Towards a Standardized Classification of the Hepatobiliary Manifestations in Cystic Fibrosis (CFHBI): A Joint ESPGHAN/NASPGHAN Position Paper
CME module may be found at https://learnonline.naspghan.org/jpgn2
Disclaimer: See complete disclaimers on page 165.
Dr Bodewes served on medical advisory board for Vertex Pharmaceuticals and has research funding from the European Society for Paediatric Gastroenterology Hepatology and Nutrition and the Dutch CF Foundation. Dr Narkewicz serves as a consultant for Vertex and has research funding from Gilead and AbbVie and the Cystic Fibrosis Foundation. Dr Scheers has research funding from Fondation Contre le Cancer, Salus Sanguinis, and Fond National pour la Recherche Scientifique. Dr Debray serves as a consultant for Alexion Pharmaceuticals and Orphalan, and has research funding from Vaincre la Mucoviscidose. Dr Verkade has served as consultant for Ausnutria, Albireo, Mirum, Friesland Campina, Vivet, Intercept, GMP-Orphan, and Shire, and his University Medical Center received compensation from Vertex for a tutorial contribution. Dr Freeman receives research funding from the Cystic Fibrosis Foundation, National Institutes of Health, AbbVie, and Travere Therapeutics. He has served as a consultant for AbbVie and Takeda. The remaining author reports no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal's Web site (www.jpgn.org).
Abstract
The broad spectrum of hepatobiliary involvement in cystic fibrosis (CF) has been commonly referred to as cystic fibrosis liver disease (CFLD). However, differences in the definitions of CFLD have led to variations in reported prevalence, incidence rates, and standardized recommendations for diagnosis and therapies.
Harmonizing the description of the spectrum of hepatobiliary involvement in all people with CF (pwCF) is deemed essential for providing a reliable account of the natural history, which in turn supports the development of meaningful clinical outcomes in patient care and research.
Recognizing this necessity, The European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) commissioned and tasked a committee to develop and propose a systematic classification of the CF hepatobiliary manifestations to increase uniformity, accuracy, and comparability for clinical, registry, and research purposes. This report describes the committee's combined expert position statement on hepatobiliary involvement in CF, which has been endorsed by NASPGHAN and ESPGHAN.
We recommend using CFHBI (Cystic Fibrosis Hepato-Biliary Involvement) as the updated term to describe and classify all hepatobiliary manifestations in all pwCF. CFHBI encompasses the current extensive spectrum of phenotypical, clinical, or diagnostic expressions of liver involvement observed in pwCF. We present a schematic categorization of CFHBI, which may also be used to track and classify the changes and development of CFHBI in pwCF over time. The proposed classification for CFHBI is based on expert consensus and has not been validated for clinical practice and research purposes. Achieving validation should be an important aim for future research.
Highlights
What Is Known
-
Hepatobiliary involvement is common in people with cystic fibrosis (CF).
-
The manifestations of hepatobiliary involvement can significantly vary in form, extent, and severity among people with CF (pwCF).
-
The presently commonly utilized term “cystic fibrosis liver disease” (CFLD) is not comprehensive enough to accurately encompass all the liver and biliary involvement observed in pwCF.
What Is New
-
We recommend using CFHBI (Cystic Fibrosis Hepato-Biliary Involvement) as the up-to-date all-encompassing term for all liver and biliary presentations in all pwCF.
-
CFHBI encompasses the full range of phenotypical, clinical, and biochemical presentations of liver and biliary involvement in pwCF.
-
We provide a comprehensive, structured categorization for CFHBI that may also be used to track and document the progression of CFHBI in pwCF over time.
-
We recommend using this new classification system for CFHBI in pwCF to enhance uniformity, accuracy, and comparability in clinical, registry, and research contexts.
During an expert meeting held in January 2016 at a European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) monothematic conference on cystic fibrosis-related liver disease (CFLD), the need for a universal consensus on the definition of CFLD to clarify disease stages and identify relevant biomarkers for assessing severity was emphasized. This initiative aimed to achieve a transatlantic agreement between major professional associations, ESPGHAN and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN), on a consensus definition and classification system critical for addressing epidemiology and the natural course of the disease. A deeper understanding of the pathophysiology and prognostic factors for the long-term evolution of CFLD is fundamental to move forward and has a strong bearing on identifying potential treatments (1). A joint expert committee (that includes all authors of this manuscript) composed of members of Hepatology committee and the Pancreas-CF Special interest group of ESPGHAN and NASPGHAN members with a special interest in CFLD was convened to evaluate the current classification and nomenclature for CF hepatobiliary involvement (CFHBI) for clinical, registry, and research use, and if appropriate, propose a revised classification system. Recommendations for this classification as a combined expert statement, was subsequently accepted according to established approval procedures as a combined societal position paper by both NASPGHAN and ESPGHAN.
The broad spectrum of hepatobiliary involvement in people with CF (pwCF) has been commonly referred to as CFLD. During follow-up, hepatobiliary involvement may occur in 80%–90% of pwCF, of which approximately 10% are severe and affect outcomes (2,3). However, for some signs currently included in the term CFLD, it is unknown if they represent actual liver disease or merely epiphenomena of limited clinical relevance. Moreover, the pathophysiology and the correlation between different hepatobiliary manifestations in pwCF and their clinical consequences are not fully known. Due to a lack of a uniform definition of CFLD, there are discrepancies in reported prevalence and incidence rates and variations in the suggested diagnostic and treatment approaches for the various types of liver involvement in pwCF.
