Nutrition and Intestinal Rehabilitation of Children With Short Bowel Syndrome
A Position Paper of the ESPGHAN Committee on Nutrition. Part 1
Abstract
Short bowel syndrome (SBS) is the leading cause of intestinal failure (IF) in children. The mainstay of treatment for IF is parenteral nutrition (PN). The aim of this position paper is to review the available evidence on managing SBS and to provide practical guidance to clinicians dealing with this condition. All members of the Nutrition Committee of the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) contributed to this position paper. Some renowned experts in the field joined the team to guide with their experience. A systematic literature search was performed from 2005 to May 2021 using PubMed, MEDLINE, and Cochrane Database of Systematic Reviews. In the absence of evidence, recommendations reflect the expert opinion of the authors. Literature on SBS mainly consists of retrospective single-center experience, thus most of the current papers and recommendations are based on expert opinion. All recommendations were voted on by the expert panel and reached >90% agreement. The first part of this position paper focuses on the physiological mechanism of intestinal adaptation after surgical resection. It subsequently provides some clinical practice recommendations for the primary management of children with SBS from surgical resection until discharged home on PN.
What Is Known
-
Short bowel syndrome (SBS) is the leading cause of intestinal failure in childhood.
-
First line treatment is parenteral nutrition (PN).
-
Complications of SBS may be independent or related to PN support and may occur at any time.
What Is New
-
Prevention of SBS complication from birth has a key role in helping physiological intestinal adaptation, support adequate growth, and eventually weaning from PN
-
A multidisciplinary follow-up involving teams of experts in the intestinal rehabilitation unit is crucial to prevent those complications.
DEFINITIONS
Please find below the definitions of terms that will be applied throughout the current paper.
-
Intestinal failure (IF): Critical reduction of the gut mass or its function below the minimum needed to absorb nutrients and fluids required for adequate growth in children for a minimum of 60 days within a 74 consecutive day interval (1.).
-
Short bowel syndrome (SBS): the consequence of any natural loss or surgical resection of small bowel (SB) (2.).
-
SBS intestinal failure (SBS-IF): SB length below a critical value for nutritional supply for adequate growth (2.).
-
Parenteral nutrition (PN): intravenous administration of water, macronutrients (glucose, lipids, amino acids), electrolytes, minerals, and micronutrients (trace elements and vitamins) (1.).
-
Home parenteral nutrition (HPN): administration of PN in the home setting outside the hospital after parents/carers have undergone a structured training program in children who are clinically stable and expected to require PN for at least a further 3 months (3.).
-
Enteral nutrition (EN): the administration of nutrients through the gastrointestinal tract and can be divided into: oral feeding (OF) and artificial feeding (AF) via a feeding device (nasogastric tube, nasojejunal tube, gastrostomy, or jejunostomy) (4.).
-
Nutrition with artificial feeding device can be further divided into continuous, intermittent, or bolus feeding according to the mode of feed delivery (4.).
INTRODUCTION
Short bowel syndrome (SBS) is the leading cause of intestinal failure (IF) defined as the critical reduction of the gut mass or its function below the minimum needed to absorb nutrients and fluids required for adequate growth in children for a minimum of 60 days within a 74 consecutive day interval (5.). It requires parenteral nutrition (PN) while it persists. Usually, SBS is the consequence of a congenital disorder or surgical resection leaving the SB length below a critical value for adequate nutritional supply. Severely reduced mucosal surface results in malabsorption with subsequent diarrhea, water-electrolytes imbalances, and malnutrition (5.). SBS-IF may be reversible or irreversible, depending on several factors such as the underlying cause of SBS, the length of the remaining intestine, and treatment used to develop or restore intestinal autonomy.
Due to technical refinements and steady advances in the development of highly sophisticated nutrient formulations consisting of well-balanced combinations of macronutrients and micronutrients (6.,7.), PN has become a safe and efficient mode of feeding. Home-PN is the cornerstone of long-term management of SBS patients (8.,9.).However, using the GI tract, preferably feeding orally as soon and as much as possible in order to promote physiological adaptation of the remnant intestine and to avoid feeding disorders, is of utmost importance (10.,11.).
