Efficacy and safety of crisaborole ointment in Chinese and Japanese patients aged ≥2 years with mild-to-moderate atopic dermatitis
Trial Registration: ClinicalTrials.gov, NCT04360187.
Abstract
Atopic dermatitis is a chronic inflammatory skin disease with a significant impact on the overall wellbeing of patients and their families. Crisaborole ointment, 2%, is a nonsteroidal phosphodiesterase 4 inhibitor approved for the treatment of mild-to-moderate atopic dermatitis in multiple countries. However, in the key pivotal trials, a low proportion of the overall patient population was Asian, therefore the safety and efficacy of crisaborole in the Asian population with atopic dermatitis remains unclear. CrisADe CLEAR was a multicenter, randomized, double-blind, vehicle-controlled, phase 3 study (NCT04360187) to assess the efficacy and safety of crisaborole ointment in Chinese and Japanese patients aged ≥2 years with mild-to-moderate atopic dermatitis involving ≥5% treatable body surface area. Patients were randomly assigned 2:1 to receive crisaborole or vehicle twice daily for 28 days. The primary endpoint was percentage change from baseline in the Eczema Area and Severity Index total score at day 29. Additional endpoints were improvement and success per Investigator's Static Global Assessment score at day 29 and change from baseline on the Peak Pruritus Numerical Rating Scale at week 4. Safety was assessed using rates of treatment emergent adverse events, serious adverse events, and clinically significant changes in vital signs and clinical laboratory parameters. Crisaborole-treated patients showed a significantly greater reduction versus vehicle in percentage change from baseline in Eczema Area and Severity Index total score at day 29 (P = 0.0002). Response rates for achievement of Investigator's Static Global Assessment improvement and success at day 29 were significantly higher for patients treated with crisaborole versus vehicle (P = 0.0124 and P = 0.0078, respectively). Crisaborole-treated patients showed a significantly greater reduction versus vehicle in change from baseline on the Peak Pruritus Numerical Rating Scale at week 4 (P = 0.0009). No new safety signals were identified. Treatment with crisaborole was effective and well tolerated in Chinese and Japanese patients with mild-to-moderate atopic dermatitis.
1 INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin disease that has a significant impact on the overall wellbeing of patients and their families.1, 2 AD-related symptoms such as pruritus, cosmetically visible manifestations including erythema, excoriation, and lichenification, as well as the chronic, relapsing nature of the disease have a significant effect on work, education, and social activities.3-6
Global prevalence of AD is approximately 15%–30% in children and 2%–10% in adults.7 The prevalence of AD varies worldwide; however, the prevalence of AD has increased in certain regions, particularly in the Asia-Pacific region.8, 9 As determined in a systematic review, the prevalence of AD in Asia over a period of 1 year was 1.2% in adults and ranged from 0.96% to 22.6% in children.10
In China, the overall prevalence of AD in children aged 1–7 years has been reported to be about 12.9% (ranging from 9.0% to 24.7% between metropolises), and a study of 8758 Chinese adults found that the prevalence of AD in that population is approximately 4.6%.11, 12 A trend of increasing prevalence of AD has been observed in China.9, 13 The prevalence of AD in children in Japan is approximately 10%.14 An international survey including 10 911 Japanese adults reported the prevalence of AD overall and the percentage of the study population being treated to be 2.1% and 1.5%, respectively.15
For decades, emollients and topical corticosteroids (TCSs) have been the cornerstone of treatment for AD, with topical calcineurin inhibitors (TCIs) being added more recently as a second-line option.3, 16, 17 Emerging treatment options include Janus kinase (JAK) inhibitors (ruxolitinib) and phosphodiesterase 4 (PDE4) inhibitors (roflumilast and crisaborole).18 There are only a limited number of treatment options available, especially in the pediatric population; many are associated with unfavorable side effect profiles, leaving an unmet need for new drug therapy options in AD.3, 16 Crisaborole ointment, 2%, is a nonsteroidal PDE4 inhibitor approved for the treatment of mild-to-moderate AD in multiple countries and regions.19-21 In some of the countries where crisaborole has been approved for use, it has only been approved for use in patients ≥2 years of age, while in other countries, such as Canada and the United States, crisaborole is approved for patients as young as 3 months of age.19, 21 Regulatory approvals of crisaborole have been based on the results of two identically designed, vehicle-controlled, randomized, double-blind, pivotal phase 3 clinical studies, AD-301 (CORE 1) and AD-302 (CORE 2), and a long-term safety study (AD-303).22-24
In the CORE 1 and CORE 2 studies, conducted in the United States in patients ≥2 years of age with mild-to-moderate AD, crisaborole showed improvement in Investigator's Static Global Assessment (ISGA) score and an acceptable safety profile.23, 24 However, only a low proportion (approximately 5%) of the overall patient population included in these studies were Asian, therefore the efficacy and safety of crisaborole in the Asian AD population is unclear.23 The objective of the CrisADe CLEAR study was to analyze the efficacy and safety of crisaborole in Chinese and Japanese patients aged ≥2 years with mild-to-moderate AD.