THE LIVER IN THE ERA OF CFTR MODULATOR THERAPIES
Currently, proven, effective therapy for the liver involvement in pwCF is lacking. Ursodeoxycholic acid (UDCA) has been used in the absence of randomized trials to treat CFLD and to attempt to prevent the development of advanced liver disease in pwCF. However, recent large nonrandomized cohort studies suggest that it is not effective in the prevention of the development of advanced liver disease in pwCF (4-6). In recent years, various CF transmembrane regulator (CFTR) directed modulator therapies have become available for pwCF. It is reasonable to assume that these therapies may impact the liver and liver pathology in pwCF, with potential beneficial effects or unintended hepatic side effects. Therefore, it is crucial that clinical studies evaluate the safety and efficacy of CFTR modulator therapies in relation to hepatobiliary involvement. Similar concerns may arise with other liver-specific therapies for pwCF. To systematically evaluate the impact of these therapies on CFHBI, a universally accepted classification of liver involvement in pwCF is necessary.
METHODS
Regarding the composition of the committee, representatives were suggested by NASPGHAN Hepatology and Pancreas committees and approved, and ESPGHAN appointed members from the Hepatology committee and the special interest group on CF and Pancreas. All members of the committee took part in the committee meetings regarding the basic concept and outline of the current position paper. Each member of the committee was the primary reviewer for one subheading of the manuscript based on their individual expertise. All committee members reviewed and approved the manuscript.
The consensus process involved several stages, including initial discussions through email correspondence, teleconferences, and a joint meeting where the purpose, main characteristics, and structure of the classification were discussed and recorded. Each committee member was assigned to a specific aspect of the classification system to review the relevant literature and draft the relevant section, ensuring equal participation and diverse perspectives. One author (FAJAB) compiled the drafts into a cohesive document, which the entire committee then reviewed and provided feedback on through email correspondence and teleconferences. Revisions were made based on feedback, and the process continued until consensus was reached among all committee members. The draft manuscript was circulated to ESPGHAN members, including the Hepatology committee and the ESPGHAN special interest group on CF and Pancreas for comments and review.
The committee initially reviewed and discussed the current published definitions of CFLD (Table 1). The Colombo (7), Debray (8), and Koh (9) criteria focus on clinical signs for disease classification, while the Flass (10) criteria are a phenotypic classification. The classification of Colombo and Debray targeted identification of pwCF for potential therapeutic interventions, in particular the use of UDCA; the Flass and Koh classifications were primarily intended for evaluation of natural history and use in research.
| Colombo | 2002 | CFLD considered if at least 2 of the following conditions present on at least 2 consecutive examinations spanning a 1-year period:
Ultrasonographic pattern of steatosis and liver biopsy were not included in the definition |
| Debray | 2011 | Diagnosis of CFLD should be considered if 2 or more categories are present:
|
| Flass | 2013 | Classification of CFLD
|
| Koh | 2017 | Diagnosis of CFLD should be considered if 2 or more categories are present:
|
- ALP = alkaline phosphatase; APRI = AST to Platelet Ratio Index; CFLD = cystic fibrosis liver disease; CT = computed tomography, ERCP, endoscopic retrograde cholangiopancreatography; MRI = magnetic resonance imaging.
In the following consensus process, literature was reviewed considering the present relevant hepatic manifestations and signs in pwCF and their potential significance for the revised classification. The joint committee deliberated on this information and proposed an alternative classification system that encompasses key physical examination findings, commonly available laboratory values, liver imaging findings, liver stiffness measurements (LSM), and liver histology associated with clinically significant hepatobiliary outcomes. The summary boxes at the end of each paragraph represent the committee's current understanding of the topics discussed, based on existing knowledge, and are intended to support future research and help develop improved recommendations for managing CFHBI.
CFHBI IN PWCF
The joint committee recommends the use of “Cystic Fibrosis Hepatobiliary Involvement” (CFHBI) to refer to all liver and biliary tract-related signs, clinical and/or biochemical diagnostic findings observed in pwCF.
-
Understanding the progression of CFHBI over time.
-
Establishing links between various manifestations of CFHBI, such as whether steatosis can lead to severe liver disease in certain cases.
-
Assessing and evaluating the potential impact of treatments for pwCF on CFHBI.
Table 2 provides a detailed description of the CFHBI classification based on different elements, including biochemistry, imaging, histology, LSM, and clinical signs.
