third and subsequent doses); only 3.6% (3/83) of patients received adalimumab at the alternative dose regimen at least once during the study (i.e., 160 mg [first dose], 80 mg [second dose], and 80 mg every 2 weeks [third and subsequent doses]). Overall, 43.4% (36/83) of patients discontinued adalimumab treatment for one or more reasons. The reasons for treatment discontinuation (multiple selections allowed) were “changed hospital or did not visit hospital” (38.9%, 14/36 patients), “symptom improvement” (27.8%, 10/36 patients), “insufficient effect” (13.9%, 5/36 patients), “AE” (13.9%, 5/36 patients), surgery for HS (11.1%, 4/36 patients), and other (2.8%, 1/36 patients).
3.4 Safety
Serious infection was reported by two (2.4%) patients, with one patient having a serious infection classified as an ADR (Table 2). Overall, there were 39 AEs reported by 21 (25.3%) patients, and an AE of any infection was reported by 11 (13.3%) patients (Table 2 and Supporting Information Table S3). Nineteen ADRs were reported by 10 (12.0%) patients and ADRs of any infection were reported by five (6.0%) patients. Two patients reported a serious ADR, both of whom discontinued adalimumab treatment: one patient was hospitalized for serious infection (subcutaneous abscess, abscess on the left forearm, and a staphylococcal infection) and one patient died of liver disorder. Dermatitis psoriasiform (physician-reported term: rash psoriasiform, one patient), a safety event of special interest, was observed in a patient who did not have previous or comorbid psoriasis. The incidence of ADRs evaluated by patient characteristics is shown in Supporting Information Table S4.
| AE | ADR | |||
|---|---|---|---|---|
| Any | Serious | Any | Serious | |
| Patients with serious infection | 2 (2.4) | 2 (2.4) | 1 (1.2) | 1 (1.2) |
| Patients with ADR | – | – | 10 (12.0) | 2 (2.4) |
| Patients with any infections | 11 (13.3) | 2 (2.4) | 5 (6.0) | 1 (1.2) |
| Patients with any safety events of special interest other than serious infection | ||||
| Reactivation of hepatitis B | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Tuberculosis | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Demyelinating disorders | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Lupus-like syndrome | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Serious allergic reaction | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Interstitial pneumonia | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Serious blood disorder | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Liver disorder (fulminant hepatitis, liver dysfunction, jaundice, liver failure) | 1 (1.2) | 1 (1.2) | 1 (1.2) | 1 (1.2) |
| Malignant tumor | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Dermatitis psoriasiform (exacerbation and new onset of psoriasis) | 1 (1.2) | 0 (0) | 1 (1.2) | 0 (0) |
| Exacerbation of sarcoidosis | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Immunogenicity | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
- Note: Data are n (%).
- Abbreviations: ADR, adverse drug reaction; AE, adverse event.
- a Per the Medical Dictionary for Regulatory Activities/Japanese edition (MedDRA/J) Version 24.0.
3.5 Effectiveness
3.5.1 Achievement of HiSCR
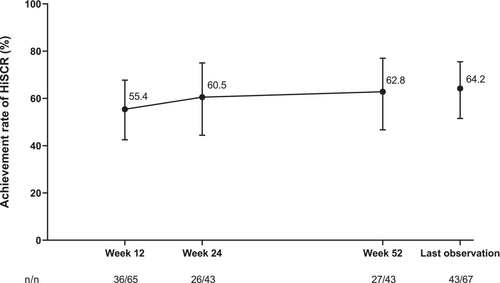
In this final analysis, 55.4% (95% CI 42.5–67.7) of patients achieved HiSCR at 12 weeks of adalimumab treatment (Figure 1). This increased to 60.5% (95% CI 44.4–75.0) at 24 weeks and was maintained through 52 weeks of adalimumab treatment; at 52 weeks and last observation, the achievement rates of HiSCR were 62.8% (95% CI 46.7–77.0) and 64.2% (95% CI 51.5–75.5), respectively. Although the sample size was too small to conduct statistical significance tests, the achievement rate of HiSCR at last observation was generally similar regardless of baseline characteristics (Supporting Information Table S5). The proportion of patients achieving HiSCR at last observation was 68.4%, 57.1%, and 58.8% for patients with a body mass index (BMI) of <25, ≥25 to <30, and ≥30 kg/m2, respectively. However, the achievement rate of HiSCR was 72.7% in patients without a smoking history, which was numerically higher than those with a smoking history (57.1%; Supporting Information Table S5). Among patients who did not receive concomitant antibacterial drugs (i.e., received previous antibacterial drugs but discontinued them and never received them at the start of and during adalimumab treatment), 60.0% and 50.0% achieved HiSCR at 12 and 52 weeks, respectively (Supporting Information Table S6). In those who continued to receive concomitant antibacterial drugs, 30.0% and 50.0% achieved HiSCR at 12 and 52 weeks, respectively (Supporting Information Table S6). Among patients who received previous nonpharmacological HS therapy, the achievement rates of HiSCR in patients who did not receive concomitant nonpharmacological therapy were 58.8%, 64.7%, and 64.7% at 12, 24, and 52 weeks, respectively (Supporting Information Table S6). The achievement rates of HiSCR in patients who received both previous and concomitant nonpharmacological therapy were 42.9%, 44.4%, and 55.6% at 12, 24, and 52 weeks, respectively.
