Sustained efficacy and safety of guselkumab in patients with palmoplantar pustulosis through 1.5 years in a randomized phase 3 study
[Correction added on 20th September 2021 after first online publication: The supplementary figure S1 was replaced in the article]
Abstract
The safety and efficacy of guselkumab for palmoplantar pustulosis (PPP) have been established through week (W)52; however, no sufficient information is available beyond 1 year. This study was conducted to assess the efficacy and safety of guselkumab through W84, and to explore factors associated with the sustainability of its efficacy in Japanese PPP patients. Patients received guselkumab 100 or 200 mg at W0, W4, W12, and every 8 weeks (q8w) until W60, or placebo at W0, W4, and W12. At W16, patients receiving placebo were re-randomized to receive guselkumab 100/200 mg at W16, W20, and q8w until W60. Efficacy end-points included PPP Area and Severity Index (PPPASI), PPP Severity Index (PPSI), Physician’s Global Assessment scores, and patient reported outcomes (PRO) (Dermatology Life Quality Index, EuroQoL-5 Dimensions, and 36-item Short Form Health Survey). Post-hoc comparison of patient characteristics was performed between PPPASI-75/90 responders and non-responders at W60, and sustained responders and non-responders at W84. Safety was evaluated through W84. A total of 45, 43, 21, and 24 patients from the guselkumab 100 mg, guselkumab 200 mg, placebo→guselkumab 100 mg, and placebo→guselkumab 200 mg groups, respectively, completed the study through W84. Overall, the mean improvement in the guselkumab groups from baseline in the PPPASI and PPSI total scores at W84 was ~79% and ~66%, respectively. All PRO improved through W84. The proportion of responders through W60 was higher in patients who had not received prior phototherapy and non-biologic systemic therapy for PPP. Non-smokers and patients with no prior non-biologic systemic treatment tended numerically towards sustained efficacy through W84. The majority of treatment-emergent adverse events (TEAE) were mild to moderate (~88%) with low incidence of serious TEAE (7.6%). Overall, guselkumab showed sustained efficacy and safety with improvement in the health-related quality of life through W84 in Japanese PPP patients.
1 INTRODUCTION
Palmoplantar pustulosis (PPP) is a chronic, recurrent skin disease affecting the palms and/or soles. It clinically presents as vesicles with erythematous scaling, followed by appearance of sterile pustules.1 In the West, it is often classified by its localized form due to manifestations similar to other subtypes of pustular psoriasis; nevertheless, it can be differentiated based on its genetic features (low prevalence of interleukin [IL]-36 receptor antagonist mutations in comparison to generalized pustular psoriasis [GPP] and acrodermatitis continua of Hallopeau).1-4 Overall prevalence of PPP is approximately 0.05–0.12%; however, population-based studies are lacking.5 The Japanese prevalence of PPP is approximately 0.12%, with a male to female ratio of 0.53.6 Palmoplantar pustulosis leads to physical disability, restricting the use of palms and soles, and impairment of health-related quality of life (HRQOL).7, 8
The pathophysiology of PPP is complex and not fully understood. The IL-23/IL-17 pathway (via proliferation of type 17 helper T cells [Th17]) is suggested to stimulate cytokine production and play a crucial role in neutrophil infiltration and pustule formation.4, 9-13 Acrosyringium may be involved in vesicle formation in PPP.14 Treatment of PPP is challenging due to lack of standard therapies and curative responses.11 The current treatment in Japan includes topical therapy (vitamin D3 analog and corticosteroids), excimer or ultraviolet phototherapy, oral retinoids, methotrexate and cyclosporin, dental infection control or tonsillectomy, and granulocyte and monocyte adsorption apheresis, which often result in inadequate treatment outcomes.15-20 Biologic therapy seems to be an effective option but more studies are required to assess its long-term efficacy and safety.21
Guselkumab is a human immunoglobulin G1λ monoclonal antibody that binds to the p19 subunit of IL-23, thereby blocking the IL-23 signaling pathway and subsequent release of cytokines.9, 22 The efficacy and safety of guselkumab has been demonstrated in global studies in patients with moderate-to-severe plaque psoriasis and psoriatic arthritis (PsA),23-26 including Japanese studies for moderate-to-severe plaque psoriasis,27, 28 GPP and erythrodermic psoriasis,29 and PPP.30 Guselkumab is the first IL-23 inhibitor approved in the USA and Europe for the treatment of adult patients with moderate-to-severe plaque psoriasis and active PsA.31-33 In Japan, it is approved for the treatment of plaque psoriasis, GPP and erythrodermic psoriasis, and PsA in patients with inadequate response to conventional therapies,34 and was approved for PPP in November 2018.35
This phase 3 study was conducted to evaluate the efficacy and safety of guselkumab (100 and 200 mg), administrated at week (W)0, W4, W12, and every 8 weeks (q8w) thereafter until W60 in Japanese patients with PPP through W84. Previously published data demonstrated a favorable efficacy and safety profile of guselkumab in PPP through W52.1 This report describes the efficacy and safety of guselkumab through W84. Further, a post hoc analysis explored factors associated with the sustained efficacy of guselkumab by comparing the patient characteristics between Palmoplantar Pustulosis Area and Severity Index (PPPASI)-75/90 responders and non-responders at W60, and between PPPASI-75/90 sustained responders and non-sustained responders at W84.
2 METHODS
The detailed study methodology was published earlier.1 The study protocol was approved by institutional review boards at all 40 study sites, and the study was conducted in accordance with the ethical principles defined in the Declaration of Helsinki, International Council for Harmonization (Good Clinical Practices) guidelines, and applicable regulatory requirements. Written informed consents were obtained from all patients before enrollment. The study protocol is available at the trial registry site (ClincalTrials.gov identifier: NCT02641730).
2.1 Patient population
Japanese patients (aged ≥20 years) diagnosed with PPP (with or without pustulotic arthro-osteitis [PAO]) at least ≥24 weeks before the screening, having an inadequate response to conventional therapies, and a PPPASI total score of ≥12 and a PPPASI severity score of pustules/vesicles of ≥2 at screening and baseline were enrolled. Patients with plaque-type psoriasis, ≥5 PPPASI total score improvement during screening, or drug-induced PPP were excluded.
2.2 Study design
This was a phase 3, randomized, double-blind, multicenter, placebo-controlled study consisting of three phases: a screening phase (~6 weeks), a blinded treatment phase (W0–W60), and an observational phase (W60–W84) (Figure 1).