| Elevation of liver enzymes (> 1.5× ULN†) | |||
| E0 | No elevation of liver enzymes | Either AST/ALT/GGT | |
| E1 | Transient elevation of liver enzymes | ||
| E2 | Persistent elevation of liver enzymes >6 months | ||
| Imaging of the liver | Either ultrasound/MRI | ||
| I0 | No imaging abnormalities | ||
| I1 | Heterogeneous increased signal | ||
| I2 | Nodular imaging abnormalities | ||
| I3 | Homogeneous increased signal | ||
| In | No imaging available | ||
| Histopathology of the liver | |||
| H0 | No histopathological abnormalities | ||
| H1 | a | Fibrosis F1–F2 | METAVIR classification |
| b | Fibrosis F3–F4 | ||
| H2 | Obliterative portal venopathy | ||
| H3 | Steatosis | ||
| H4 | Cholestatic histopathology | ||
| Hn | No histology available | ||
| Stiffness of the liver | Various modalities of elastography | ||
| S0 | Normal liver stiffness | ||
| S1 | Increased liver stiffness | ||
| Sn | Liver stiffness was not measured | ||
| Portal hypertension‡ | |||
| PO | No portal hypertension | ||
| P1 | Cirrhotic portal hypertension | - Histology consistent with cirrhosis (F4) AND/OR - Severe increase of liver stiffness (see Table 3A, Supplemental Digital Content 1, http://links.lww.com/MPG/D344 for cutoff values) Supportive cirrhosis: macronodular liver including an irregular edge, inhomogeneous parenchyma on imaging |
|
| P2 | Non-cirrhotic portal hypertension | - Histology not consistent with fibrosis or cirrhosis Supportive of non-cirrhotic: normal or mildly elevated HVPG and/or no macronodular appearance of the liver on imaging |
|
| Biliary manifestations | |||
| B0 | No biliary involvement | ||
| B1 | Cholelithiasis and hepatolithiasis | ||
| B2 | Biliary strictures | MRCP or ERCP | |
| Malignancies of the liver and biliary tract | |||
| M0 | No malignancies | ||
| M1 | Hepatocellular carcinoma | ||
| M2 | Cholangiocarcinoma | ||
- ALT = alanine aminotransferase; AST = aspartate aminotransferase; ERCP = endoscopic retrograde cholangiopancreatography; GGT = gamma-glutamyl transpeptidase; HVPG = hepatic venous pressure gradient; MRCP = magnetic resonance cholangiopancreatography; MRI = magnetic resonance imaging; ULN = upper limit of normal.
- * Ranking in numbering and lettering are used for classification and do not represent hierarchy in severity.
- † ULN cutoff value of 1.5 times the normal range to avoid misclassifying, particularly those with borderline results.
- ‡ Portal hypertension is defined by any 1 of the following criteria: 1. Persistent splenomegaly either by physical exam or by imaging; 2. Persistent hypersplenism (platelet count <150 × 109/L); 3. Esophageal or gastric varices or portal hypertensive gastropathy; 4. Hepatic venous pressure gradient >10 mm Hg.
HEPATOBILIARY INVOLVEMENT IN PWCF
Elevation of Liver Enzymes (E)
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transpeptidase (GGT) are frequently assessed as part of standard follow-up. For pwCF, there has been an ongoing debate about whether elevated liver enzyme levels are useful biomarkers or predictors of the structural liver involvement in pwCF. The finding of elevated liver enzymes in pwCF must be evaluated considering factors such as recurrent respiratory infections, nutritional issues (including malnutrition and obesity), and potential drug-induced liver injury.
AST and ALT
Elevated transaminases are a frequent finding in pwCF (2). A study conducted on children diagnosed with CF through newborn screening revealed that a persistent elevation of AST or GGT >1.5 × ULN (upper limit of normal) before the age of 5 was linked to a 6-fold increase in the risk of identifying the clinically significant liver disease, defined as the presence of cirrhosis, portal hypertension (PHT), or stage F3/4 fibrosis on liver biopsy (2). Patriquin et al (11) also reported a correlation between elevated AST levels and the appearance of signs of cirrhosis on liver ultrasound (US). Ling et al (12) conducted a recent study on 244 children with CF, which reported higher mean ALT/AST levels in children and adolescents having liver US abnormalities compared to those without such imaging abnormalities. However, the transaminase levels were mostly within the normal range, and there was a significant overlap in those with or without imaging abnormalities (12). Cipolli et al reported in their study cohort that elevated ALT, GGT, or alkaline phosphatase (ALP) of 2 or more occasions before age 6.5 were linked to a higher risk of PHT. Elevated ALT had a hazard ratio of 2.7 but only had a sensitivity of ~20% and positive predictive value (PPV) of 10%–20% (13). Overall, there is insufficient evidence to suggest that changes in ALT and/or AST levels over time can reliably predict the development of structural hepatic abnormalities in pwCF. However, when transaminases remain persistently elevated in pwCF, it is necessary to consider a broader range of differential diagnoses.
GGT
GGT is expressed in high concentration in cholangiocytes and an increase in serum GGT concentration may indicate bile duct or cholangiocyte damage, such as in the case of bile duct obstruction or other cholangiopathies. In children with CF, sustained elevation of GGT, defined as values above 30 IU/L, have been associated with the occurrence or presence of structural nodular liver transformation and cirrhosis (2,12,14).
Gamma-glutamyl transpeptidase-to-platelet ratio (GPR)

GPR is reported as a potentially valuable diagnostic tool in fibrosis liver disease (15). Calvopina et al showed that elevated levels of GPR indicate liver damage in pediatric pwCF, with good diagnostic accuracy for detecting hepatic fibrosis severity and predicting the presence of PHT. These findings suggest that GPR can aid in better identification of patients at risk for PHT and timely follow-up and treatment (16).
AST to Platelet Ratio Index (APRI) score
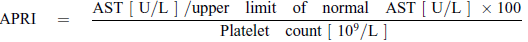
Leung et al (19) reported in a retrospective, cross-sectional, biopsy-validated study in pediatric pwCF with suspected liver disease, defined by the Debray criteria, that a 50% increase in APRI score was associated with a 2.3-fold risk of having severe fibrosis. APRI scores above a cutoff of 0.462 were a significant indicator of severe fibrosis. However, in their study APRI overestimated the fibrosis stage in almost half of the cases and underestimated fibrosis in almost 20% (19). In a recent study by Ling et al (12), it was found that the APRI was approximately 2-fold higher in individuals with a nodular liver on US than in those with a normal US pattern. It should be noted that the APRI values for advanced fibrosis in pwCF are much lower than those described for viral hepatitis.