2 METHODS
2.1 Study design
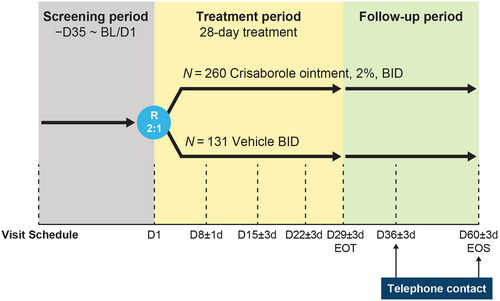
This was a multicenter, randomized, double-blind, vehicle-controlled phase 3 study (NCT04360187) that included Chinese and Japanese patients aged ≥2 years with mild-to-moderate AD involving ≥5% treatable body surface area (BSA). At baseline (day 1), patients were randomly assigned 2:1 to receive crisaborole or vehicle twice daily (BID), respectively, for a 28-day treatment course. Patient follow-up was on days 36 and 60 after the end of the treatment period (Figure 1).
2.2 Patients and treatment
All patients were aged ≥2 years at the time of informed consent/assent and had a confirmed clinical diagnosis of AD at screening and baseline (day 1) per Hanifin and Rajka criteria.25 Patients were required to have mild-to-moderate AD, defined as an ISGA score of 2 (mild) or 3 (moderate) and a percentage of treatable body surface area (%BSA) of ≥5, excluding the scalp.
Patients with clinically significant medical conditions (including non-AD dermatological conditions and genetic dermatological conditions overlapping with AD), patients using any prohibited medications as per the study protocol that may alter the course of AD without the required minimum washout periods, and patients who had participated in previous crisaborole clinical studies were excluded from this study. The use of systemic corticosteroids was prohibited within 28 days before baseline/day 1, and the application of TCS or TCIs was prohibited 14 days before baseline/day 1.
Patients and/or their parents or legal guardians were instructed to apply the study treatment (crisaborole ointment or vehicle) to cover each lesion twice daily during the 28-day treatment period. Patients were also instructed to apply the study drug to newly identified AD lesions that appeared after baseline/day 1. This included all treatable areas affected by AD (excluding the scalp) identified at baseline/day 1 regardless of whether or not they became clinically clear prior to day 29. All relevant medication precautions were communicated to the patient and/or parent/legal guardians.
Patients were permitted to use emollients, moisturizer, and sunscreen during the study to manage dry skin in areas surrounding but not on or overlapping the treatable AD-involved areas.
2.3 Endpoints and assessments
2.3.1 Efficacy assessments
Efficacy assessments were performed during scheduled study visits at baseline (day 1) and on days 8, 15, 22, and 29 (end of treatment) (Figure 1). The primary endpoint was the percentage change from baseline on the Eczema Area and Severity Index (EASI) on day 29. The EASI quantifies the disease severity based on both severity of lesion clinical signs and %BSA. The EASI is a composite scoring of the degree of erythema, induration/papulation, excoriation, and lichenification (each scored separately) for each of four body regions (head and neck, trunk, upper limbs, lower limbs), with adjustment for the %BSA involved for each body region and for the proportion of the body region relative to the entire body.26
The ISGA assesses the severity of AD on a five-point clinician-reported severity scale, ranging from 0 (clear) to 4 (severe). Key secondary endpoints included in the study were achievement of ISGA improvement and achievement of success in the ISGA. Improvement in ISGA score is defined as an ISGA score of 0 (clear) or 1 (almost clear) at day 29. Success in ISGA is defined as an ISGA score of 0 or 1 with a ≥2-grade improvement from baseline at day 29.27
Another key secondary endpoint is the change from baseline on the Peak Pruritus Numerical Rating Scale (PP-NRS; used with permission from Regeneron Pharmaceuticals, Inc., and Sanofi) at week 4 (for patients aged ≥12 years). This is a patient-rated pruritus score of lesions, rating the severity of pruritus (suffered in the past 24 h) on an 11-point scale, where 0 represents no pruritus and 10 represents the most severe pruritus.28
Other secondary endpoints included improvement and success in ISGA over time, percentage change from baseline in EASI total score over time, and change from baseline in %BSA over time (other than day 29).