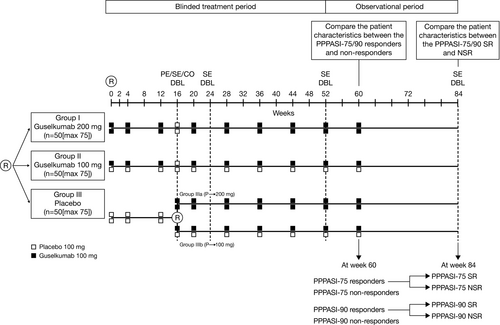
Eligible patients were randomized to one of three treatment groups (1:1:1 ratio): guselkumab 100 mg, guselkumab 200 mg (both at W0, W4, W12, and q8w thereafter until W60), or placebo (at W0, W4, and W12) administrated as s.c. injections. At W16, patients from the placebo group were re-randomized to receive guselkumab 100 or 200 mg (1:1 ratio) at W16, W20, and q8w thereafter until W60. Central randomization was implemented and balanced by randomly permuted blocks. Patients were stratified by PPPASI total scores at baseline (≤20, 21–30, ≥31) and by smoking status.
Treatments for PPP (except topical moisturizers) that could affect the course of PPP or study evaluations were prohibited from W0 to W60; there was no restriction on the use of concomitant therapies after W60.
2.3 Efficacy evaluations
The primary, major secondary, and exploratory efficacy end-points are described in the previously published manuscripts.1, 36 Other secondary efficacy evaluations through W84 included: (i) changes from baseline in the PPPASI total score and Palmoplantar Pustulosis Severity Index (PPSI) total score; (ii) proportions of patients achieving the following responses over time: PPPASI-50/75/90/100, PPSI-50/75/90/100, PPSI subscores of 0 (none) or 1 (slight) for erythema, pustular/vesicle, and desquamation/scale, Physician’s Global Assessment (PGA) score of clear (0) or minimal (1), and PGA score of 0/1 with ≥2-grade improvement (score range 0 [clear] to 5 [very severe]) from baseline; and (iii) Patient-reported outcomes (PRO)1: change from baseline over time in the 36-item Short Form Health Survey (SF-36) score including Physical Component Summary (PCS) and Mental Component Summary (MCS),37 EuroQoL-5 Dimensions (EQ-5D) Questionnaire score,38 and Dermatology Life Quality Index (DLQI) score.39
A post hoc comparison of patient characteristics (demographics, disease characteristics, smoking, medical history, and prior and concomitant medications), SF-36, EQ-5D, and DLQI scores between PPPASI-75/90 responders and non-responders at W60, and between PPPASI-75/90 sustained and non-sustained responders at W84 was performed.
2.4 Safety evaluations
Treatment-emergent adverse events (TEAE), laboratory parameters, vital signs, physical examinations, electrocardiograms (ECG), injection-site reactions, allergic reactions, infections, major adverse cardiovascular events (MACE), malignancies, anaphylactic reactions or serum sickness-like reactions, and tuberculosis review were evaluated from the signing of informed consent through W84. From W72 to W84, only TEAE considered related to the study treatment were collected.
2.5 Statistical analysis
2.5.1 Sample size and analysis sets
The sample size rationale is described in the previously published manuscript.1
The preplanned summaries and analyses “through W84” were based on patients randomized to guselkumab at W0 or W16. The post hoc analysis was based on a subset of patients who completed the study through W84. The safety analysis included patients who received ≥1 injections of study treatment.
2.5.2 Statistical evaluations
Efficacy analyses through W16 are described in the previous publication.1 The PPPASI, PPSI, PGA, SF-36, EQ-5D, and DLQI were analyzed through W84 using descriptive statistics.
The post hoc analysis was performed descriptively by comparing the patient characteristics between the PPPASI-75/90 responders and non-responders at W60, and between the PPPASI-75/90 sustained and non-sustained responders at W84. The PPPASI-75/90 sustained responders at W84 were defined as patients achieving PPPASI-75/90 response at both W60 and W84. The PPPASI-75/90 non-sustained responders at W84 were defined as patients achieving PPPASI-75/90 response at W60 but not at W84. Data from the guselkumab combined group were presented as results for a single overall guselkumab group. This post hoc analysis was based on observed data and no missing data imputation was performed. The statistical tests of the post hoc results were considered nominal and statistical values are not shown in the manuscript.
Safety data were descriptively summarized. TEAE were summarized by the treatment group using the Medical Dictionary for Regulatory Activities (version 19.1) system organ class and preferred terms.
3 RESULTS
3.1 Patient disposition
This study was conducted from 15 December 2015 to 17 July 2018. Overall, 159 patients were randomized at W0 and 133 patients completed the study at W84 (45, 43, 21, and 24 patients in the guselkumab 100 mg, guselkumab 200 mg, placebo→guselkumab 100 mg, and placebo→guselkumab 200 mg groups, respectively). Through W60, 25/159 patients (15.7%) discontinued study treatment, with the most common reason being TEAE. Through W60–W84, one patient from the guselkumab 200 mg group withdrew from the study (Figure 2).
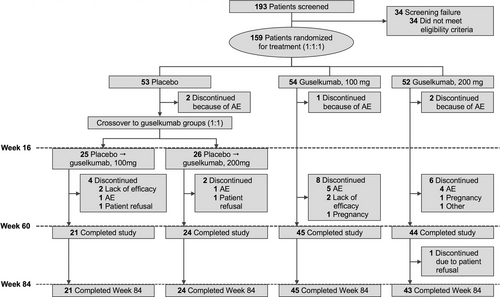
The demographics and baseline characteristics were published previously.1
3.2 Efficacy
3.2.1 PPPASI and PPSI
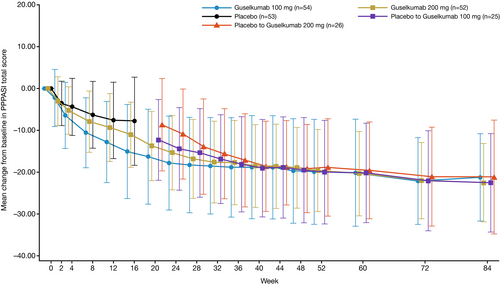
Continuous improvements in the PPPASI and PPSI total scores were observed through W60 and sustained in the observational phase (W60–W84) across all treatment groups, including the placebo-crossover groups (Figure 3 and Figure S1). At W84, the mean percent improvements in the PPPASI and PPSI total scores were 77.53%, 85.13%, 75.98%, and 74.28%, and 58.9%, 74.2%, 68.9%, and 63.0%, respectively, for the guselkumab 100 mg, guselkumab 200 mg, placebo→guselkumab 100 mg, and placebo→guselkumab 200 mg groups (Table 1).