FIB-4 Score
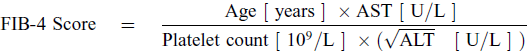
Position Statements Regarding Elevation of Liver Enzymes in pwCF
- 1.
Elevations of transaminases (AST and ALT) are a common and frequent manifestation of CFHBI in pwCF.
- 2.
Elevation of transaminases (AST and ALT) is neither sensitive nor a specific marker for variants of CFHBI in pwCF.
- 3.
Persistent elevation of transaminases (AST and ALT) should warrant a broader diagnostic approach to the underlying cause.
- 4.
GGT, GRP, APRI, and FIB-4 are useful for detecting advanced fibrotic liver disease and cirrhosis with PHT and are less discriminative for milder forms of fibrotic liver involvement.
Recommended CFHBI Classification* for Elevation of Liver Enzymes (E)
- E0
No elevation of liver enzymes
- E1
Transient elevation of liver enzymes
- E2
Persistent elevation of liver enzymes (> 6 months)
*Please refer to Table 2 for more information.
HEPATIC IMAGING (I)
Liver imaging is a noninvasive method for obtaining information about the overall appearance of the parenchyma and any structural changes in the parenchyma and/or biliary system. Over time, imaging in pwCF has transitioned from a targeted diagnostic tool used to identify cirrhotic morphology to a screening instrument employed for routine follow-up (1). Although this has led to a more comprehensive imaging depiction of CFHBI, the challenge remains in consistently categorizing imaging observations and determining the progression and prognosis of liver disease.
Ultrasonography of the Liver
- 1.
Normal
- 2.
Heterogeneous increased echogenicity
- 3.
Homogeneous increased echogenicity
- 4.
Nodularity of the liver.
The assessment of ultrasonographic findings related to liver echogenicity, such as homogeneous or heterogeneous increases, can be influenced by interobserver variability. A heterogeneous pattern on US can be a potential indicator of fibrosis, but it can also be caused by patchy steatosis (8). Additionally, a heterogeneous US pattern of the liver has been suggested to indicate patients at risk for cirrhosis (25-27), and could potentially be used as a clinical outcome measure.
Use of US has not been recommended universally for routine screening but is recommended if there is a suspicion for liver involvement based on clinical exam or biochemical indices (8,28). Sellers et al (29) demonstrated that targeted use of US based on indices of fibrosis can increase the detection of advanced liver disease in children with CF.
The finding of a nodular liver on US has good specificity for structural liver disease in adults with CF (30). However, a nodular US liver pattern may not discriminate between cirrhotic or non-cirrhotic disease of the liver (31,32). Patients with a heterogeneous pattern of the liver on US had a 5.2-fold increased incidence of cirrhosis and a 6.1-fold increased incidence of PHT compared to children with a normal pattern on liver US (25). A longitudinal study of the use of liver US to predict the development of advanced liver disease has confirmed that a heterogeneous pattern of the liver on US as determined by the consensus of 3 study radiologists is associated with a significant, 9-fold, increased risk for development of a nodular liver compared to children who had a normal US pattern at entry to the study (27).
Computed Tomography (CT) Imaging of the Liver
Abdominal CT imaging has been utilized to assess liver architecture and can differentiate fibrosis and steatosis more accurately than US (33). However, in pwCF, its use is restricted to reduce radiation exposure, and it frequently is being replaced by magnetic resonance imaging (MRI).
MRI of the Liver
Position Statements Regarding Imaging of the Liver in pwCF
- 1.
Liver US is currently the most frequently used and advised imaging method for characterizing the liver in pwCF.
- 2.
A normal US result correlates well with absence of structural CFHBI at the time of examination.
- 3.
Nodular parenchymal pattern on liver US, CT, or MRI has good specificity for the presence of structural CFHBI including cirrhosis and nodular regenerative hyperplasia (NRH).
- 4.
A liver US showing a heterogeneous increased echogenicity pattern suggests an elevated risk of advanced CFHBI.
- 5.
The clinical and prognostic importance of a homogeneous increased pattern of the liver on US suggestive for steatosis is uncertain.
- 6.
The additional significance and applicability of CT scanning of the liver in CFHBI is limited.
- 7.
Although the role of MRI in CFHBI shows promise, its precise indication and benefit in standard clinical practice and follow up of pwCF are yet to be determined.
Recommended CFHBI Classification* for Imaging of the Liver (I)
- I0
No imaging abnormalities
- I1
Heterogeneous increased signal
- I2
Nodular imaging abnormalities
- I3
Homogenous increased signal
- In
No imaging available
*Please refer to Table 2 for more information.
HEPATIC HISTOPATHOLOGY (H)
- 1.
Need for differentiation between cirrhotic and non-cirrhotic liver disease (31).
- 2.
Evaluation for other (potentially treatable) causes of liver disease such as autoimmune hepatitis, alcoholic liver disease, nonalcoholic fatty liver disease, alpha-1 antitrypsin deficiency (39,40), Wilson disease (41), drug-induced liver disease (42), and others.
- 3.
Evaluation of liver-lung transplantation candidates regarding clinical consequences and therapeutic options.
- 1.
Preponderant fibrotic and cirrhotic features including
- a.
Multilobular cirrhosis
- b.
Focal biliary cirrhosis
- c.
Portal or cholangial inflammation with fibrosis
- a.
- 2.
Obliterative portal venopathy
- 3.
Steatosis with or without inflammation or fibrosis
- 4.
(Neonatal) cholestatic features
Cholestatic histopathology is reported in neonates and infants, the other histopathologic patterns are typically identified during later childhood and adulthood.
