Cannabinoid Hyperemesis Syndrome and Hypophosphatemia in Adolescents
C.P. Alexander and P. Jhaveri are members of NASPGHAN. The remaining authors report no conflicts of interest.
Guarantor of the article: Chandran P. Alexander, MBBS, MD.
Abstract
We report 3 adolescents with cannabis hyperemesis syndrome and recurrent hypophosphatemia complicating their clinical course with potential for significant consequences. They serve as reminders for providers to consider the diagnosis of cannabis hyperemesis syndrome and to monitor serum electrolytes closely in the setting of adolescent hyperemesis.
What Is Known
- Cannabis is the most commonly used illicit drug in the US adolescent population.
- Cannabinoid hyperemesis syndrome (CHS) is increasingly recognized in adolescents who chronically use Cannabis.
- Severe hypophosphatemia has been described in a few adults with CHS.
What Is New
- We report the first set of adolescents who had CHS and severe hypophosphatemia who required parenteral phosphate replenishments.
- Common etiologies of hypophosphatemia were ruled out, suggesting an unknown mechanism of transcellular phosphate shift.
- Pediatric and adolescent care providers need to be aware of this potentially serious though transient side effect of CHS.
INTRODUCTION
Cannabis continues to be the most commonly used illicit drug by adolescents (1), with rates of cannabis use disorder increasing in all age groups from 2018 to 2019 (2). One manifestation of chronic cannabis use is cannabis hyperemesis syndrome (CHS), characterized by recurrent episodes of intractable vomiting, nausea, and abdominal pain. The diagnosis of CHS is often missed at initial acute healthcare visits and may incur unnecessary diagnostic investigations (3). Once identified, symptoms can be managed through supportive therapy and anti-emetics, but prolonged cannabis abstinence is required for full resolution (4).
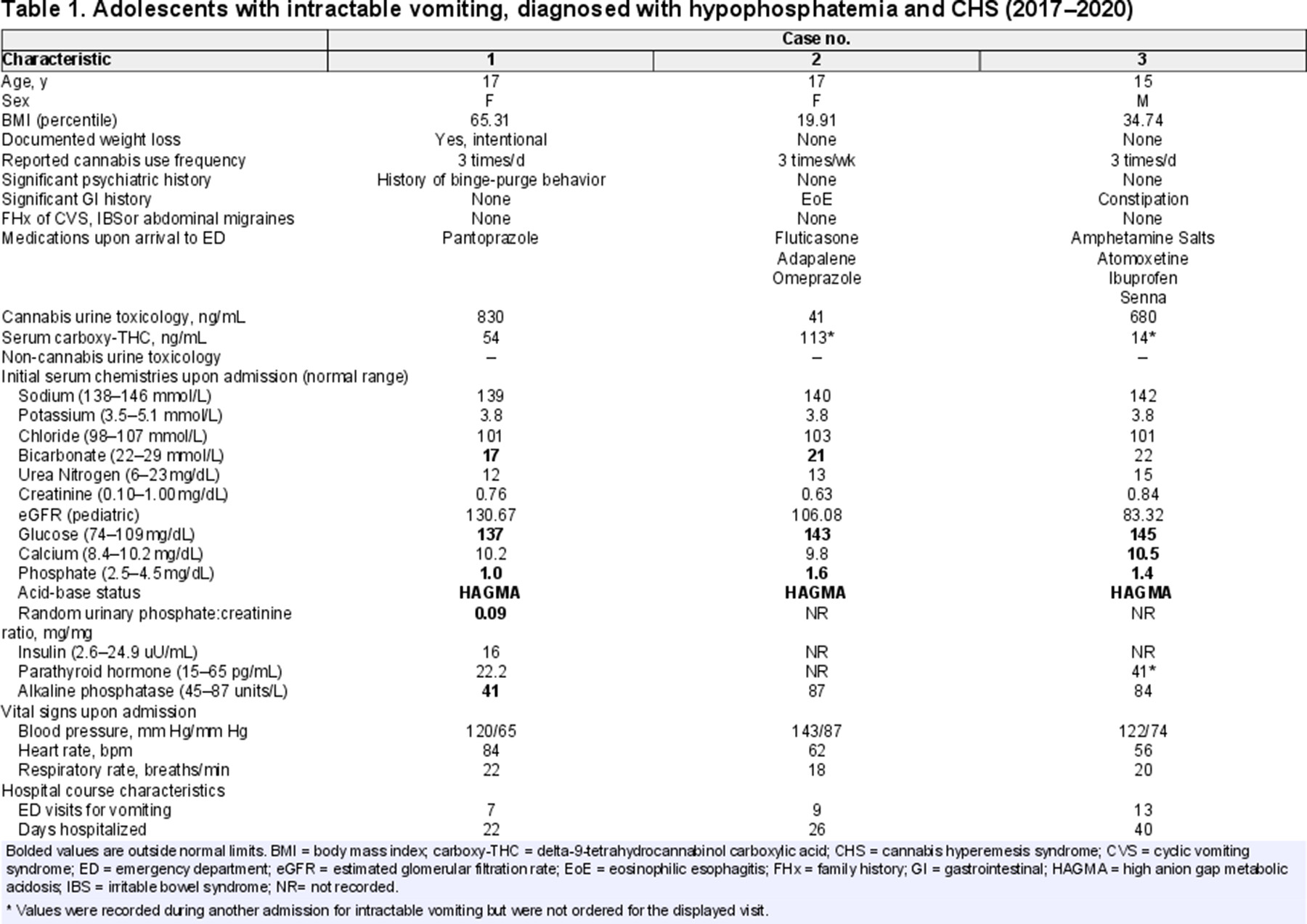
Although a single-center 10-year case series(5) provided insight into adolescent CHS, electrolyte comorbidities were not assessed. One underrecognized metabolic consequence of CHS is hypophosphatemia, with few cases reported in adults (6). In this report, we present 3 adolescents with CHS who had moderate-to-severe hypophosphatemia on arrival to our emergency department between 2017 and 2020 and required parenteral phosphate replacement. This is the first case series documenting not only low serum phosphate levels across time and visits (Fig. 1), but also confirming marijuana use through quantitative urine and serum toxicology while excluding known common causes of hypophosphatemia (Table 1).