| End-points |
Placebo→guselkumab 100 mg (N = 21) |
Placebo→guselkumab 200 mg (N = 24) |
Guselkumab 100 mg (N = 45) |
Guselkumab 200 mg (N = 43) |
Guselkumab combined (N = 133) |
|---|---|---|---|---|---|
| Percent improvement from baseline in PPPASI total score, mean (SD) | 75.98 (24.818) | 74.28 (35.206) | 77.53 (21.319) | 85.13 (16.675) | 79.16 (23.827) |
| PPPASI-50/75/90/100 responders, n (%) | |||||
| PPPASI-50 | 18 (85.7%) | 21 (87.5%) | 40 (88.9%) | 41 (95.3%) | 120 (90.2%) |
| PPPASI-75 | 13 (61.9%) | 16 (66.7%) | 31 (68.9%) | 35 (81.4%) | 95 (71.4%) |
| PPPASI-90 | 10 (47.6%) | 10 (41.7%) | 14 (31.1%) | 22 (51.2%) | 56 (42.1%) |
| PPPASI-100 | 1 (4.8%) | 0 | 1 (2.2%) | 6 (14.0%) | 8 (6.0%) |
| Percent improvement from baseline in PPSI total score, mean (SD) | 68.9 (26.19) | 63.0 (34.07) | 58.9 (27.70) | 74.2 (23.20) | 66.1 (27.84) |
| PPSI-50/75/90/100 responders, n (%) | |||||
| PPSI-50 | 17 (81.0%) | 19 (79.2%) | 30 (66.7%) | 36 (83.7%) | 102 (76.7%) |
| PPSI-75 | 12 (57.1%) | 11 (45.8%) | 15 (33.3%) | 27 (62.8%) | 65 (48.9%) |
| PPSI-90 | 6 (28.6%) | 7 (29.2%) | 8 (17.8%) | 14 (32.6%) | 35 (26.3%) |
| PPSI-100 | 2 (9.5%) | 1 (4.2%) | 3 (6.7%) | 9 (20.9%) | 15 (11.3%) |
| PPSI subscores, n (%) | |||||
| PPSI subscore (erythema) of none (0) or slight (1) | 16 (76.2%) | 17 (70.8%) | 31 (68.9%) | 35 (81.4%) | 99 (74.4%) |
| PPSI subscore (pustular/vesicle) of none (0) or slight (1) | 15 (71.4%) | 15 (62.5%) | 27 (60.0%) | 32 (74.4%) | 89 (66.9%) |
| PPSI subscore (desquamation/scale) of none (0) or slight (1) | 14 (66.7%) | 13 (54.2%) | 21 (46.7%) | 28 (65.1%) | 76 (57.1%) |
| PGA score, n (%) | |||||
| PGA score of clear (0) or almost clear (1) | 9 (42.9%) | 13 (54.2%) | 17 (37.8%) | 23 (53.5%) | 62 (46.6%) |
| PGA score of clear (0) or almost clear (1) and at least a 2-grade improvement from baseline | 9 (42.9%) | 13 (54.2%) | 17 (37.8%) | 23 (53.5%) | 62 (46.6%) |
Note
- Patients in the placebo group were re-randomized to receive guselkumab 100 or 200 mg starting at W16.
- Abbreviations: n, number of patients; N, total number of patients evaluated in each group; PGA, Physician’s Global Assessment; PPPASI, Palmoplantar Pustulosis Area and Severity Index; PPPASI-50/75/90/100, 50%/75%/90%/100% reduction in Palmoplantar Pustulosis Area and Severity Index; PPSI, Palmoplantar Pustulosis Severity Index; PPSI-50/75/90/100, 50%/75%/90%/100% reduction in Palmoplantar Pustulosis Severity Index; SD, standard deviation; W, week.
The proportions of PPPASI-50 responders increased through W60 (Table 2) and were increased/sustained through W84, reaching a maximum of 88.9%, 95.3%, 85.7%, and 87.5%, respectively, for the guselkumab 100 mg, guselkumab 200 mg, placebo→guselkumab 100 mg, and placebo→guselkumab 200 mg groups. At W84, the proportions of PPPASI-75 responders were numerically higher in the guselkumab 200 mg group (81.4%) than the guselkumab 100 mg (68.9%), placebo→guselkumab 200 mg (66.7%), and placebo→guselkumab 100 mg (61.9%) groups. Similar trends were observed for the PPPASI-90/100 responders.
| End-points |
Placebo→guselkumab 100 mg (N = 25) |
Placebo→guselkumab 200 mg (N = 26) |
Guselkumab 100 mg (N = 54) |
Guselkumab 200 mg (N = 52) |
Guselkumab combined (N = 157) |
|---|---|---|---|---|---|
| Percent improvement from baseline in PPPASI total score at W60, mean (SD) | 66.40 (26.327) | 70.30 (24.576) | 71.35 (29.916) | 76.58 (24.242) | 72.12 (26.705) |
| PPPASI-50/75/90/100 responders, n (%) | |||||
| PPPASI-50 | 20 (80.0%) | 21 (80.8%) | 43 (79.6%) | 44 (84.6%) | 128 (81.5%) |
| PPPASI-75 | 10 (40.0%) | 13 (50.0%) | 34 (63.0%) | 36 (69.2%) | 93 (59.2%) |
| PPPASI-90 | 5 (20.0%) | 9 (34.6%) | 19 (35.2%) | 23 (44.2%) | 56 (35.7%) |
| PPPASI-100 | 1 (4.0%) | 1 (3.8%) | 5 (9.3%) | 2 (3.8%) | 9 (5.7%) |
| Percent improvement from baseline in PPSI total score, mean (SD) | 51.0 (30.59) | 57.1 (24.25) | 56.0 (30.87) | 64.7 (28.41) | 58.3 (29.15) |
| PPSI-50/75/90/100 responders, n (%) | |||||
| PPSI-50 | 14 (56.0%) | 17 (65.4%) | 33 (61.1%) | 39 (75.0%) | 103 (65.6%) |
| PPSI-75 | 5 (20.0%) | 8 (30.8%) | 20 (37.0%) | 25 (48.1%) | 58 (36.9%) |
| PPSI-90 | 5 (20.0%) | 4 (15.4%) | 7 (13.0%) | 13 (25.0%) | 29 (18.5%) |
| PPSI-100 | 2 (8.0%) | 1 (3.8%) | 5 (9.3%) | 4 (7.7%) | 12 (7.6%) |
| PPSI subscores, n (%) | |||||
| PPSI subscore (erythema) of none (0) or slight (1) | 14 (56.0%) | 17 (65.4%) | 32 (59.3%) | 35 (67.3%) | 98 (62.4%) |
| PPSI subscore (pustular/vesicle) of none (0) or slight (1) | 14 (56.0%) | 18 (69.2%) | 33 (61.1%) | 34 (65.4%) | 99 (63.1%) |
| PPSI subscore (desquamation/scale) of none (0) or slight (1) | 8 (32.0%) | 10 (38.5%) | 20 (37.0%) | 29 (55.8%) | 67 (42.7%) |
| PGA score, n (%) | |||||
| PGA score of clear (0) or almost clear (1) | 6 (24.0%) | 7 (26.9%) | 19 (35.2%) | 26 (50.0%) | 58 (36.9%) |
| PGA score of clear (0) or almost clear (1) and at least a 2-grade improvement from baseline | 6 (24.0%) | 7 (26.9%) | 19 (35.2%) | 26 (50.0%) | 58 (36.9%) |
Note
- Patients in the placebo group were re-randomized to receive guselkumab 100 or 200 mg starting at W16.