Fibrosis and Cirrhosis (H1)
Biliary cirrhosis has long been described as a typical pathology of liver involvement in pwCF. A distinction has been made between “focal biliary cirrhosis” and “multilobular (biliary) cirrhosis,” the latter associated with cirrhotic PHT. The most-reported fibrotic hepatic histopathological findings in older children with CF are various degrees of portal fibrosis, bile duct proliferation, cholangitis, peri-cholangitis, and portal inflammatory infiltration (mainly neutrophils), as well as ductular inspissation of mucus, often with eosinophilic material (43-45). Several authors observed that ductular proliferation and inflammatory cells were more prominent in younger patients (44,46,47). To histologically assess fibrosis in CFLD, the METAVIR scoring system is most frequently utilized and reported, which was initially developed to evaluate fibrosis in viral hepatitis (16,38).
Obliterative Portal Venopathy (H2)
As an alternative to the biliary obstructive theory, other studies have reported findings to support the concept that liver injury is related to alterations in gut-liver axis (48) and/or to vasculopathy (31,32). In a study by Witters et al, 12 pwCF and PHT underwent liver biopsy or examination of the liver explant. In 7 participants, the liver samples showed no evidence of cirrhosis, but the absence of portal vein branches in over 40% of portal tracts, consistent with non-cirrhotic (presinusoidal) PHT (31). Subsequently, the same group reported a small case series with comparable outcomes (49). Hillaire et al (50) confirmed these findings by identifying non-cirrhotic (presinusoidal) PHT caused by obliterative portal venopathy with nodular regeneration in explants obtained from 8 out of 10 adult liver transplant recipients with CF. A global panel of experts has proposed discontinuing the use of the term “obliterative portal venopathy” and replacing it with “porto-sinusoidal vascular disease (PSVD)” (51).
Steatosis (H3)
Although mild to moderate macro-vesicular steatosis is a common finding in pwCF, its underlying pathophysiology is not well understood. Steatosis has been related to malnutrition (53,54) or, more specifically, to essential fatty acid deficiency (55), but other, yet undiscovered factors may be involved, as steatosis is also observed in children with good nutritional status (56). It was suggested more than 50 years ago (45), that malnutrition is likely not the only cause of hepatic steatosis in pwCF and that other factors (such as intercurrent illness, choice of supplemental nutrition, and the underlying disease itself) could play a role. Although there are reports of pwCF in whom steatosis rapidly progressed to fibrosis and cirrhosis (45), the significance of steatosis as a potential risk factor for more severe forms of CFHBI, including cirrhosis, is not known.
(Neonatal) Cholestatic Features (H4)
Position Statements Regarding Histopathology of the Liver in pwCF
- 1.
There are 4 major hepatic histopathological variants recognized in pwCF:
- (a)
Preponderant fibrotic and cirrhotic features
- -
Multilobular cirrhosis
- -
Focal biliary cirrhosis
- -
Portal or cholangial inflammation with fibrosis
- -
- (b)
Obliterative portal venopathy
- (c)
Steatosis
- (d)
(Neonatal) cholestatic features
- (a)
- 2.
Liver fibrosis in pwCF should be assessed using the METAVIR scoring system
Recommended CFHBI Classification* for Histopathology of the Liver (H)
- H0
No histopathological abnormalities
- H1a
Fibrosis F1–F2
- H1b
Fibrosis F3–F4
- H2
Obliterative portal venopathy
- H3
Steatosis
- H4
Cholestatic histopathology
- Hn
No histology available
*Please refer to Table 2 for more information.
LIVER STIFFNESS (S)
LSM are used to determine the elastic properties of the liver. An increase in liver stiffness is a noninvasive surrogate sign of a fibrotic change of the liver parenchyma. Liver stiffness is reported as a continuous quantitative variable and is used to estimate hepatic fibrosis stage or change of fibrosis over time.
Multiple modalities including vibration controlled transient elastography (VCTE), US elastography [acoustic radiation force impulse (ARFI)], or 2D shear wave elastography (SWE) and magnetic resonance elastography (MRE) are used. Due to variations in technology and algorithms used, inter-modality comparisons cannot be made on a 1:1 basis and, accordingly, cutoff values are modality specific (58). Below, we summarize the experience of the various available modalities for LSM in pwCF.
VCTE
The first study to evaluate the use of VCTE in pwCF was performed in 2009 (59). Since that time, several studies have reported on the utility of VCTE to identify liver disease and liver disease progression in pwCF. However, the collective experience with VCTE is complicated by inconsistent definitions of CFLD. This has resulted in a wide range of cutoff values (reported cutoff values summarized in Table 3, Supplemental Digital Content 1, http://links.lww.com/MPG/D344). Proposed cutoff values for advanced CFLD with PHT vary from 6.2 to 22.5 kPa with varying sensitivity and specificity (Table 4, Supplemental Digital Content 2, http://links.lww.com/MPG/D345). Although VCTE samples a far larger portion of the liver than biopsy, it still only samples ~1% of the liver and sampling error may occur given the described patchy nature of liver involvement in pwCF (10). In pwCF who have severe, restrictive lung disease, right-sided cardiac dysfunction, or hepatic inflammation, LSM are elevated and may not indicate hepatic fibrosis. Therefore, it is important to interpret these results within the context of the individual's overall medical status and disease condition.