2.3.2 Safety assessments
Patients were assessed for treatment-emergent adverse events (TEAEs) during study visits (Figure 1). Unscheduled safety assessments could have been performed at any time during the study to assess any perceived safety concerns. TEAEs were defined as adverse events that first occurred on or after the day of the first study drug dose. TEAEs were classified as treatment-related if they were determined by the study investigator to be definitely, probably, or possibly related to the treatment with crisaborole. AEs were recorded and classified using the Medical Dictionary for Regulatory Activities terminology.
Safety was assessed using rates of TEAEs, serious adverse events (SAEs), and clinically significant changes in vital signs and clinical laboratory parameters.
2.4 Statistical analysis
Efficacy analyses were performed on the full analysis set, which encompassed all patients who were randomly assigned to the study drug or vehicle, regardless of discontinuation. The safety populations contained all randomly assigned patients who received one or more doses of the study drug. Statistical significance was defined as P ≤ 0.05.
Percentage change from baseline in EASI total score at day 29 and change from baseline to week 4 in the weekly average of the PP-NRS score for patients aged ≥12 years were analyzed using a linear mixed-effect model for repeated measures that included treatment group, visit, and treatment group-by-visit interactions as factors and baseline value as a covariate.
The percentages of patients who achieved improvement or success in ISGA at day 29 were compared between the crisaborole arm and the vehicle arm, and the differences were tested based on normal approximation to response rates.
2.5 Ethics statement
The final protocol, any amendments, and informed consent documents were reviewed and approved by the institutional ethics committees (IECs) at each of the investigational centers participating in the study. The two leading sites’ IEC approval numbers are [2020] Y011A-03 (Beijing Children's Hospital, Capital Medical University, National Center for Children's Health) and 2019PHA026-005 (Peking University People's Hospital). All patients and/or their parents/guardians provided written informed consent for participation in the study. This study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with all International Conference on Harmonization Good Clinical Practice (ICH GCP) Guidelines.
3 RESULTS
3.1 Baseline characteristics
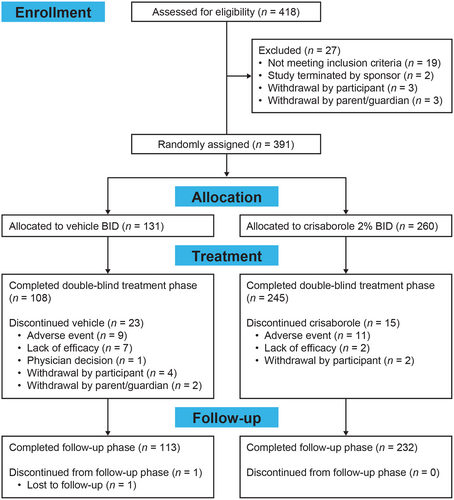
The full analysis set of the phase 3 study consisted of 391 patients. Of the 391 enrolled patients, 260 and 131 were randomly assigned to crisaborole and vehicle groups, respectively (Figure 2). The demographic and baseline characteristics between the two groups were balanced and are shown in Table 1.