3.5.2 HS lesions (inflammatory nodules, abscesses, and drainage fistulas)
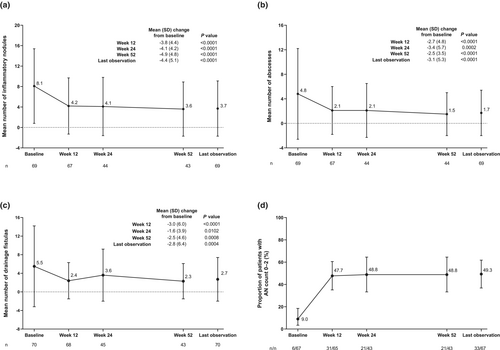
Compared with baseline, the number of HS lesions (inflammatory nodules, abscesses, and drainage fistulas) decreased at 12, 24, and 52 weeks of treatment (Figure 2a–c); the reduction from baseline was significant for all time points and all lesions. The reduction in the mean (SD) number of HS lesions from baseline to 52 weeks was 8.1 (7.3) to 3.6 (5.3) for inflammatory nodules, 4.8 (7.4) to 1.5 (3.5) for abscesses, and 5.5 (8.7) to 2.3 (3.8) for drainage fistulas, with mean (SD) change from baseline to 52 weeks in inflammatory nodules, abscesses, and drainage fistulas of −4.9 (4.8), −2.5 (3.5), and −2.5 (4.6), respectively. Furthermore, the proportion of patients achieving AN count of 0–2 increased from 9.0% at baseline to 47.7% at 12 weeks and remained stable at 24 weeks (48.8%) and through to 52 weeks (48.8%) of adalimumab treatment (Figure 2d).
3.5.3 Physician-assessed overall improvement and CRP
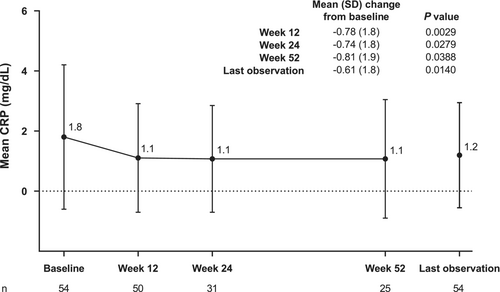
The proportion of patients with a status of “improved” for physician-assessed overall improvement was 93.8% at 12 weeks (Supporting Information Figure S1). The proportion remained relatively stable through 52 weeks of adalimumab treatment, with 93.6% of patients having a physician-assessed overall improvement status of “improved” at 52 weeks. Mean (SD) CRP decreased from baseline to 12 weeks; the reduction was maintained through 52 weeks of adalimumab treatment (Figure 3). Mean (SD) CRP (mg/dL) was 1.8 (2.4) at baseline, 1.1 (1.8) at 12 weeks, 1.1 (1.8) at 24 weeks, and 1.1 (2.0) at 52 weeks. Mean (SD) change from baseline in CRP was significant at all time points.