- Abbreviations: n, number of patients; N, total number of patients evaluated in each group; PGA, Physician’s Global Assessment; PPPASI, Palmoplantar Pustulosis Area and Severity Index; PPPASI-50/75/90/100, 50%/75%/90%/100% reduction in Palmoplantar Pustulosis Area and Severity Index; PPSI, Palmoplantar Pustulosis Severity Index; PPSI-50/75/90/100, 50%/75%/90%/100% reduction in Palmoplantar Pustulosis Severity Index; SD, standard deviation; W, week.
The proportions of PPSI-50 responders increased through W60 (Table 2) and were sustained through W84 (66.7%, 83.7%, 81.0%, and 79.2% in the guselkumab 100 mg, guselkumab 200 mg, placebo→guselkumab 100 mg, and placebo→guselkumab 200 mg groups, respectively). At W84, the proportions of responders in the guselkumab 200 mg group were numerically higher than the guselkumab 100 mg group for PPSI-75 (62.8% vs 33.3%) and PPSI-90 (32.6% vs 17.8%), while the proportions of PPSI-75/90 responders were comparable in the placebo→guselkumab 100 mg and 200 mg groups. A similar trend was observed for the PPSI-100 responders.
An improvement in the PPSI subscores of erythema, pustular/vesicle, and desquamation/scale was observed through W60 and sustained through W84 (Table 1).
3.2.2 PGA
The proportions of patients achieving PGA scores of 0/1 increased through W60 (Table 2), and from W60 to W84 in the guselkumab treatment groups (Table 1). The proportions of patients achieving PGA scores of 0/1 and having ≥2-grade improvement from baseline at W84 were 37.8%, 53.5%, 42.9%, and 54.2% in the guselkumab 100 mg, guselkumab 200 mg, placebo→guselkumab 100 mg, and placebo→guselkumab 200 mg groups, respectively.
3.2.3 Patient reported outcomes
An improvement was observed from baseline through W84 in SF-36 (PCS and MCS), EQ-5D (visual analog scale [VAS] and index), and DLQI scores in all treatment groups (Table 3).
| End-points | Placebo→guselkumab 100 mg (N = 21) | Placebo→guselkumab 200 mg (N = 24) | Guselkumab 100 mg (N = 45) | Guselkumab 200 mg (N = 43) | Guselkumab combined (N = 133) |
|---|---|---|---|---|---|
| Change from baseline, mean (SD) | |||||
| SF-36, PCS | 9.29 (19.175) | 9.01 (12.964) | 7.06 (15.034) | 6.75 (13.619) | 7.66 (14.849) |
| SF-36, MCS | 1.72 (8.740) | 0.78 (6.975) | 2.51 (8.428) | 2.58 (7.977) | 2.10 (8.028) |
| EQ-5D index score | 0.1283 (0.21561) | 0.1706 (0.19590) | 0.1622 (0.20457) | 0.1553 (0.14282) | 0.1561 (0.18532) |
| EQ-5D VAS score | 6.7 (24.71) | 10.0 (19.55) | 10.5 (21.53) | 8.7 (18.42) | 9.2 (20.57) |
| DLQI score | −6.0 (5.29) | −5.6 (4.66) | −6.4 (5.59) | −6.3 (5.41) | −6.2 (5.27) |
Note
- Patients in the placebo group were re-randomized to receive guselkumab 100 or 200 mg starting at week 16.
- Abbreviations: DLQI, Dermatology Life Quality Index; EQ-5D, EuroQoL-5 Dimensions; MCS, Mental Component Summary; N, total number of patients evaluated in each group; PCS, Physical Component Summary; SD, standard deviation; SF-36, 36-Item Short Form Health Survey; VAS, visual analog scale.
Post hoc evaluation of SF-36 scores
The mean increases from baseline in SF-36 (both PCS and MCS) scores (indicates improvement) at W52/W72/W84 were numerically higher in PPPASI-75/90 responders compared with non-responders at W60 (Table 4). A similar trend was observed in the PPPASI-75/90 sustained responders compared with non-sustained responders at W84, except the increase from baseline in SF-36 MCS scores at W72 was numerically higher in the PPPASI-75/90 non-sustained responders compared with sustained responders at W84 (Table 5).
| PPPASI-75 R at W60 | PPPASI-75 NR at W60 | PPPASI-90 R at W60 | PPPASI-90 NR at W60 | |
|---|---|---|---|---|
| Change from baseline in SF-36 PCS score, mean (SD) | ||||
| N | 88 | 45 | 55 | 78 |
| W52 | 8.93 (14.940) | 5.75 (12.201) | 10.52 (15.479) | 5.98 (12.826) |
| W72 | 8.84 (14.211) | 4.85 (12.281) | 10.58 (14.976) | 5.31 (12.311) |
| W84 | 9.26 (15.440) | 4.55 (13.233) | 11.48 (15.490) | 4.97 (13.856) |
| Change from baseline in SF-36 MCS score, mean (SD) | ||||
| N | 88 | 45 | 55 | 78 |
| W52 | 2.16 (8.112) | −0.04 (8.030) | 2.30 (8.796) | 0.80 (7.608) |
| W72 | 1.53 (7.942) | 0.73 (7.971) | 1.96 (8.357) | 0.76 (7.632) |
| W84 | 2.66 (7.671) | 0.99 (8.665) | 2.76 (7.679) | 1.63 (8.281) |
| Change from baseline in EQ-5D Index score, mean (SD) | ||||
| N | 88 | 45 | 55 | 78 |
| W52 | 0.1924 (0.18585) | 0.1109 (0.13331) | 0.2070 (0.18552) | 0.1351 (0.15954) |
| W72 | 0.1849 (0.20937) | 0.1040 (0.12266) | 0.2013 (0.21727) | 0.1266 (0.15878) |
| W84 | 0.1876 (0.20295) | 0.0945 (0.12538) | 0.1987 (0.19540) | 0.1261 (0.17288) |
| Change from baseline in EQ-5D VAS score, mean (SD) | ||||
| N | 88 | 45 | 55 | 78 |
| W52 | 13.1 (20.91) | 8.1 (14.65) | 14.8 (22.99) | 9.0 (15.56) |
| W72 | 12.7 (22.05) | 8.8 (16.43) | 14.3 (24.66) | 9.3 (16.52) |
| W84 | 10.7 (22.62) | 6.4 (15.69) | 13.1 (23.78) | 6.5 (17.63) |
| Change from baseline in DLQI score, mean (SD) | ||||
| N | 88 | 45 | 55 | 78 |
| W52 | −6.2 (5.56) | −4.7 (4.98) | −6.9 (5.79) | −4.8 (4.97) |
| W72 | −6.6 (5.66) | −4.8 (4.30) | −7.1 (6.15) | −5.2 (4.47) |
| W84 | −6.6 (5.58) | −5.4 (4.57) | −7.1 (5.96) | −5.6 (4.68) |
| Patients with DLQI score of 0 or 1, n (%) | ||||
| N | 81 | 42 | 49 | 74 |
| W52 | 45 (55.6%) | 15 (35.7%) | 29 (59.2%) | 31 (41.9%) |
| W72 | 49 (60.5%) | 16 (38.1%) | 32 (65.3%) | 33 (44.6%) |
| W84 | 50 (61.7%) | 20 (47.6%) | 33 (67.3%) | 37 (50.0%) |
Note
- Data of responders and non-responders presented in each column are from the guselkumab combined group.