| Vehicle BID N = 131 | Crisaborole, 2%, BID N = 260 | Total N = 391 | |
|---|---|---|---|
| Age, years, n (%) | |||
| 2–11 | 69 (52.7) | 123 (47.3) | 192 (49.1) |
| 12–17 | 15 (11.5) | 25 (9.6) | 40 (10.2) |
| ≥18 | 47 (35.9) | 112 (43.1) | 159 (40.7) |
| Mean age (SD) | 16.0 (13.58) | 19.4 (15.8) | 18.3 (15.16) |
| Sex, n (%) | |||
| Male | 67 (51.1) | 138 (53.1) | 205 (52.4) |
| Female | 64 (48.9) | 122 (46.9) | 186 (47.6) |
| Country, n (%) | |||
| China | 80 (61.1) | 157 (60.4) | 237 (60.6) |
| Japan | 51 (38.9) | 103 (39.6) | 154 (39.4) |
| EASI score, mean (SD) | 10.8 (8.5) | 10.2 (7.0) | 10.4 (7.5) |
| ISGA score, n (%) | |||
| 1 (mild) | 56 (42.7) | 110 (42.3) | 166 (42.5) |
| 2 (moderate) | 75 (57.3) | 150 (57.7) | 225 (57.5) |
| PP-NRS, mean (SD)a | 5.4 (2.2) | 5.2 (2.0) | 5.3 (2.0) |
- Abbreviations: BID, twice daily; EASI, Eczema Area and Severity Index; ISGA, Investigator's Static Global Assessment; PP-NRS, Peak Pruritus Numerical Rating Scale; SD, standard deviation.
- a Data for patients ≥12 years old.
The mean ages (SD) were 19.4 (15.8) and 16.0 (13.6) for the crisaborole and vehicle groups, respectively. The mean EASI scores (SD) were 10.2 (7.0) for the crisaborole group and 10.8 (8.5) for the vehicle group. In the crisaborole group, 110 patients (42.3%) had an ISGA score of 2 (mild) and 150 patients (57.7%) had an ISGA score of 3 (moderate). In the vehicle group, 56 patients (42.7%) had an ISGA score of 2 (mild) and 75 patients (57.3%) had an ISGA score of 3 (moderate). The mean PP-NRS scores (SD) were 5.2 (2.0) and 5.4 (2.2) for the crisaborole and vehicle groups, respectively (Table 1).
The medications most used by patients prior to baseline/day 1 included hydrocortisone butyrate, a TCS, and tacrolimus monohydrate, a TCI (Table 2).
| Medication, n (%) | Vehicle BID N = 131 | Crisaborole, 2%, BID N = 260 |
|---|---|---|
| Heparinoid | 42 (32.1) | 75 (28.8) |
| Emollients and protectives | 15 (11.5) | 39 (15) |
| Hydrocortisone butyrate | 17 (13.0) | 25 (9.6) |
| Oloptadine hydrochloride | 16 (12.2) | 27 (10.4) |
| Herbal preparation | 15 (11.5) | 28 (10.8) |
| Tacrolimus monohydrate | 15 (11.5) | 14 (5.4) |
- Abbreviation: BID, twice daily.
During the study treatment period, 23 (17.6%) of the vehicle-treated patients and 15 (5.8%) of the patients receiving crisaborole discontinued treatment. The most common reason was the occurrence of AEs, with prevalences of 6.9% and 4.2% in the vehicle and crisaborole-treated groups, respectively. During the follow-up phase, only one patient who received vehicle discontinued from the study (Figure 2).
3.2 Efficacy endpoints
3.2.1 Primary endpoint: Percentage change from baseline in EASI total score at day 29
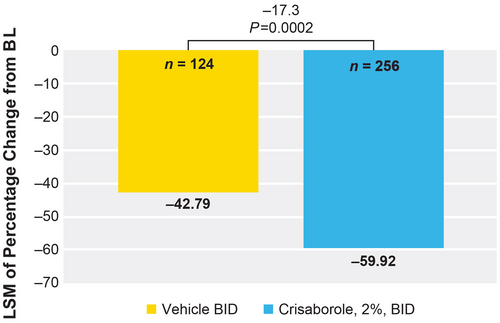
Crisaborole-treated patients showed a significantly greater reduction versus vehicle-treated patients in least squares mean (LSM) of percentage change from baseline in EASI total score at day 29 (−59.9% vs −42.8%, respectively; LSM difference of −17.1, P = 0.0002; Figure 3).