3.5.4 Skin pain and QOL
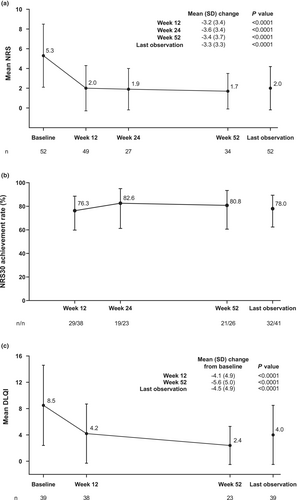
Mean (SD) NRS of skin pain was 5.3 (3.2) at baseline, 2.0 (2.3) at 12 weeks, 1.9 (2.1) at 24 weeks, and 1.7 (1.8) at 52 weeks (Figure 4a). Mean (SD) change from baseline in NRS of skin pain was significant at all time points (P < 0.0001). The proportion of patients achieving NRS30 also remained relatively stable during adalimumab treatment (Figure 4b); the achievement rates of NRS30 were 76.3% at 12 weeks, 82.6% at 24 weeks, and 80.8% at 52 weeks. Furthermore, an improvement in mean (SD) DLQI score was observed during adalimumab treatment (Figure 4c). Mean (SD) DLQI was 8.5 (6.1), 4.2 (4.5), and 2.4 (2.9) at baseline, 12, and 52 weeks, respectively; the improvement was significant at all time points (P < 0.0001).
4 DISCUSSION
This 52-week PMS was the first study to evaluate the long-term safety and effectiveness of adalimumab in patients with HS in real-world clinical settings in Japan. In this final analysis, the incidence of ADRs was 12.0%; most were nonserious, and almost all patients with ADRs were recovering or had recovered. Furthermore, no new safety concerns were observed over 52 weeks of adalimumab treatment. Adalimumab was also effective in improving clinical response, HS symptoms, and QOL. The effects observed at 12 weeks were maintained at 24 weeks and through 52 weeks of adalimumab treatment. These results provide real-world evidence of the long-term safety and effectiveness of adalimumab in the treatment of patients with HS in Japan.
In this study, the incidence of any ADR with adalimumab treatment was 12.0%, which was lower than that reported in a clinical trial but higher than the incidences reported in recent observational studies. The Japanese phase III, open-label, single-arm study reported that 47% of patients had any ADR.14 However, a multicenter post-marketing observational study of adalimumab in mainly White patients with HS (HARMONY study) reported an incidence rate of any ADR of 9.5%.15 Moreover, in a 1-year, Canadian, prospective observational PMS (SOLACE study), 7.1% of patients reported any ADR.16 The difference in the overall incidence rate of ADRs between the current study and previous studies may be explained by the differences in study design (clinical trials vs. observational studies). Furthermore, patient characteristics, including ethnicity, sex, and patients' comorbidity and previous disease, may also explain these differences given that the present study demonstrated that the incidence of ADRs was higher in women than in men and in patients with previous disease or comorbidity than in those without. However, further studies are needed to confirm this because no statistical test was conducted for the subgroup analyses.
Increased risks of serious infections, malignancies, and tuberculosis have been reported previously with TNF-α inhibitors, including adalimumab.19, 20 However, in this study, AE of serious infection was reported in only two (2.4%) patients, and AEs of liver disorder and dermatitis psoriasiform were reported in one (1.2%) patient each; no patients reported malignancies or tuberculosis. These results were not markedly different from the safety results observed in previous studies,13-16 therefore the results from this study suggest that long-term adalimumab treatment is well tolerated and safe in Japanese patients with HS.
Consistent with the results from a previous clinical trial and observational studies,14-16 the present study reported that the proportion of patients achieving HiSCR increased and stabilized over time, reaching an achievement rate of 62.8% at 52 weeks of adalimumab treatment. This was also supported by a significant reduction in HS lesions and improvements in inflammation (CRP) and skin pain through 52 weeks relative to baseline. Moreover, QOL in this study significantly improved over time (P < 0.0001 at all time points); mean DLQI score of 8.5 at baseline continued to decrease after 12 weeks and was <3 at 52 weeks of adalimumab treatment. This is similar to the results observed in the HARMONY study,15 which reported an approximately 50% reduction in DLQI from baseline (16.9) to 52 weeks (8.6), although the mean DLQI scores at baseline and at 12 and 52 weeks were substantially lower in this study than in the HARMONY study. Health-related QOL is considerably affected by bodily pain,8 therefore the greater reduction in skin pain observed in this study compared with that observed in the HARMONY study15 (NRS 1.7 vs. 2.69 at 52 weeks) may have contributed to the difference observed in DLQI scores. Furthermore, given that the baseline DLQI score in this study was similar to that reported by a recent Japanese multicenter questionnaire-based study (8.5 vs. 9.9),8 the difference in DLQI scores between the current study and the HARMONY study may also be due to the difference in ethnicity (Japanese vs. White). Nonetheless, these results indicate that adalimumab treatment demonstrates long-term effectiveness in Japanese patients with HS.