- Abbreviations: DLQI, Dermatology Life Quality Index; EQ-5D, EuroQoL-5 Dimensions; MCS, Mental Component Summary; n, number of patients; N, total number of patients evaluated in each group; NR, non-responders; PCS, Physical Component Summary; PPPASI-75/90, 75%/90% reduction in Palmoplantar Pustulosis Area and Severity Index; R, responders; SD, standard deviation, SF-36, 36-Item Short Form Health Survey; VAS, visual analog scale; W, week.
| PPPASI-75 SR at W84 | PPPASI-75 NSR at W84 | PPPASI-90 SR at W84 | PPPASI-90 NSR at W84 | |
|---|---|---|---|---|
| Change from baseline in SF-36 PCS score, mean (SD) | ||||
| N | 78 | 10 | 38 | 17 |
| W52 | 9.44 (15.593) | 4.98 (7.599) | 11.08 (15.348) | 9.25 (16.171) |
| W72 | 9.31 (14.935) | 5.18 (5.196) | 11.41 (16.075) | 8.74 (12.415) |
| W84 | 9.92 (16.117) | 4.11 (6.982) | 12.92 (16.4.30) | 8.25 (13.019) |
| Change from baseline in SF-36 MCS score, mean (SD) | ||||
| N | 78 | 10 | 38 | 17 |
| W52 | 2.26 (8.576) | 1.39 (2.476) | 2.92 (8.685) | 0.91 (9.151) |
| W72 | 1.37 (7.990) | 2.78 (7.851) | 1.56 (8.871) | 2.85 (7.244) |
| W84 | 2.91 (7.829) | 0.71 (6.292) | 3.57 (8.182) | 0.94 (6.258) |
| Change from baseline in EQ-5D index score, mean (SD) | ||||
| N | 78 | 10 | 38 | 17 |
| W52 | 0.1999 (0.18886) | 0.1342 (0.15653) | 0.2157 (0.18774) | 0.1876 (0.18459) |
| W72 | 0.1890 (0.21410) | 0.1526 (0.17404) | 0.2129 (0.21243) | 0.1753 (0.23222) |
| W84 | 0.1982 (0.20552) | 0.1056 (0.16836) | 0.2283 (0.18915) | 0.1326 (0.19848) |
| Change from baseline in EQ-5D VAS score, mean (SD) | ||||
| N | 78 | 10 | 38 | 17 |
| W52 | 13.2 (21.37) | 12.6 (17.86) | 14.1 (23.80) | 16.2 (21.69) |
| W72 | 12.6 (22.10) | 13.3 (22.83) | 15.5 (23.51) | 11.5 (27.62) |
| W84 | 10.9 (21.89) | 8.6 (28.98) | 16.0 (23.25) | 6.6 (24.40) |
| Change from baseline in DLQI score, mean (SD) | ||||
| N | 78 | 10 | 38 | 17 |
| W52 | −6.6 (5.69) | −3.2 (3.29) | −6.4 (5.09) | −7.9 (7.17) |
| W72 | −6.8 (5.83) | −4.3 (3.59) | −6.7 (5.32) | −8.0 (7.81) |
| W84 | −7.1 (5.57) | −2.6 (4.06) | −6.9 (5.01) | −7.4 (7.84) |
| Patients with DLQI score of 0 or 1, n (%) | ||||
| N | 71 | 10 | 35 | 14 |
| W52 | 41 (57.7%) | 4 (40.0%) | 21 (60.0%) | 8 (57.1%) |
| W72 | 45 (63.4%) | 4 (40.0%) | 24 (68.6%) | 8 (57.1%) |
| W84 | 49 (69.0%) | 1 (10.0%) | 27 (77.1%) | 6 (42.9%) |
Note
- (i) PPPASI-75/90 SR at W84 were defined as patients who achieved PPPASI-75/90 response at both W60 and W84. PPPASI-75/90 NSR at W84 were defined as patients who achieved PPPASI-75/90 response at W60 but not at W84. (ii) Data of responders and non-responders presented in each column are from the guselkumab combined group.
- Abbreviations: DLQI, Dermatology Life Quality Index; EQ-5D, EuroQoL-5 Dimensions; MCS, Mental Component Summary; n, number of patients; N, total number of patients evaluated in each group; NSR, non-sustained responders; PCS, Physical Component Summary; PPPASI-75/90, 75%/90% reduction in Palmoplantar Pustulosis Area and Severity Index; SD, standard deviation; SF-36, 36-Item Short Form Health Survey; SR, sustained responders; VAS; visual analog scale; W, week.
Post hoc evaluation of EQ-5D scores
The mean increases from baseline in EQ-5D scores (both index and VAS) scores (indicates improvement) at W52/W72/W84 were numerically higher in PPPASI-75/90 responders compared with non-responders at W60 (Table 4). A similar trend was observed in the PPPASI-75/90 sustained responders compared with non-sustained responders at W84, except the increase from baseline in EQ-5D VAS scores at W52 and W72 was numerically higher, respectively, in the PPPASI-90 and PPPASI-75 non-sustained responders compared with sustained responders at W84 (Table 5).
Post hoc evaluation of DLQI scores
The mean decreases from baseline in DLQI scores (i.e., improvement in DLQI) at W52/W72/W84 were numerically higher in PPPASI-75/90 responders compared with non-responders at W60 (Table 4). A similar trend was observed in the PPPASI-75 sustained responders compared with non-sustained responders at W84. The improvement from baseline in the DLQI scores at W52/W72/W84 was numerically higher in the PPPASI-90 non-sustained responders compared with sustained responders at W84 (Table 5). Patients achieving a DLQI score of 0/1 (no/little effect on the patient’s life) at W52/W72/W84 were numerically higher in PPPASI-75/90 responders compared with non-responders at W60. A similar trend was observed in PPPASI-75/90 sustained responders compared with non-sustained responders at W84. The scores of SF-36, EQ-5D, and DLQI at W52/W72/W84 are shown in Tables S1 and S2.