3.2.2 Key secondary endpoint: Achievement of improvement and success in ISGA at day 29
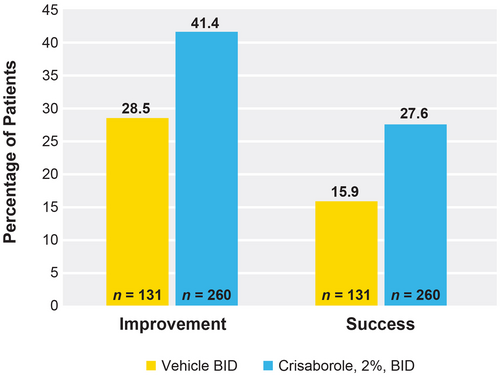
Response rates for achievement of ISGA score improvement at day 29 were significantly higher for patients treated with crisaborole than vehicle-treated patients (41.4% vs 28.5%). The percentage of patients who achieved improvement in ISGA score was 12.9% higher for crisaborole-treated patients versus vehicle-treated patients (P = 0.0124). The percentage of patients who achieved ISGA success was also found to be significantly higher in the crisaborole-treated group versus the vehicle-treated group (27.6% vs 15.9%). The percentage of patients who achieved success in ISGA score was 11.7% higher for the crisaborole-treated group versus vehicle-treated patients (P = 0.0078) (Figure 4).
3.2.3 Key secondary endpoint: Change from baseline in weekly average of PP-NRS at week 4 for participants 12 years
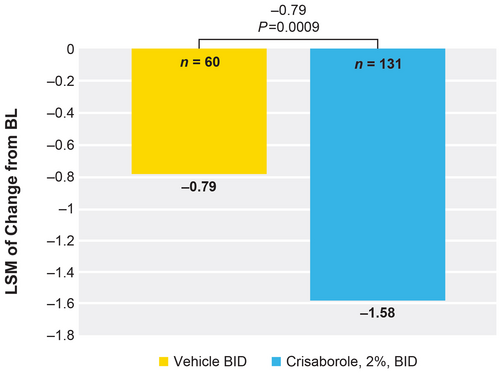
Crisaborole-treated patients showed a significantly greater reduction versus vehicle-treated patients in change from baseline in PP-NRS at week 4 (−1.58 vs −0.79). The LSM of change from baseline in the weekly average of PP-NRS was 0.79 lower for the crisaborole-treated group versus the vehicle-treated group (P = 0.0009) (Figure 5).
3.2.4 Safety
Overall, 46.2% and 44.3% of patients who were treated with crisaborole and vehicle, respectively, experienced all-causality TEAEs (Table 3); most AEs were mild. The majority of TEAEs (82.9% and 82.7% in the crisaborole- and vehicle-treated groups, respectively) had resolved by the end of the study. Application site pain was the most frequently reported TEAE and was experienced by 13.1% and 3.8% of the patients in the crisaborole- and vehicle-treated groups (Table 3), respectively, and all application site pain TEAEs were considered treatment-related. Application site pain greatly affected the face and neck in the crisaborole-treated group (20 of 34 and 14 of 34 patients experienced application site pain in each of these areas, respectively). In vehicle-treated patients, there was little difference in the number of patients who experienced application site pain in the various impacted areas, ranging from zero of five patients to three of five patients. Most of the reported application site pain cases were mild, with median durations of 6.5 and 16 days for crisaborole- and vehicle-treated patients, respectively (Table 4).
| No. of patients evaluable for AEs, n (%)a | Vehicle BID N = 131 | Crisaborole, 2%, BID N = 260 |
|---|---|---|
| No. of patients per system organ class and preferred term | ||
| Any AE | 58 (44.3) | 120 (46.2) |
| General disorders and administration site conditions | 12 (9.2) | 57 (21.9) |
| Application site pain | 5 (3.8) | 34 (13.1) |
| Skin and subcutaneous tissue disorders | 26 (19.8) | 34 (13.1) |
| Dermatitis atopic | 15 (11.5) | 20 (7.7) |
- Abbreviations: AE, adverse event; BID, twice daily; TEAE, treatment-emergent adverse event.