Guidelines for PN are available providing basic knowledge for the PN management of these complex patients (6.,7.). European Guidelines for PN in neonates, infants, and children were published in 2005 and updated in 2018 (6.,7.).
There are currently almost no randomized controlled trials (RCTs) or systematic reviews available to inform about optimal EN management. Diverse approaches are promoted by different teams and can vary significantly between countries and continents (12.,13.).
This position paper highlights, in its first part, the pathophysiology of SBS and provides basic practical advice for the optimal management of pediatric patients with SBS-IF. The target group are medical and surgical care givers. The patients require long lasting and specialized multidisciplinary management by an “Intestinal rehabilitation Centre” (IRC). The different clinical situations as well as the complications related to both long lasting IF and/or PN will be covered in the second part of this position paper.
Classification and Etiology of Short Bowel Syndrome
Exact measurement of the remnant intestine remains difficult even with the help of radiographic assessment (14.). The residual bowel length can be expressed as the percentage of the theoretical small bowel length (15.) instead of the actual length remaining.
Classification of SBS into 3 types is helpful for understanding the different outcomes: type 1 with end enterostomy or entero-rectal anastomosis with absence of the colon, type 2 with jejuno-colic anastomosis, and type 3 with jejuno-ileocolic anastomosis preserving the ileocecal valve (ICV) and the colon (16.) (Fig. 1).
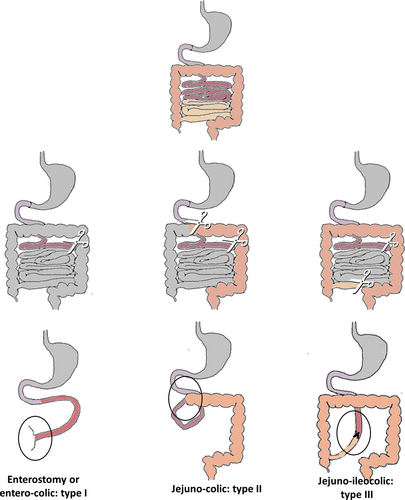
Classification of short bowel syndrome. Scissors represent the surgical resection point. Black circle highlights intestinal anastomosis.
In children, the conditions that most commonly lead to extensive small bowel resections are necrotizing enterocolitis (NEC), midgut volvulus, gastroschisis, intestinal atresia (including apple peel syndrome), and extensive aganglionosis. Total/Near Total intestinal aganglionosis (TIA/NTIA) is an uncommon and life-threatening form of Hirschsprung disease (HD). Most of the time, neonates with TIA/NTIA have SBS type 1 with less than 50 cm of normally innervated small intestine beyond the ligament of Treitz (2.) (Table 1 and Fig. 1).
Numerous factors determine SBS prognosis and duration of PN dependency: the underlying diagnosis, the type and condition of intestinal segments preserved, the presence/absence of the ICV and/or colon, a long-term enterostomy versus a primary anastomosis, any associated motility disorders, especially in intestinal atresia and gastroschisis (17.), the number of surgical procedures, as well as the age of the patient at the time of surgery (18.,19.). However, information about long-term growth and final adult height is lacking.
Epidemiology
Accurate estimates of neonatal SBS incidence are difficult to determine because of variation in the definition of SBS between institutions, difficulty of tertiary care referral centers to accurately determine their referral population, and problems ensuring complete follow-up of the cohort. Those difficulties are indicated by differing estimations of the incidence of SBS by different teams around the world.
The Canadian Collaborative Study Group estimated a neonatal SBS prevalence of 4.8/1 million population with an incidence of 24.5 per 100,000 live births [95% confidence interval (CI) 12.1–36.9] that was much higher in infants born before 37-weeks’ gestation compared with term newborns (353.7/100,000 live births vs 3.5/100,000 live births) (20.). In a report from the beginning of the century, overall mortality rate of neonates with SBS was 37.5%, 3 times higher than that of a matched cohort of infants without SBS. Intestinal failure associated liver disease (IFALD) was the cause of death in 60% of SBS (21.); this picture has changed due to advance in PN support (22.).
In the United States, the annual incidence was estimated to be 3–5/100,000 live births with a mortality rate of 26% (13.).