3.2.4 Post hoc evaluation of patient characteristics
Detailed post hoc evaluations are summarized in Tables 6 and 7.
| Patient characteristics | PPPASI-75 R at W60 | PPPASI-75 NR at W60 | PPPASI-90 R at W60 | PPPASI-90 NR at W60 |
|---|---|---|---|---|
| Number of patients in each group | 88 | 45 | 55 | 78 |
| Men, n (%) | 14 (15.9%) | 12 (26.7%) | 7 (12.7%) | 19 (24.4%) |
| Women, n (%) | 74 (84.1%) | 33 (73.3%) | 48 (87.3%) | 59 (75.6%) |
| Weight, mean (SD) kg | 58.52 (9.944) | 63.53 (11.630) | 57.53 (9.449) | 62.11 (11.284) |
| BMI, mean (SD) kg/m2 | 23.223 (3.2380) | 24.887 (4.3008) | 23.132 (3.3201) | 24.247 (3.9032) |
| PPP disease duration, mean (SD), years | 7.15 (8.003) | 5.62 (8.515) | 6.92 (8.517) | 6.43 (7.982) |
| PPPASI total score (0–72), mean (SD) | 28.01 (10.566) | 26.30 (10.485) | 26.96 (11.088) | 27.76 (10.179) |
| Prior phototherapya, n (%) | ||||
| Nb | 86 | 45 | 54 | 77 |
| Never used | 54 (62.8%) | 23 (51.1%) | 35 (64.8%) | 42 (54.5%) |
| Ever used | 32 (37.2%) | 22 (48.9%) | 19 (35.2%) | 35 (45.5%) |
| Prior non-biologics systemicsc, n (%) | ||||
| Never used | 70 (79.5%) | 32 (71.1%) | 44 (80.0%) | 58 (74.4%) |
| ≥1 therapy | 15 (17.0%) | 12 (26.7%) | 9 (16.4%) | 18 (23.1%) |
| ≥2 therapies | 3 (3.4%) | 1 (2.2%) | 2 (3.6%) | 2 (2.6%) |
| Smoking habit, n (%) | ||||
| Smoking | 51 (58.0%) | 21 (46.7%) | 30 (54.5%) | 42 (53.8%) |
| Non-smoking | 37 (42.0%) | 24 (53.3%) | 25 (45.5%) | 36 (46.2%) |
| Medical history | ||||
| Hyperlipidemia | 20 (22.7%) | 13 (28.9%) | 11 (20.0%) | 22 (28.2%) |
| Hypertension | 26 (29.5%) | 12 (26.7%) | 16 (29.1%) | 22 (28.2%) |
| PAO | 43 (48.9%) | 14 (31.1%) | 31 (56.4%) | 26 (33.3%) |
| Concomitant medicationsd (from W60 through W84, n [%]) | ||||
| Ultra-high-potency topical corticosteroid (total) | NA | NA | NA | |
| For palmoplantar pustulosis after W60 | NA | NA | NA | NA |
Note
- Data of responders and non-responders presented in each column are from the guselkumab combined group.
- Abbreviations: BMI, body mass index; n, number of patients; N, total number of patients evaluated in each group; NA, not available; NR, non-responders; PAO, pustulotic arthro-osteitis; PPP, palmoplantar pustulosis; PPPASI, Palmoplantar Pustulosis Area and Severity Index; PPPASI-75/90, 75%/90% reduction in Palmoplantar Pustulosis Area and Severity Index; PUVA, psoralen and ultraviolet A therapy; R, responders; SD, standard deviation; UV, ultraviolet; W, week.
- a Includes PUVA or UV-B.
- b N is presented separately here due to number of patients evaluated in each group for prior phototherapy evaluation is different from number of total patients evaluated in each group.
- c Includes PUVA, methotrexate, cyclosporin, acitretin, apremilast, or tofacitinib.
- d Excluding those started after study treatment discontinuation.
| Patient characteristics | PPPASI-75 SR at W84 | PPPASI-75 NSR at W84 | PPPASI-90 SR at W84 | PPPASI-90 NSR at W84 |
|---|---|---|---|---|
| Number of patients in each group | 78 | 10 | 38 | 17 |
| Men, n (%) | 12 (15.4%) | 2 (20.0%) | 7 (18.4%) | 0 |
| Women, n (%) | 66 (84.6%) | 8 (80.0%) | 31 (81.6%) | 17 (100.0%) |
| Weight, mean (SD), kg | 58.53 (9.769) | 58.46 (11.806) | 59.76 (9.173) | 52.54 (8.275) |
| BMI, mean (SD), kg/m2 | 23.243 (3.2039) | 23.066 (3.6734) | 23.407 (3.2765) | 22.517 (3.4343) |
| PPP disease duration, mean (SD), years | 7.05 (8.203) | 7.89 (6.531) | 5.83 (7.591) | 9.36 (10.121) |
| PPPASI total score (0–72), mean (SD) | 27.77 (10.852) | 29.87 (8.203) | 27.80 (11.549) | 25.09 (10.052) |
| Prior phototherapya, n (%) | ||||
| Nb | 76 | 10 | 37 | 17 |
| Never used | 46 (60.5%) | 8 (80.0%) | 25 (67.6%) | 10 (58.8%) |
| Ever used | 30 (39.5%) | 2 (20.0%) | 12 (32.4%) | 7 (41.2%) |
| Prior non-biologic systemicsc, n (%) | ||||
| Never used | 64 (82.1%) | 6 (60.0%) | 32 (84.2%) | 12 (70.6%) |
| ≥1 therapy | 11 (14.1%) | 4 (40.0%) | 5 (13.2%) | 4 (23.5%) |
| ≥2 therapies | 3 (3.8%) | 0 | 1 (2.6%) | 1 (5.9%) |
| Patients with smoking, n (%) | ||||
| Smoking | 44 (56.4%) | 7 (70.0%) | 20 (52.6%) | 10 (58.8%) |
| Non-smoking | 34 (43.6%) | 3 (30.0%) | 18 (47.4%) | 7 (41.2%) |
| Medical history, n (%) | ||||
| Hyperlipidemia | 19 (24.4%) | 1 (10.0%) | 5 (13.2%) | 6 (35.3%) |
| Hypertension | 22 (28.2%) | 4 (40.0%) | 8 (21.1%) | 8 (47.1%) |
| PAO | 37 (47.4%) | 6 (60.0%) | 21 (55.3%) | 10 (58.8%) |
| Concomitant medicationsd (from W60 through W84, n [%]) | ||||
| Ultra-high-potency topical corticosteroid (total) | 22 (28.2%) | 4 (40.0%) | 6 (15.8%) | 7 (41.2%) |
| For palmoplantar pustulosis after W60 | 20 (25.6%) | 1 (10.0%) | 4 (10.5%) | 7 (41.2%) |
Note
- (i) PPPASI-75/90 SR at W84 were defined as patients who achieved PPPASI-75/90 response at both W60 and W84. PPPASI-75/90 NSR at W84 were defined as patients who achieved PPPASI-75/90 response at W60 but not at W84. (ii) Data of responders and non-responders presented in each column are from the guselkumab combined group.
- Abbreviations: BMI, body mass index; n, number of patients; N, total number of patients evaluated in each group; NA, not available; NSR, non-sustained responders; PAO, pustulotic arthro-osteitis; PGA, Physician’s Global Assessment; PPP, palmoplantar pustulosis; PPPASI, Palmoplantar Pustulosis Area and Severity Index; PPPASI-75/90, 75%/90% reduction in Palmoplantar Pustulosis Area and Severity Index; PUVA, psoralen and ultraviolet A therapy; SD, standard deviation; SR, sustained responders; UV, ultraviolet; W, week.
- a Includes PUVA or UV-B.