ARFI Elastography
ARFI utilizes radiation-forced impulses to determine LSM during routine US examination. Among 9 studies of ARFI in pwCF since 2011 (60-68), a similar experience to VCTE was noted, with varying definitions for CFLD degrees of severity used and a wide range of LSM values between groups (reported cutoff values summarized in Table 3, Supplemental Digital Content 1, http://links.lww.com/MPG/D344). Only 2 studies proposed cutoff values for the presence of CFLD ranging from 1.28 to 1.45 m/s (mild differences between left and right lobes), and only 1 study proposed a cutoff for advanced CFLD at 1.3 m/s. ARFI does not appear to have a unique advantage or disadvantage compared to VCTE in the evaluation of LSM in pwCF. Given the significant overlap, ARFI does not appear to be sufficiently reliable in determining the severity of CFHBI and is unlikely to be of benefit in following progression of disease severity over time.
2D SWE
Like ARFI, SWE has the benefit of being obtained with routine B-mode US. It is suggested that SWE may be better at detecting incremental changes in liver disease among children. In a single center study of 125 children (29 controls, 41 pwCF without liver disease, and 55 with CFLD based on the Debray criteria (8)), using SWE, the median LSM was significantly higher among individuals with CFLD (8.1 kPa, 6.7–11.9) compared to pwCF without liver disease (6.2 kPa, 5.6–7.0; P < 0.001) and controls (5.3 kPa, 4.9–5.8; P < 0.001) (69). The authors proposed a cutoff of 6.85 kPa which had a sensitivity and specificity of 75% and 71%, respectively, in detecting CFLD. Further evaluation of SWE in pwCF is required to determine to what extent this methodology would be of benefit in following progression of disease severity over time.
MRE
MRE utilizes a mechanical wave from a passive driver secured to the patient's chest to indirectly measure both regional and global LSM (70). A stiffness heat map is then generated through post-processing to provide a global visual assessment of liver stiffness. Although MRI sequences are getting faster, children unable to stay still throughout the study may need sedation. Normal ranges for children without liver disease have been reported (71). The experience of MRE in pwCF is limited to single-center studies (72,73). There is insufficient data to determine if MRE has improved diagnostic performance compared to TE (transient elastography) or SWE. MRE has the advantage to globally assess liver stiffness. Still, experience is limited in pwCF and MRE may lack broader clinical utility due to cost and the potential need for sedation in young children.
There are several studies where imaging findings are combined with serum markers to better delineate severity of liver involvement in pwCF (12,27,29,63,74,75). Further evaluation is needed to determine if combining modalities to assess fibrosis improves sensitivity and specificity.
Controlled Attenuation Parameter (CAP)
Position Statements Regarding Liver Stiffness in pwCF
- 1.
Increased liver stiffness is a diagnostic indicator of CFHBI in pwCF.
- 2.
Measuring liver stiffness can be used as a surrogate quantitative measurement to grade the degree of liver fibrosis in pwCF. Multiple modalities for measuring liver stiffness are available.
- 3.
High liver stiffness values obtained by VCTE, after taking into consideration other causes of increased liver stiffness, likely indicate the presence of severe fibrotic or cirrhotic variants of CFHBI in pwCF, but the optimal cutoff values have yet to be established.
- 4.
Recent technologies such as 2D SWE and MRE may offer new strategies in detecting incremental changes in liver stiffness in pwCF but need further evaluation and validation.
- 5.
With more studies investigating CAP in pwCF, this method may in the future gain significance in diagnosing steatosis in pwCF and determining the clinical implications of steatosis in pwCF.
Recommended CFHBI Classification* for Stiffness of the Liver (S)
- S0
Normal liver stiffness
- S1
Increased liver stiffness
- Sn
Liver stiffness was not measured
*Please refer to Table 2 for more information.
PHT (P)
PHT is the most clinically significant and severe manifestation of CFHBI. The development of PHT is associated with increased morbidity and mortality (78,79). While present in only 3%–10% of pwCF, advanced liver disease with PHT is the 3rd leading cause of mortality in pwCF (3,80,81).
In pwCF, there are 2 distinct described pathophysiological etiologies for PHT, including a cirrhotic and a non-cirrhotic variant.
Cirrhotic PHT (P1)
Cirrhosis is histologically characterized by progressive biliary fibrosis. Cirrhosis is the most prevalent cause of PHT in pwCF and typically develops in children and adolescents with classic multinodular cirrhosis (56,82).
Non-cirrhotic PHT (P2)
Non-cirrhotic PHT is thought to be related to CF-related hepatic venopathy and is described more commonly later in adulthood (9,31,49,50). In non-cirrhotic PHT, histopathology shows signs of obliterative portal venopathy and NRH (32,49,50).
The treatments for PHT in pwCF focus on preventing and managing complications that result from gastrointestinal variceal bleeding and include variceal band ligation (78), portosystemic shunt procedures (83), and liver transplantation (84,85).
Assessment of the Presence of PHT
Splenomegaly
Often, the initial and most common sign of PHT in pwCF is splenomegaly (3), identified by either physical exam or imaging. The use of imaging to assess splenomegaly in pwCF is preferred because thoracic hyperinflation, which can be found in pwCF, may lead to a more readily palpable spleen. For assessment of splenomegaly with imaging modalities, there are specific cutoff values of the spleen span for adults and children. In adults, the ULN spleen span on imaging is ~14 cm (86). In children, a measured spleen span greater than 2 SD is regarded as enlarged (87,88)
Thrombocytopenia
Splenomegaly is often accompanied, or followed by, signs of hypersplenism such as a progressive decrease in the platelet count. Thrombocytopenia is strongly associated with PHT in pwCF (89). In addition, a steady decrease in platelet count over time is regarded as a relevant sign of development/progression of PHT. It is also important to consider that platelet count may vary as part of an acute phase response related to inflammatory processes and infections. Therefore, it is advised to monitor the platelet count over longer periods of time. Although clinically clearly recognizable, there is currently no standardized threshold value for either platelet count or the rate of platelet decline that can be used to diagnose PHT.