- a Includes all data collected since the first dose of study drug.
| Location, n | Vehicle BID | Crisaborole, 2%, BID | ||||
|---|---|---|---|---|---|---|
| Age, years | Age, years | |||||
| 2–11 | 12–17 | ≥18 | 2–11 | 12–17 | ≥18 | |
| Scalp | 0 | 0 | 0 | 0 | 0 | 0 |
| Face | 0 | 1 | 2 | 8 | 1 | 11 |
| Periorbital area | 0 | 1 | 2 | 1 | 0 | 2 |
| Eyelid | 0 | 1 | 2 | 0 | 0 | 3 |
| Ear | 0 | 0 | 1 | 0 | 0 | 0 |
| Perioral area | 0 | 0 | 3 | 0 | 0 | 4 |
| Neck | 0 | 1 | 1 | 6 | 1 | 7 |
| Shoulder | 0 | 1 | 1 | 2 | 0 | 0 |
| Axilla | 0 | 0 | 2 | 0 | 0 | 1 |
| Arm | 1 | 1 | 1 | 4 | 1 | 1 |
| Antecubital fossa | 0 | 1 | 1 | 3 | 0 | 0 |
| Hand | 0 | 0 | 0 | 1 | 1 | 1 |
| Back | 0 | 1 | 1 | 5 | 0 | 2 |
| Chest | 0 | 1 | 1 | 2 | 0 | 0 |
| Nipple | 0 | 0 | 1 | 0 | 0 | 0 |
| Abdomen | 0 | 1 | 1 | 3 | 0 | 0 |
| Buttock | 0 | 0 | 1 | 2 | 0 | 0 |
| Perianal region | 0 | 0 | 0 | 0 | 0 | 0 |
| Groin | 0 | 1 | 0 | 0 | 0 | 0 |
| Genitals | 1 | 0 | 0 | 1 | 0 | 0 |
| Leg | 0 | 1 | 1 | 4 | 0 | 0 |
| Popliteal fossa | 1 | 1 | 1 | 4 | 0 | 0 |
| Feet | 0 | 0 | 0 | 0 | 0 | 0 |
| Duration, days | ||||||
| n | 5 | 34 | ||||
| Mean | 20.2 | 10.7 | ||||
| Median | 16 | 6.5 | ||||
| Outcome | ||||||
| Recovered/resolved | 5 | 34 | ||||
| Onset date, daysa | ||||||
| n | 5 | 34 | ||||
| Mean | 1.6 | 1 | ||||
| Median | 1 | 1 | ||||
- Abbreviation: BID, twice daily.
- a Days from initiation of treatment (D1).
Treatment-related AEs occurred in 23.5% of patients in the crisaborole-treated group and 18.3% of the vehicle-treated group; none were serious (Table 5). Treatment-related AEs were application site discoloration, irritation, pain, and paresthesia, folliculitis, atopic dermatitis, contact dermatitis, and pruritus.
| Evaluable for AEs, n (%) | Vehicle BID N = 131 | Crisaborole, 2%, BID N = 260 |
|---|---|---|
| Number of patients per system organ class and preferred term | ||
| With any AE | 24 (18.3) | 61 (23.5) |
| General disorders and administration site conditions | 10 (7.6) | 51 (19.6) |
| Application site pain | 5 (3.8) | 34 (13.1) |
| Skin and subcutaneous tissue disorders | 12 (9.2) | 10 (3.8) |
- Abbreviations: AE, adverse event; BID, twice daily; TEAE, treatment-emergent adverse event.
One SAE was reported in each group (abnormal myocardial enzymes in the vehicle-treated group and exacerbation of carpal tunnel syndrome in the crisaborole-treated group); both SAEs were considered unrelated to treatment. The abnormal myocardial enzymes and exacerbated carpal tunnel syndrome SAEs resolved within 6 and 14 days, respectively.
No safety signals were identified from vital signs and laboratory testing in either group.
4 DISCUSSION
This phase 3 study addressed the efficacy and safety of crisaborole in Chinese and Japanese patients aged ≥2 years with mild-to-moderate AD.
Crisaborole demonstrated superior efficacy in all primary and key secondary endpoints versus vehicle. Percentage change from baseline in EASI total score at day 29 showed a statistically significant reduction for patients treated with crisaborole versus vehicle (LSM difference of −17.1, P = 0.0002). Response rates for achievement of ISGA improvement and success at day 29 were significantly higher in patients treated with crisaborole versus vehicle. Crisaborole-treated patients also showed a significantly greater reduction in change from baseline in PP-NRS versus vehicle-treated patients at week 4.