- b N is presented separately here due to number of patients evaluated in each group for prior phototherapy evaluation is different from number of total patients evaluated in each group.
- c Includes PUVA, methotrexate, cyclosporin, acitretin, apremilast, or tofacitinib.
- d Excluding those started after study treatment discontinuation.
Demographics and disease characteristics
Numerically, there was no gender impact on the sustenance of PPPASI-75/90 response at W84 in responders at W60. The mean baseline bodyweight and body mass index (BMI) of PPPASI-75/90 responders at W60 were slightly lower as compared with non-responders. No consistent trend was observed in the baseline PPP disease duration. No notable difference was observed in the baseline PPPASI total score between PPPASI-75/90 responders and non-responders at W60 or PPPASI-75/90 sustained and non-sustained responders at W84.
Medical history
The majority of patients had a medical history of PAO (>31.0%), hypertension (>21.0%), and hyperlipidemia (>10.0%). The proportions of patients with hyperlipidemia and hypertension were comparable between PPPASI-75/90 responders and non-responders at W60; the proportions of patients with PAO were numerically higher in PPPASI-75 (48.9% vs 31.1%) and PPPASI-90 responders (56.4% vs 33.3%) than non-responders. No consistent trend was observed in the proportions of patients with these medical histories on comparing PPPASI-75/90 sustained and non-sustained responders at W84.
Prior and concomitant medications
The proportions of patients with prior phototherapy (37.2% vs 48.9%) and non-biologic systemic therapy (17.0% vs 26.7%) were lower in PPPASI-75 responders compared with non-responders at W60. A similar trend was observed for PPPASI-90 in patients with prior phototherapy (35.2% vs 45.5%) and non-biologic systemic therapy (16.4% vs 23.1%). The proportion of patients with prior non-biologic systemic therapy was relatively lower in PPPASI-75/90 sustained responders compared with non-sustained responders at W84. No consistent trend was observed with the concomitant use of ultra-high-potency topical corticosteroid for PPP from W60 to W84.
Smoking history
The proportion of smokers was numerically higher in PPPASI-75 responders than non-responders (58.0% vs 46.7%) but no obvious tendency was observed in PPPASI-90 responders at W60. The proportion of smokers was relatively lower in PPPASI-75/90 sustained responders than non-sustained responders at W84.
3.3 Safety
An overall summary of TEAE through W84 is presented in Table 8. No deaths were reported through W84. The proportions of patients with ≥1 TEAE were 88.9% (48/54), 100.0% (52/52), 92.0% (23/25), and 84.6% (22/26) in the guselkumab 100 mg, guselkumab 200 mg, placebo→guselkumab 100 mg, and placebo→guselkumab 200 mg groups, respectively. In the guselkumab combined group, the majority of TEAE were mild (61.8%) or moderate (26.8%) and nasopharyngitis was the most common TEAE (48.4% [76/157] of patients). Serious TEAE were infrequent (7.6% [12/157] of patients); 5.6%, 9.6%, 12.0%, and 3.8% of patients in the guselkumab 100 mg, guselkumab 200 mg, placebo→guselkumab 100 mg, and placebo→guselkumab 200 mg groups, respectively.
| Variables | Placebo→guselkumab 100 mg (N = 25) | Placebo→guselkumab 200 mg (N = 26) | Guselkumab 100 mg (N = 54) | Guselkumab 200 mg (N = 52) | Guselkumab combined (N = 157) |
|---|---|---|---|---|---|
| n (%)a | |||||
| Average duration of follow-up (weeks) | 62.0 | 65.8 | 76.5 | 76.0 | 72.3 |
| ≥1 TEAE | 23 (92.0%) | 22 (84.6%) | 48 (88.9%) | 52 (100.0%) | 145 (92.4%) |
| Serious TEAE | 3 (12.0%) | 1 (3.8%) | 3 (5.6%) | 5 (9.6%) | 12 (7.6%) |
| TEAE that were reasonably related to study treatmentb | 7 (28.0%) | 9 (34.6%) | 19 (35.2%) | 22 (42.3%) | 57 (36.3%) |
| TEAE leading to permanent discontinuation of study treatment administration | 1 (4.0%) | 1 (3.8%) | 7 (13.0%) | 6 (11.5%) | 15 (9.6%) |
| TEAE leading to death | 0 | 0 | 0 | 0 | 0 |
| TEAE of severe intensity | 2 (8.0%) | 1 (3.8%) | 1 (1.9%) | 2 (3.8%) | 6 (3.8%) |
| >10% TEAE in any of the treatment arms | |||||
| Nasopharyngitis | 17 (68.0%) | 14 (53.8%) | 20 (37.0%) | 25 (48.1%) | 76 (48.4%) |
| Oral herpes | 0 | 3 (11.5%) | 0 | 3 (5.8%) | 6 (3.8%) |
| Eczema | 3 (12.0%) | 2 (7.7%) | 6 (11.1%) | 8 (15.4%) | 19 (12.1%) |
| Urticaria | 2 (8.0%) | 1 (3.8%) | 2 (3.7%) | 7 (13.5%) | 12 (7.6%) |
| Dental caries | 2 (8.0%) | 3 (11.5%) | 4 (7.4%) | 4 (7.7%) | 13 (8.3%) |
| Injection site erythema | 3 (12.0%) | 5 (19.2%) | 4 (7.4%) | 10 (19.2%) | 22 (14.0%) |
| Injection site pruritus | 2 (8.0%) | 3 (11.5%) | 1 (1.9%) | 5 (9.6%) | 11 (7.0%) |
| Injection site swelling | 0 | 3 (11.5%) | 2 (3.7%) | 3 (5.8%) | 8 (5.1%) |
| Hyperlipidaemia | 3 (12.0%) | 0 | 2 (3.7%) | 1 (1.9%) | 6 (3.8%) |
| TEAE of special interest | |||||
| Injection-site reaction | 4 (16.0%) | 6 (23.1%) | 5 (9.3%) | 13 (25.0%) | 28 (17.8%) |
| Infections | 18 (72.0%) | 17 (65.4%) | 35 (64.8%) | 35 (67.3%) | 105 (66.9%) |
| Infections that required oral or parenteral antibiotic treatment | 7 (28.0%) | 8 (30.8%) | 19 (35.2%) | 20 (38.5%) | 54 (34.4%) |
| Serious infections | 0 | 0 | 0 | 2 (3.8%) | 2 (1.3%) |
Note
- (i) From W72 to W84, only the TEAE considered related to the study treatment were collected. (ii) Patients in the placebo group were re-randomized to receive guselkumab 100 or 200 mg starting at W16. For patients who received placebo at W0, events that occurred from W16 to W84 were included in placebo→guselkumab 100 mg or placebo→guselkumab 200 mg groups.
- Abbreviations: n, number of patients; N, total number of patients evaluated in each group; TEAE, treatment-emergent adverse event; W, week.
- a Incidence is based on the number of patients experiencing at least 1 adverse event, not the number of events.