A previous Italian cohort study reported that a significant risk factor for nonresponse to adalimumab treatment is therapeutic delay.21 In this study, although clinical response by disease duration did not show a clear trend, patients with Hurley stage I disease, as well as patients with no previous medications or nonpharmacological therapy, had a numerically higher clinical response than those with Hurley stage II/III disease or those who had previous medications or nonpharmacological therapy. The results from this study may indirectly support the correlation between therapeutic delay and a lack of response to adalimumab, suggesting the potential benefit of early treatment with adalimumab. Given that the current Japanese guidelines recommend adalimumab for patients with Hurley stage II/III disease unresponsive to all existing treatment options, but treatment can be tailored to the individual situation,22 these results indicate that adalimumab may potentially be used before starting other medications and nonpharmacological therapy for HS or in patients with mild disease.
In this study, clinical response at last observation was numerically higher in patients with lower BMI and in those with no smoking history. These results are consistent with the results from a post hoc analysis of the PIONEER I and II studies,23 in which significantly reduced odds of achieving HiSCR were observed for every unit increase in BMI (odds ratio [OR] 0.93, 95% CI 0.89–0.97, P < 0.001) and current smokers were associated with a significant reduction in the achievement of HiSCR compared with nonsmokers (OR 0.56, 95% CI 0.31–0.98, P = 0.04), therefore these results may justify recommending smoking cessation and weight loss in patients with HS. Furthermore, in the present study, the achievement rate of HiSCR in this study was higher in patients with mild disease (Hurley stage I) than in those with more severe disease (Hurley stage II/III). A lower achievement rate of HiSCR was observed in patients who had the perianal region as the affected site than in those with other affected sites; although patients may have had multiple affected sites, this suggests that patients with perianal lesions may have more severe disease. These findings therefore suggest that baseline and clinical factors may predict the effectiveness of adalimumab. However, given that baseline characteristics were not adjusted between the groups, and that no significance testing was conducted, the results from the subgroup analyses should be interpreted with caution.
The mainstay treatments for HS include topical and systemic antibacterial drugs. Topical clindamycin is recommended as first-line treatment for less severe HS to reduce inflammation and inhibit biofilm formation.24 Systemic antibacterial drugs provide anti-inflammatory and antibacterial effects in HS, and oral tetracyclines are recommended as first-line systemic therapy.24 However, the use of antibacterial drugs has been reported to be associated with a reduced achievement rate of HiSCR (OR 0.47, 95% CI 0.23–0.93, P = 0.03),23 and the prevalence of antibiotic resistance is also high in patients with HS.25 In the present study, use of systemic antibacterial drugs decreased after the start of adalimumab treatment, suggesting that use of adalimumab may prevent or reduce the use of antibacterial drugs. Furthermore, although a significance test was not conducted, the achievement rate of HiSCR at 52 weeks was similar between patients who received and did not receive concomitant antibacterial drugs (i.e. continued and discontinued previous antibacterial drugs prior to the initiation of adalimumab). These results suggest that adalimumab alone is effective in eliciting clinical response, and that unnecessary antibacterial drug use with adalimumab should be avoided to prevent antibiotic resistance in Japanese patients with HS.
In the randomized controlled SHARPS study,26 a significantly greater proportion of patients receiving adalimumab with surgery achieved HS clinical response than those receiving placebo with surgery in patients with moderate-to-severe HS; no increased risk of postoperative wound infection, complication, or hemorrhage between the treatment groups was observed. Results from the SHARPS study26 suggested that discontinuation of adalimumab in patients undergoing surgery for HS is not necessary, and that the use of adalimumab in combination with surgery could be considered at an appropriate time with the aim of curing the disease. In the present study, 25 patients had surgery for HS prior to starting adalimumab treatment, which possibly reflects the fact that surgery was the standard treatment for HS before the start of this study. During the study, 12 patients received adalimumab concomitantly with surgery, and four patients discontinued adalimumab treatment to undergo surgery for HS; however, no significance test was conducted, therefore additional real-world studies are required to confirm the benefit of continuing adalimumab during surgery in patients with HS in Japan.