In France, the prevalence was estimated according to the number of patients on long-term home-PN. The French Home-PN registry demonstrated a prevalence of IF from all etiologies of around 10/million individuals <18 years of age and an annual incidence around 20–30 new cases per year (9.). Among this total population of children on home-PN 40%–50% were suffering from SBS. The reported mortality rate was 3.6% including all home parenteral nutrition (HPN) patients irrespective of the cause for long-term IF (9.).
Furthermore, the most recent report from the British Association of Parenteral and Enteral Nutrition (BAPEN) reported a steady increase of children with SBS-IF in the United Kingdom from 18 reported in 1992 passing through 99 reported in a single point prevalence survey in 2012 to the final number of 185 reported from the prospective registry in 2019 (23.)
Consequences of Intestinal Resection
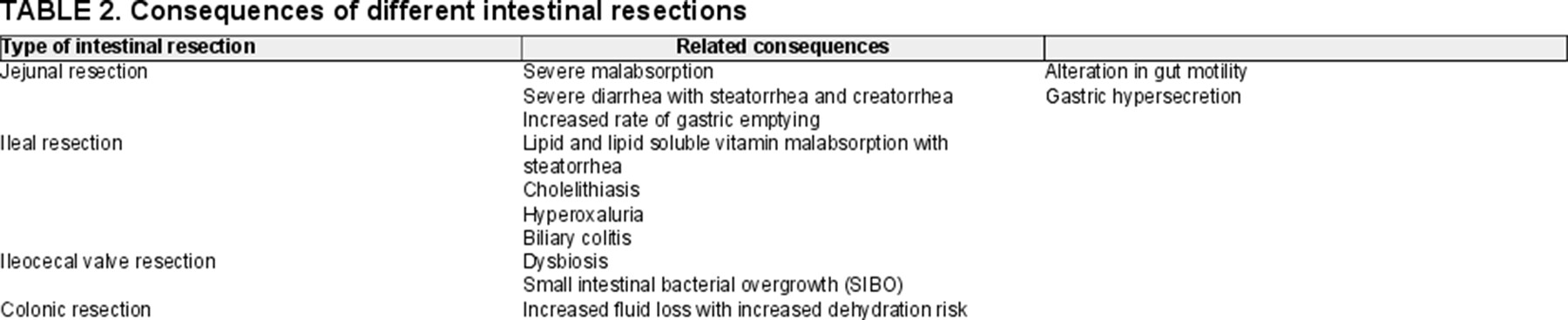
SBS is characterized by a compromised intestinal absorptive capacity due to a severely reduced mucosal surface area resulting in diarrhea, fluid and electrolyte imbalance, and failure to thrive (5.). The functional consequences of SBS depend not only on the length, surface area, and site of the resected small intestine but also on the functional capacity of the remaining intestine (Table 2). The cause of resection and age of the patient at the time at which surgery was carried out also influence the capacity of the remaining gut to function and the potential for adaptation. Term neonates have a small bowel length of 250 ± 40 cm which doubles during the last trimester of gestation, suggesting that a short bowel remnant does not have the same prognosis in a preterm infant as in a full-term baby (15.). The same difference exists between neonates and older children. Thus, if the surgical resection occurs earlier, the opportunity for adaptive growth is greater (5.). The severity of intestinal resection can be classified according to the length of the small intestine measured along the anti-mesenteric border at surgery. Although, currently a clear definition of SBS according to length is lacking, the majority of recent literature defines ultrashort bowel syndrome as a remnant small bowel of <25–10 cm (or <20%–10% of expected small bowel) (24.-26.). Other determinants of IF in SBS are the portion of the small bowel resected (jejunum, ileum, ileocecal valve, colon) (Fig. 2), and the functional integrity of the residual intestine (27.-29.).
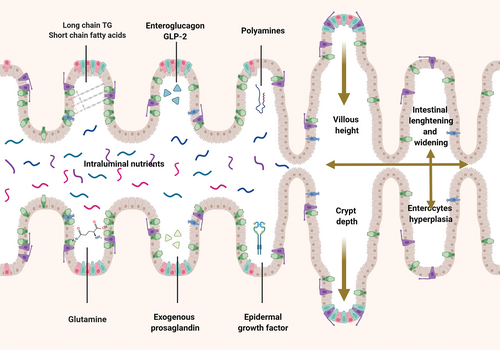
Mechanism responsible for intestinal adaptation in SBS children. SBS = short bowel syndrome.