Spleen Stiffness Measurement
Another noninvasive diagnostic modality for PHT is spleen stiffness measurement. Although promising in both adults and children with PHT, spleen stiffness measurement is not widely available and still requires validation for pwCF with PHT (90,91).
Gastrointestinal Varices and Variceal Bleeding
Confirmation of PHT can be made through endoscopic diagnosis of gastrointestinal varices or documented episodes of gastrointestinal variceal bleeding. While varices and variceal bleeding are frequently observed in the esophagus and stomach cardia, they can also emerge in other regions of the gastrointestinal tract.
Direct and Indirect Portal Vein Pressure Measurements
Direct invasive measurement in the portal vein provides the most precise quantification of portal venous pressure. However, introducing a catheter directly into the portal vein is a complex procedure. A less hazardous invasive technique involves catheter placement in the hepatic vein to measure the hepatic venous pressure gradient (HVPG). In adults with cirrhosis, HVPG ≥10 mmHg is diagnostic of clinically significant PHT that has been associated with the presence of esophageal varices (92,93). In pediatric liver disease, direct or indirect measurements of portal pressure are rarely performed. No specific pediatric cutoff values have been established; therefore, the adult criteria are applied (94). It is worth noting that both obliterative portal venopathy and NRH, significant causes of PHT in pwCF, are presinusoidal lesions. Even with a normal or minimally elevated HVPG, clinically significant PHT may still be present. In pwCF, invasive portal vein pressure measurements can be considered in the evaluation for liver and/or lung transplantation to assess the severity of the PHT.
Additional Complications of Cirrhosis and PHT in pwCF
Position Statements Regarding PHT in pwCF
- 1.
Liver involvement presenting with PHT is the most clinically significant manifestation of CFHBI associated with increased morbidity and mortality.
- 2.
The committee advises classifying CFHBI with PHT as either cirrhotic or non-cirrhotic.
- 3.
There are 4 distinct signs indicative for PHT in pwCF:
- a.
Splenomegaly determined by either physical examination or preferably by imaging.
- b.
Persistent thrombocytopenia and/or decline of platelet count*.
- c.
Gastrointestinal varices and/or variceal bleeding.
- d.
Increased HVPG*.
- a.
*Uniform cutoffs have yet to be determined.
Recommended CFHBI Classification* for PHT (P)
- P0
No PHT
- P1
Cirrhotic PHT
- P2
Non cirrhotic PHT
*Please refer to Table 2 for more information.
BILIARY MANIFESTATIONS (B)
PwCF can exhibit cholangiopathy and other bile duct-related abnormalities as part of CF-related hepatobiliary involvement (CFHBI). Multiple theories have been proposed regarding the underlying cause of cholangiopathies in pwCF. In the liver, CFTR is solely present at the apical membrane of cholangiocytes that line the bile ducts and gallbladder. Consequently, local dysfunction of CFTR protein could lead to changes in bile composition, such as alterations in bile acid concentration and pH, resulting in cholangiocyte injury or precipitation of bile salts. Other theories about cholangiopathies include dysregulation of the liver-gut axis, such as variations in gut-liver signaling, intestinal inflammation, and microbial factors (48,95). As with other forms of CFHBI, it is not clear if cholangiopathies represent a distinct pathophysiological mechanism or a continuous and interrelated spectrum of the hepatobiliary disease in pwCF.
Gallstones (B1)
Gallstones are common in pwCF, presenting primarily as cholelithiasis but may also present as intrahepatic hepatolithiasis (96). Asymptomatic gallstones have been reported in approximately 5% of routine yearly US in pwCF (97). Gallstones requiring intervention were reported in only 0.2% of the CF population in the United States CF Foundation patient registry (3). However, others have reported symptomatic gallstones in up to 4% of pwCF (98).
Biliary Strictures (B2)
Recommended CFHBI Classification* for Biliary Manifestations (B)
- B0
No biliary involvement
- B1
Cholelithiasis and hepatolithiasis
- B2
Biliary strictures
*Please refer to Table 2 for more information.
Malignancies of Liver and Biliary Tract (M)
Hepatocellular Carcinoma (M1)
Hepatocellular disease and cirrhosis are established risk factors for the onset of hepatocellular carcinoma (HCC) in the general population. Although the incidence of HCC is notably low in pwCF, its likelihood of occurrence is greater in comparison to the general population (102). HCC in pwCF can develop in (young) adults (2nd–4th decade of life) with known CF-related cirrhosis (103-106). With increased survival, the cumulative risk of developing HCC in pwCF with cirrhosis may increase. Thus far, HCC has not been reported in non-cirrhotic CF liver involvement. As in other forms of liver cancer, a high alpha-fetoprotein might suggest the presence of HCC, but normal levels do not exclude it. Specific screening protocols for HCC in pwCF are limited, mainly due to the fact that US and MRI criteria used to identify HCC may be less reliable in pwCF (106).
Cholangiocarcinoma (M2)
Recommended CFHBI Classification* for Malignancies of the Liver and Biliary Tract (M)
- M0
No malignant manifestations
- M1
Hepatocellular carcinoma
- M2
Cholangiocarcinoma
*Please refer to Table 2 for more information.