The real-world discontinuation rate of adalimumab was reported to be 23.3% in the Canadian SOLACE study16; reasons for discontinuation were withdrawal by patients (37.8%), lost to follow-up (35.6%), and AEs (11.1%). In another observational study conducted in the USA,27 32.9% of patients discontinued adalimumab treatment due to a lack of therapeutic improvement (64%) and AEs (28%). Compared with these previous studies, a higher discontinuation rate was reported in this current study (43.4% for one or more reasons); however, this included 27.8% of patients who discontinued due to symptom improvement. Also, 38.9% of patients discontinued adalimumab due to patients changing hospital or not visiting the hospital. These patients may have continued adalimumab treatment after changing hospital; however, the treatment status of these patients could not be followed up, which was a limitation of this study. Furthermore, in this real-world study of Japanese patients, discontinuation rates of adalimumab due to AEs or lack of efficacy were lower than or similar to those reported in the Canadian and US studies,16, 27 suggesting that discontinuation rates of adalimumab are similar across diverse patient populations, including Japanese patients with HS.
This study evaluated real-world long-term (up to 52 weeks) data of >80 patients with HS in Japan. Unlike clinical trials, which have strict eligibility criteria and exclude patients who are using concomitant medications or have mild disease, the patients assessed in this study better reflect the real patient population in Japan. In this study, the effectiveness of adalimumab was also evaluated using a variety of outcome measures, including HiSCR, CRP, NRS, and DLQI, and a subgroup analysis was conducted for both efficacy and safety outcomes. However, this study was limited by the absence of a control or comparator group and the exclusion of patients with a history of adalimumab use for HS and other diseases. The severity of HS was also not reported using the International Hidradenitis Suppurativa Severity Score System, and the results of some outcomes were not analyzed or were based on a small sample size due to missing patient data. Furthermore, for the subgroup analyses, no significance test was conducted and no baseline characteristics were adjusted. Subgroup analysis of patients with and without concomitant antibacterial drugs or nonpharmacological therapy may also be subject to selection bias, therefore the clear relationship between the use of concomitant antibacterial drug or nonpharmacological therapy and the achievement of HiSCR may be lacking.
In conclusion, this PMS study demonstrated the long-term safety and effectiveness of adalimumab for the treatment of patients with HS in real-world clinical practice in Japan. No new safety concerns were observed over the 52 weeks of treatment; adalimumab was well tolerated regardless of patient demographic and clinical characteristics. Furthermore, adalimumab showed sustained improvements in clinical response, symptoms, and QOL through the 52 weeks of treatment.
ACKNOWLEDGMENTS
The authors would like to thank all study participants. This surveillance study was conducted at 65 institutions nationwide in Japan. We would like to thank all the physicians and medical staff members who cooperated in this surveillance.
FUNDING INFORMATION
This study was sponsored by AbbVie GK, manufacturer and licensee of adalimumab. Medical writing assistance was provided by Hana Nomura, BPharm (Hons), and Prudence Stanford, PhD, CMPP, of ProScribe – Envision Pharma Group, and was funded by AbbVie GK. ProScribe's services complied with international guidelines for Good Publication Practice. AbbVie GK was involved in the study design, data collection, data analysis, and preparation.
CONFLICT OF INTEREST STATEMENT
N.H. has received consultancy fees from AbbVie GK and Maruho, and speaker honoraria from AbbVie GK, Maruho, and Sun Pharma Japan. K.H. has received grants, speaker honoraria, and consultancy fees from AbbVie GK; speaker honoraria and consultancy fees from Boehringer Ingelheim; grants and speaker honoraria from Eisai, Kaken Pharmaceutical, Kyowa Kirin, Maruho, Mitsubishi Tanabe Pharma, Novartis Pharma, Sanofi, and Taiho Pharmaceutical; speaker honoraria from Janssen Pharmaceutical and Meiji Seika Pharma; and grants from Nihon Pharmaceutical and Sun Pharma Japan. K.T. has served as a paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie GK, Boehringer Ingelheim, Celgene (Bristol-Myers Squibb), Eisai, Eli Lilly Japan, Janssen Pharmaceutical, Kyowa Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe Pharma, Novartis Pharma, Taiho Pharmaceutical, and Torii Pharmaceutical. I.K. has no conflict of interest. M.O., T.K., and E.I. are employees of AbbVie GK. T.T. has received research funds from Maruho and honoraria for serving as a speaker, consultant, and advisory board member from AbbVie GK, Boehringer Ingelheim, Celgene (Bristol-Myers Squibb), Eli Lilly Japan, Janssen Pharmaceutical, Kyowa Kirin, LEO Pharma, Mitsubishi Tanabe Pharma, Novartis Pharma, and Sanofi.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.