- b Reasonably related to the study treatment is defined as the relationship in “very likely”, “probably”, and “possible”.
Through W72 (12 weeks or ~5 half-lives after the last dose of study treatment), the proportions of patients with TEAE that were considered reasonably related to study treatment were 35.2%, 42.3%, 28.0%, and 34.6% in the guselkumab 100 mg, guselkumab 200 mg, placebo→guselkumab 100 mg, and placebo→guselkumab 200 mg groups, respectively. The TEAE that led to study treatment discontinuation were reported in 13.0%, 11.5%, 4.0%, and 3.8% of patients in the guselkumab 100 mg, guselkumab 200 mg, placebo→guselkumab 100 mg, and placebo→guselkumab 200 mg groups, respectively. No TEAE were reasonably related to study treatment from W72 to W84. The incidence of treatment-emergent infections was similar across all treatment groups. Serious treatment-emergent infections were reported only in 3.8% (2/52) of patients receiving guselkumab 200 mg. The most frequently reported injection-site reactions were injection-site erythema and injection-site pruritus, with higher rates in the placebo→guselkumab 200 mg (23.1%) and guselkumab 200 mg groups (25%) compared to placebo→guselkumab 100 mg (16%) and guselkumab 100 mg groups (9.3%). No patients had anaphylactic reactions, serum sickness-like reactions, MACE, or active tuberculosis. One serious TEAE of gastric cancer was reported in the placebo→guselkumab 100 mg group.
Changes in vital signs and ECG were not clinically relevant. No laboratory abnormalities were reported as serious TEAE or led to study treatment discontinuation.
4 DISCUSSION
There is a lack of evidence beyond 1-year efficacy and safety of guselkumab in PPP. The present study demonstrated the efficacy and safety of guselkumab for the treatment of PPP through 84 weeks. Efficacy was observed as early as W16,1 continued through W60 (treatment phase), and sustained until W84 (observational phase).
The mean percent improvement from baseline in the PPPASI and PPSI total scores at W84 was ~79% and ~66%, respectively, in the guselkumab combined group. The mean percent improvements from baseline in the PPPASI total scores were comparable between guselkumab 100 and 200 mg groups, while numerically higher improvement was seen for PPSI total score in the guselkumab 200 mg group compared to guselkumab 100 mg group at W60, W72, and W84. This variation may be attributed to different ways of score calculation between PPPASI and PPSI responses; the PPPASI evaluation includes the “area” of PPP that is not included in the PPSI evaluation.30 No such trend was noted for the PPSI total score in the placebo-crossover groups.
Patients in the guselkumab 200 mg group did not show a statistically significant difference in the PPPASI-50 response (p = 0.78) at W16 compared with the placebo group, while a significantly higher proportion of patients in the guselkumab 100 mg group was PPPASI-50 responders (p = 0.02).1 After W24, higher improvements were seen with guselkumab 200 mg, and a comparable PPPASI-50 response rate was observed with guselkumab 100 mg at W60 and W84, which offset the relatively lower efficacy at earlier assessment points. A similar trend was noted for PPPASI-75/90/100 response rates at W60. The PGA scores continued to improve through W60 (treatment phase) and W60–W84 (observation phase) in the guselkumab treatment groups.
All PRO improved through W84, consistent with the improvement in the PPPASI, PPSI, and PGA scores. The post hoc analysis showed an improvement in the SF-36 (PCS and MCS) and EQ-5D (index and VAS) scores in PPPASI-75/90 responders at W60 and sustained responders at W84 compared with the non-responders and non‑sustained responders, respectively, indicating better health status. At W84, an improvement in the HRQOL (assessed by DLQI) was observed across all treatment groups. The post hoc analysis indicated that PPPASI-75 responders at W60 and sustained responders at W84 achieved greater HRQOL improvements (assessed by DLQI) compared with the non-responders and non-sustained responders, respectively. Since DLQI clinically correlates with PPPASI, PPPASI sustained responders tended to obtain better DLQI response.
Based on post hoc analysis, hyperlipidemia, hypertension, and PAO were the commonly reported comorbidities, consistent with the previous studies;7, 40 however, these diseases did not impact the efficacy response through W84. The PPPASI-75/90 responders at W60 tended to have lower bodyweight and BMI; lower proportions of patients received prior phototherapy and non-biologic systemic therapy compared with the non-responders. The statistical tests were performed to measure the intensity of association between PPPASI-75/90 responders and non-responders at W60 or PPPASI-75/90 sustained responders and non-sustained responders at W84 and each patient characteristics. The weight/BMI and PAO (yes/no) emerged as factors with statistical significance, in line with what was observed in the results from the double-blind placebo-controlled period (through W16). However, due to the nature of post hoc analyses and the limited number of patients, statistical values were considered nominal and thus not shown in the manuscript. Guselkumab demonstrated numerical trends for sustainability of efficacy in non-smokers and patients with no prior non-biologic systemics.
The safety profile observed up to W84 was consistent with the previous guselkumab clinical programs for PPP as well as other diseases including moderate-to-severe plaque psoriasis and PsA.23-25, 41 Overall, guselkumab (100 and 200 mg) was well tolerated with no apparent differences in safety profiles between guselkumab doses through W84.
4.1 Limitations
As patients in the placebo group were re-randomized to receive guselkumab at W16, placebo-controlled comparisons after W16 were not made. After W60, there was no restriction on the use of concomitant medications, which makes it difficult to determine the true response rates to guselkumab. The post hoc analysis included a relatively small sample size.
4.2 Conclusions
Guselkumab was efficacious for the treatment of PPP through W60, with the sustenance of efficacy until W84. Guselkumab also led to an improvement in HRQOL. Overall, a favorable benefit-to-risk profile for guselkumab in PPP was demonstrated through W84 in Japanese patients.
ACKNOWLEDGMENTS
This study was funded by Janssen Pharmaceutical, Tokyo, Japan. Takayuki Kimura (an ex-employee of Janssen Pharmaceutical) provided medical advice for this manuscript. The writing support for manuscript, was provided by Vivek Rane, Vasudha Chachra, and Kiran Chawla from Kinapse (a Syneos Health® Company).
CONFLICT OF INTEREST
Hitomi Morishima and Richuan Zheng are employees of Janssen Pharmaceutical K.K., Tokyo, Japan. Yukari Okubo and Tadashi Terui have received research support and performed consulting work for Janssen Pharmaceutical K.K. Yukari Okubo has received scholarship grants from Eisai, Torii, and Maruho. Yukari Okubo has received a clinical commissioned or joint research grant from Shiseido. Yukari Okubo has received honoraria for lectures from AbbVie, Eli Lilly Japan, Janssen Pharmaceutical, Kyowa Kirin, Maruho, Novartis Pharma, Taiho, and Celgene. Tadashi Terui has received honoraria for speaking and consultancy from AbbVie, Eisai, Novartis, Janssen Pharmaceutical, Maruho, Taiho, Eli Lilly, Bristol-Myers Squibb, and Mitsubishi Tanabe Pharma.