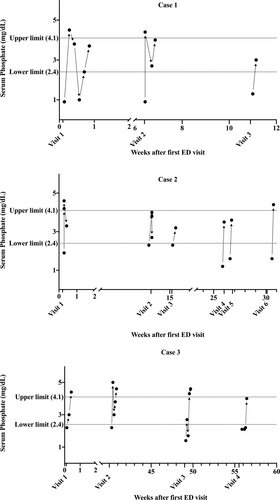
Serum phosphate levels over time for cases 1–3 during each admission for intractable vomiting after the patient's first contact with our ED. ED = emergency department.
CASE REPORTS
Case 1
A 17-year-old female patient with at least 3 prior emergency department visits at another hospital presented with nausea, epigastric pain, and intractable vomiting. She acknowledged smoking 3 blunts of cannabis per day since age 14 to self-treat nausea and anxiety. She had history of binge-purging behavior and intentional weight loss and was diagnosed in the previous hospital with CHS. Trials of topical capsaicin cream, hyoscyamine, dicyclomine, morphine, promethazine, famotidine, and divalent cation antacids did not help. Warm showers, haloperidol, metoclopramide, lorazepam, and intravenous fluid replacement therapy relieved her symptoms. Further studies performed included an upper gastrointestinal contrast study with small bowel follow through, upper endoscopy, and head computerized tomography, which all returned normal. Additional serum chemistries obtained upon her second visit at our hospital revealed critically low serum phosphate (1.0 mg/dL), associated with a low spot urine phosphate:creatinine ratio (0.09, below the fifth percentile for adolescents 14–17 years old (7)). She was administered immediate intravenous sodium phosphate repletion without a 24-hour urine collection. Serum phosphate corrected to 4.5 mg/dL (Fig. 1). It was less likely that urinary loss contributed to the hypophosphatemia because concurrent urine phosphate:creatinine ratio was low. After stabilization, she was discharged with encouragement and resources for cannabis cessation but returned within 2 months with hyperemesis and again had critically low serum phosphate (0.9 mg/dL) that was corrected parenterally. She returned 1 month later with hyperemesis and low-normal serum phosphate. Despite counseling regarding cannabis smoking cessation, patient continued at-home daily use, and was lost to follow up.