Pathophysiology of SBS, including the consequences of anatomical changes, has been extensively reviewed (30.).
Resection of the Jejunum
Both the transit time and the direct contact of intraluminal nutrients within the jejunal epithelium determine the extent of malabsorption following extensive jejunal resection. The resulting malabsorption affects all nutrients including minerals, electrolytes, trace elements, and most vitamins (31.). Severe diarrhea following extensive jejunal resection is associated with steatorrhea and creatorrhea. Moreover, the jejunal resection reduces secretion of cholecystokinin (CCK) and secretin leading to a decrease in biliary and exocrine pancreatic secretions which may lead in turn to a reduction in nutrient absorptive capacity (31.,32.). The reduced production of CCK, vasoactive intestinal peptide (VIP), gastric inhibitory peptide, and serotonin may cause gastric hypersecretion, seen more frequently after jejunal as opposed to other areas of intestinal resection (33.). The degree of malabsorption is proportional to the length of jejunum resected and will be compensated, to some extent, by the ileum and/or by the process of adaptation in response to loss or intestinal surface (34.,35.).
Resection of the Ileum and Ileocecal Valve
Even though most nutrients are absorbed in the proximal jejunum, the residual ileum can adapt and assume the role of macronutrient absorption (31.). However, the specialized cells of the terminal ileum, where vitamin B12/intrinsic factor receptors are located and where bile salts are reabsorbed, cannot be replaced by jejunal hypertrophy (36.). Thus, the ileum has specific functions which the jejunum cannot substitute (36.). The impaired vitamin B12 absorption can cause macrocytic anemia and neuropathy (37.). Malabsorption of bile salts is responsible for specific complications:
- (1)
Reduction in bile salt reabsorption by the ileum reduces their circulating pool and leads to lipid malabsorption and steatorrhea (38.). The consequences are proportional to the concentration and dehydroxylation of bile salts in the colonic lumen (deoxycholic and chenodeoxycholic acids). Because of the decreased pool of bile salts, fat soluble vitamins (A, D, E, and K) are also prone to malabsorption (39.).
- (2)
Cholelithiasis seems to be the direct consequence of the reduced concentration of bile salts in bile and of the decreased mobility of the gallbladder induced by a lack of CCK production which are characteristic of extensive ileal resection in SBS (40.). Moreover, soya-based lipid emulsion administration may be harmful for gallstones development (41.).
- (3)
Hyperoxaluria with formation of kidney stones results from ileal and subsequent lipid malabsorption (long-chain triglycerides) (42.). Undigested long-chain triglycerides in the colon are able to bind dietary calcium, resulting in increased free oxalate, which is then absorbed into the bloodstream (43.). This complication became rare with increased use of medium-chain triglyceride (MCT) rich diets (see below) (44.).
- (4)
Biliary colitis resulting from disruption of the entero-hepatic circulation of biliary acids, alters colonic water and electrolyte absorption from the colon, worsens diarrhea, and excoriation of the perianal/nappy area (45.). This phenomenon is due to increased number of BA reaching the colon where their biochemical structure stimulates increased secretion of electrolytes/water and increased mucosal permeability, as well as causes high-amplitude propagating contractions arising in the proximal colon (46.).
The ileum is the site of enteric hormone release such as enteroglucagon which are essential in the process of adaptation after extensive resection (see below) (47.).
Finally, extensive ileal loss reduces intestinal transit time by suppressing the “ileal brake,” which is the mechanism of slowing gastrointestinal transit when the terminal ileum is perfused (48.). The resection of terminal ileum usually includes also ICV resection with a further decrease in transit time, since the ICV acts as a brake. In addition, loss of the ICV, allows colonic bacteria to enter and populate the small intestine, increasing the likelihood of the development of a severe form of SBS (49.-51.) malabsorption with small intestinal bacterial overgrowth (SIBO). SIBO impacts negatively on digestion and nutrient absorption, as bacteria compete with enterocytes for nutrients and may increase the risk of SIBO-related sepsis that occurs more frequently in infants without the ICV than in those with an intact caecum (24.,52.). Thus, ICV resection represents an additional major cause of malabsorption of nutrients, water and electrolytes, dehydroxylation of bile salts, mucosal injury, increased intestinal permeability, and disordered motility. The lack of the ICV appears to greatly influence the period required to achieve intestinal autonomy (24.,27.-29.).