Both crisaborole- and vehicle-treated patients had improved primary and key secondary efficacy endpoints at the end of the treatment period versus baseline. Although the vehicle did not contain active pharmaceutical ingredients, several inert excipients have been shown to improve AD-related symptoms.29-33 Because damage to the skin barrier and any additional or subsequent innate immune system dysfunction play a pivotal role in the development of AD,29 it is likely that excipients included in vehicles often enhance the skin's innate immune defenses and bring about additional changes within the skin, which promotes restoration of the skin barrier.29-33 White petrolatum is used as the main excipient and base of crisaborole ointment 2%, which has previously been shown to upregulate antimicrobial peptides, induce expression of key barrier differentiation markers, increase stratum corneum thickness, and significantly decrease T-cell infiltrates.30
Crisaborole was well tolerated in Chinese and Japanese patients ≥2 years of age with mild-to-moderate AD. Most TEAEs were mild, and the safety profiles of the vehicle-treated and crisaborole BID groups were found to be similar. In addition, skin and subcutaneous tissue disorders (mainly AD) occurred in only 13.1% of crisaborole-treated patients versus 19.8% of vehicle-treated patients (Table 2). Compared with the safety data from the CORE 1/CORE 2 studies, the overall TEAE rates were higher, but the severity and classification of TEAEs were similar.23 Previous studies have reported a higher level of intolerance to certain dermal preparations, with Asian patients (particularly Japanese patients) having greater response rates than White patients. The CrisADe CLEAR study population comprising only Asian patients may thus have had some contribution to the higher application site pain rate in the CrisADe CLEAR Study.34, 35 Application site pain may be mitigated by storing the ointment in the fridge, applying a moisturizer prior to use, and applying the ointment on a small test area and observing any reactions before applying it to affected areas.36
Overall, the CrisADe CLEAR, pooled CORE 1/CORE 2 (which all comprised patients ≥2 years with mild-to-moderate AD), and CrisADe CARE 1 (which comprised infants aged 3 to <24 months with mild-to-moderate AD) studies had similar efficacy and safety results, but the differences noted might be explained by the differences in baseline characteristics and patient demographics. The CrisADe CLEAR study consisted of an Asian population group. Asian patients have been found to be more sensitive to chemical stimuli on their skin, potentially due to a higher sweat gland density or a thinner stratum corneum.23, 37, 38 This may account for the higher incidence of dermal adverse effects noted in the CrisADe CLEAR study.
Because patients from Western countries were not included in this study, these results cannot be directly compared with those from Western populations. The study duration was not long enough to observe the long-term safety and efficacy of treatment in the Asian population studied.
5 CONCLUSIONS
Previous studies mainly evaluated populations in Western countries, thus it was important to evaluate the safety and efficacy of crisaborole ointment in an Asian patient population. In the CrisADe CLEAR study, crisaborole-treated patients had favorable outcomes in all primary and key secondary endpoints versus vehicle-treated patients, and no new safety signals were identified. Crisaborole was effective and well tolerated in Chinese and Japanese patients aged ≥2 years with mild-to-moderate AD.
ACKNOWLEDGMENTS
Editorial and medical writing support, under the guidance of the authors, was provided by Lisa M. Klumpp Callan, PhD, and Chantell Hayward, PharmD, at ApotheCom, San Francisco, CA, USA, and was funded by Pfizer Inc., New York, NY, USA, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med. 2022; 10.7326/M22-1460).
FUNDING INFORMATION
This study was funded by Pfizer Inc.
CONFLICT OF INTEREST STATEMENT
Lin Ma, Jianzhong Zhang, and Hao Cheng have received fees for serving as consultants and speakers for Pfizer Inc. Yangmei Zhou is an employee of Pfizer R&D China and was the statistician of the CrisADe CLEAR study. Yayuan Chen is an employee and shareholder of Pfizer R&D China and was the clinician of the CrisADe CLEAR study.
Open Research
DATA AVAILABILITY STATEMENT
On request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.