Case 2
A 16-year-old female patient with eosinophilic esophagitis (EoE) presented with severe nausea and vomiting unresponsive to oral ondansetron. Due to her history, endoscopies to assess EoE flare-ups, abdominal radiographs, and a brain magnetic resonance imaging were performed, all of which were normal. The patient had hypophosphatemia at 6 of 10 arrivals (ranging from 1.2 to 2.3 mg/dL). These resolved with repletion using intravenous sodium phosphate and potassium phosphate. In some of these encounters, she needed repeated replenishments during a single admission over 2 to 3 days (Fig. 1). She reported smoking up to 3 joints per week and received cannabis cessation counseling to resolve symptoms. During 40% of her visits for vomiting, she denied recent marijuana use; however, urine drug screens consistently showed the presence of cannabinoids. This was confirmed using serum delta-9-tetrahydrocannabinol (THC) testing at one of the visits (Table 1). After prolonged cessation of cannabis use, her vomiting resolved. She continued outpatient follow-up care for EoE, remained asymptomatic, and hence phosphate levels were not measured. Her care was transitioned to adult service after her 18th birthday.
Case 3
A 15-year-old male patient with a history of significant constipation presented with nausea, intractable non-bilious, non-bloody vomiting, and abdominal pain. Hot showers, haloperidol, and prochlorperazine helped relieve the vomiting and nausea, but ondansetron and metoclopramide did not. He reported smoking 3 bowls per day since age 14. During his hospitalizations, he underwent a barium enema, several abdominal computerized tomography scans, and a brain magnetic resonance imaging, all of which were normal. He had a low serum phosphate level (1.4 mg/dL) that needed phosphate repletion before discharge (Fig. 1). Cannabis cessation was encouraged at every admission, and patient unsuccessfully attempted structured recovery programs twice.
DISCUSSION
Little is known about adolescent CHS and the mechanism for hyperemesis. Potential physiologic explanations include genetic vulnerability, dysregulation of endocannabinoid neural signaling, transient receptor potential cation channel subfamily V1 dysregulation, and acute effects of high potency cannabinoids. Additionally, a gastrointestinal perspective of the syndrome suggests chronic cannabinoid use may lead to gastroparesis and emesis, overpowering the anti-emetic effects of cannabinoids (8). The link between emesis patterns and cannabis consumption has not been elucidated and requires further investigation.
Here, we highlight hypophosphatemia as a potential risk in adolescents with CHS. Common etiologies of hypophosphatemia (below 2.4 mg/dL) include decreased intake in malnutrition, malabsorption from phosphate binding medications, increased urinary excretion or transcellular shifts from refeeding syndrome or respiratory alkalosis. We did not observe these in our patients (Table 1). Furthermore, while patients were not ingesting cannabis during their inpatient stay, vomiting persisted, and serum phosphate levels dropped spontaneously during their clinical course. Two patients required multiple administrations of parenteral phosphate during a single hospitalization (Fig. 1). For an already unpredictable recovery course after cannabis cessation, physicians must be diligent in correcting serum electrolyte abnormalities to prevent potential complications of hypophosphatemia. Until more is known about hypophosphatemia in the context of CHS, clinicians must rely on laboratory monitoring of serum phosphate levels and urine toxicology.
Heavy cannabis users may have persistently positive urine toxicology for metabolites of THC, the predominant psychoactive cannabinoid of Cannabis, for 25–95 days after cessation. This highly variable time frame is likely due to differences in physiology (9), which potentially explains the discrepancies between stated cannabis use and observed toxicology results in case 2. While drug screens provide a method of discerning whether a user has recently ingested THC, there are no reliable quantitative indices of either use latency or psychoactive effects. The duration or frequency of cannabis use necessary to cause adolescent CHS is also unknown.
Currently, little evidence supports routine cannabis toxicology screening when adolescents present with hyperemesis. Physicians may consider ordering urine analysis for cannabinoids and serum electrolytes including phosphate at the initial point of care to optimize clinical management and achieve desirable patient outcomes.
ACKNOWLEDGMENTS
R.N. contributed to the conception and design, analysis, interpretation of data; drafting; final approval. K.H. contributed to the acquisition, interpretation of data; drafting; final approval. T.S. contributed to the acquisition, interpretation of data; drafting; final approval. P.J. contributed to the acquisition, interpretation of data; drafting; final approval. K.E.V. contributed to the conception and design, interpretation of data; drafting; final approval. C.A. contributed to the conception and design; acquisition, analysis, interpretation of data; drafting; final approval.
Informed consent was obtained from parents and assent from patients when available for publication of the details of these cases. In cases where informed consent was not available, all attempts were exhausted in trying to contact the parents/guardians for the purpose of attaining informed consent from the parents or guardians to publish this report.