Resection of the Colon
The colon is a crucial component of small intestinal adaptation and function in SBS patients by absorbing fluid and electrolytes, MCTs, and producing short-chain fatty acids (SCFAs) from malabsorbed carbohydrates (CHO) (16.,49.,50.). The removal of both ileum and colon substantially increases fluid loss, increasing the risk of dehydration, and sodium depletion. Conversely, preservation of the colon in cases of major small intestinal resection appears to lessen the severity of SBS. In experimental studies increased enterocyte expression and function of apical membrane Na/H exchangers in regions distal to the anastomosis play a role in the adaptive process after extensive small bowel resection. The increased luminal Na load to distal bowel regions after proximal resection may stimulate increases in apical membrane Na/H exchangers gene transcription and protein expression (53.). In addition, the colon hosts the majority of the intestinal microbiota, which plays a crucial role in intestinal function and health.
Other General Conditions
-
Gastric hypersecretion occurs in 50% of pediatric patients with SBS and is usually explained by an increased gastrin secretion (33.). However, rates of hypergastrinemia are variable (54.,55.). Acid hypersecretion occurs early after resection and depends on the extent of the resection. Hypersecretion is transitory but it increases with enteral feeding (EF) leading to a larger amount of intestinal fluid loss. Gastric acid hypersecretion, by reducing duodenal pH, decreases the activity of pancreatic enzymes such as amylase and lipase, which, in turn, increases fat malabsorption (56.).
-
Gastric emptying of liquids is more rapid following jejunal resection, although intestinal transit may remain normal because of the braking effect of the ileum and the ICV. The loss of inhibition of gastric emptying and intestinal transit in children without a colon is related to a significant decrease in peptide YY (PYY), glucagon-like peptide I (GLP-I), and neurotensin (57.-61.). PYY is normally released from L cells in the ileum and colon when stimulated by fat or bile salts. Rapid gastric emptying may contribute to fluid losses in children with SBS, with subsequent risk of dehydration. On the other hand more than 10% of children with SBS show delayed gastric emptying after intestinal surgical resection most probably due to altered fundic receptive relaxation, decreased antral contractility, and incoordination of gastric emptying and duodenal contractions (62.).
-
Alteration in gut motor activity may be observed especially in cases of prenatal small bowel malformations (gastroschisis and/or intestinal atresias) or severe postnatal pathology such as extensive NEC (17.,63.). Gastrointestinal dysmotility may be caused by decrease in the interstitial cells of Cajal, damage of the smooth muscle cells due to small bowel ischemia, and neuropathic changes with reduction of intramuscular nerve fibers, which occurs in various degree according to the underlying cause of SBS (62.). They contribute, together with repeated surgical procedures to impair motor activity leading to SIBO and complications (see below).
Adaptation After Extensive Intestinal Resection
Soon after small bowel resection, the physiological process of adaptation of the remaining small bowel starts. Adaptation is a slow process accompanied by a gradual increase in absorption capacity for nutrients, electrolytes, and minerals (25.,30.). This comprises muscular hypertrophy (increased bowel diameter and wall thickness) and hyperplasia of the intestinal mucosa. This mucosal hyperplasia is characterized by an increased number of enterocytes per unit of SB length, an increased rate of enterocyte proliferation, and an increased villous height and crypt depth (64.). In animals, it was shown that epithelial hyperplasia following gut resection results in increased mucosal mass, including higher mucosal wet weight, higher protein content as well as higher DNA and RNA content per unit of bowel length which results in increased number of enteroendocrine cells (65.,66.).
The complex regulation of gut mucosal growth involves a multitude of factors including hormonal mediators such as enteroglucagon, glucagon-like peptides neurotensin, peptide YY (PYY), growth hormone, and insulin-like growth factor. Enteroglucagon, progenitor of glucagon peptide 1 (GLP-1) and 2 (GLP-2), and PYY seem to play a pivotal role by stimulating cell turn over, motility and absorptive capacity of the small intestine especially after small bowel resection (67.-69.).