SUMMARY AND RECOMMENDATIONS
Liver and biliary involvement is common in pwCF. Currently, most described clinical hepatic and biliary presentations are grouped under the term “CF liver disease (CFLD).” To improve understanding and research of CF-related hepatobiliary involvement in pwCF, we suggest replacing the term CFLD with CF hepatobiliary involvement (CFHBI).
The Classification of CFHBI is an Ongoing and Evolving Process
CFHBI presenting as an advanced liver disease with PHT represents the most critical form of CFHBI in terms of morbidity and mortality. Individuals with this manifestation are at greater risk of liver failure, requiring liver transplantation, and experiencing elevated overall mortality rates. The implications of other manifestations of CFHBI, such as those identified by liver biochemistry liver imaging, liver histology, or elevated liver stiffness, on morbidity and mortality, as well as disease progression, are more uncertain. Our current description of CFHBI mirrors the existing clinical practice and scientific understanding of liver involvement in pwCF. As novel diagnostic techniques and therapeutic alternatives emerge, revising and augmenting the current proposal may become necessary.
Role for the Classification of CFHBI Manifestations for Describing Natural History
The natural history of the various CFHBI manifestations and their mutual pathophysiological relationship is unknown. It is well recognized that the peak age of the diagnosis of cirrhosis in pwCF is around the age of 10 years (7,89). However, the preliminary stages of hepatic disease in these patients have not been clearly identified, leading to challenges in identifying patients at risk. Recent data suggest that a consensus determination of heterogeneous US patterns of the liver identifies individuals with an increased risk for severe liver involvement, including PHT. However, a slight majority of those with a heterogeneous pattern will not develop PHT (26,27). Another example is steatosis: it is a well-described radiological and histological entity in pwCF, but it is unknown if steatosis or its ultrasonographic correlation, a homogeneously echogenic liver, plays any role in the development or progression of CFHBI manifestations.
Role for Classification for the CFHBI Manifestations in the Clinic, Registries, and Clinical Research
Using a systematic classification system for CFHBI manifestations provides a role in clinical care, patient registries, and clinical research. A more detailed categorization of CFHBI allows for improved recognition and monitoring of liver disease progression in pwCF. The proposed classification can also provide uniformity in patient registries and inclusion criteria for clinical trials. This approach can improve our understanding of CFHBI manifestations and advance treatment for CF in general like CFTR modulators and CFHBI-specific treatments.
- E
Elevation of liver enzymes
- I
Imaging findings of the liver
- H
Histopathology of the liver
- S
Stiffness of the liver
- P
Portal hypertension
- B
Biliary manifestations
- M
Malignancies of the liver or biliary tract
The defined CFHBI categories each have numbered subheadings to describe the specific representations precisely. The ranking in numbering and letter are used for classification and do not represent a hierarchy in severity. This classification allows for pwCF to be precisely categorized based on their CFHBI-related presentation. For instance, a patient with elevated isolated liver enzymes would be recorded as CFHBI: E1, while someone with non-cirrhotic PHT and increased liver stiffness would be classified as CFHBI: S1/P2. Consistent application of the new classification will benefit the CF community and enhance our understanding and treatment of liver and biliary involvement in pwCF. The current position paper is not intended to be used as a guideline. Regarding the subject of imaging in pwCF, for example, we do not advise performing MRI scans of the liver for all patients. However, if an MRI is performed and an irregular nodular liver is observed, we would suggest using this information to classify this person with CF as: I2 Nodular imaging abnormalities.
In this joint ESPGHAN-NASPGHAN position paper, we have recommended a classification system for CFHBI, based on expert consensus, that health care providers can incorporate into their clinical practice when assessing and diagnosing liver and biliary involvement in pwCF. This classification system more accurately defines CFHBI presentations and allows for a more precise categorization of patients based on their specific CFHBI signs. Consistent use of this classification system can enhance our understanding and management of CFHBI and may benefit the pwCF and the CF community. The recommended CFHBI classification has yet to be validated for use in clinical practice and research, a key objective in future studies.
REFERENCES
Disclaimer
ESPGHAN is not responsible for the practices of physicians and provides guidelines and position papers as indicators of best practice only. Diagnosis and treatment is at the discretion of physicians.
The NASPGHAN clinical practice guidelines and position papers are evidence-based decision- making tools for managing health conditions. This document is not a disease management requirement or rule and should not be construed as establishing a legal standard of care, or as encouraging, advocating for, mandating or discouraging any particular diagnostic methodology or treatment. Our clinical practice guidelines and position papers should also not be used in support of medical complaints, legal proceedings, and/or litigation, as they were not designed for this purpose. The NASPGHAN clinical practice guidelines and position papers should also not be utilized by insurance companies or pharmacy benefit managers to deny treatment that is deemed medically necessary by a patient's physician. The health care team, patient, and family should make all decisions regarding the care of a patient, after consideration of individual specific medical circumstances. While NASPGHAN makes every effort to present accurate and reliable evidence-based information, these clinical practice guidelines and position papers are provided “as is” without any warranty of accuracy, reliability, or otherwise, either express or implied. NASPGHAN does not guarantee, warrant, or endorse the products or services of any firm, organization, or person. Neither NASPGHAN nor its officers, directors, members, employees, or agents will be liable for any loss, damage, or claim with respect to any liabilities, including direct, special, indirect, nor consequential damages, incurred in connection with the clinical practice guidelines and/or position papers or reliance on the information presented.