Nutritional substances in the lumen are central in this process and they can act directly stimulating mucosal hyperplasia and expression of their own transporters (70.) or indirectly by modifying microbiota composition and stimulating trophic gastrointestinal hormone secretions (71.).
Major nutritional adaptative substances:
-
SCFAs (eg, acetate, butyrate, propionate) are produced from the fermentation of unabsorbed CHO by the colonic microbiota. SCFA could be energy substrates for the topical nutrition of intestinal cells (72.). Pectin, a water soluble, non-cellulose dietary fiber, was shown to enhance jejunal and colonic mucosal adaptation when added to the enteral diet (50.,73.,74.).
-
Glutamine is an important circulating amino acid (AA) which plays a role in the metabolism of enterocytes by preventing intestinal luminal atrophy (75.,76.). Even if many studies have proven suggested efficacy, its role is still debated (77.).
Besides nutrients, other factors seem to play an important role in the mucosal adaptation process:
-
Epidermal growth factor (EGF) present in human milk (HM) and produced in the salivary glands and duodenal cells is a trophic substance for the gastrointestinal tract (78.,79.).
-
Polyamines (spermine, spermidine), whose synthesis is dependent on ornithine decarboxylase activity, greatly enhance intestinal cell turnover and protein synthesis (80.,81.).
Thus, adaptation of the remaining small bowel is dependent on the tight interaction of endogenous (enterohormomes, EGF, polyamines) and exogenous (nutritional substances) factors; this emphasize the usefulness of early EF after resection (82.). The goal of intestinal adaptation is to develop the ability to withdraw artificial nutrition thanks to a compensatory increase in the mucosal surface area and absorption capacity. The 2 indicators which have historically been used to predict weaning from PN in children: anatomy (residual bowel length measured at most recent major surgical resection and proportion of preserved colon) and plasma citrulline may be reliable prognostic factors in SBS patients (16.,83.-89.). Recently, monitoring citrulline plasma levels during the course of SBS adaptation has been shown to predict PN weaning (90.). In clinical practice the degree of intestinal insufficiency may be measured by both citrulline plasma levels and the percentage of PN required for normal body weight gain and longitudinal growth (16.,90.). PN requirements remain, therefore, the best measure of the degree of intestinal sufficiency in this setting (Table 3).
MATERIALS AND METHODS
For this systematic literature review, the Medline database and the Cochrane Library were searched for relevant publications in English up to July 2021; a further research update was made until March 2022. Due to the limited number of RCTs, we also included cohort studies, case studies, and surveys addressing the topic. Medline (Pubmed) search included: 1. Mesh “Short bowel syndrome” and 2. Mesh “Child” alone or in association with: a. Mesh “Breastfeeding,” b. “Hydrolyzed formula,” c. Mesh “Enteral Nutrition,” d. Mesh “nutritional requirements,” e. Mesh “nutritional status,” f. Mesh “Parenteral Nutrition, Home,” g. Mesh “Parenteral Nutrition,” h. “intestinal failure,” i. “intestinal rehabilitation,” l. “multidisciplinary management,” m. “autologous intestinal reconstruction,” n. “serial transverse enteroplasty,” o. “intestinal lengthening and tailoring,” p. “intestinal transplantation,” q. “GLP-2 analogues.” The search was limited to articles published from 2000 onward. The primary search conducted up to July 2021 retrieved 1060 publications. After screening titles and/or abstracts a list of 487 publications was proposed to the authors of each chapter. After further selection by authors and integration of new relevant papers until March 2022, the final list of 205 references was included and split in the 2 parts of this position paper (Fig. 3). Literature screening revealed a lack of RCTs or even open label trials, with most literature on the topic based on retrospective cohort studies. For this reason, many of the recommendations to provide answers about nutrition and intestinal rehabilitation in children with SBS, were proposed and discussed based on cohort studies and on the wide expert panel’s clinical experience. Recommendations were then voted on by the panel of experts and any recommendation that did not reach a consensus of 90% or more was rephrased until all authors agreed for publication.
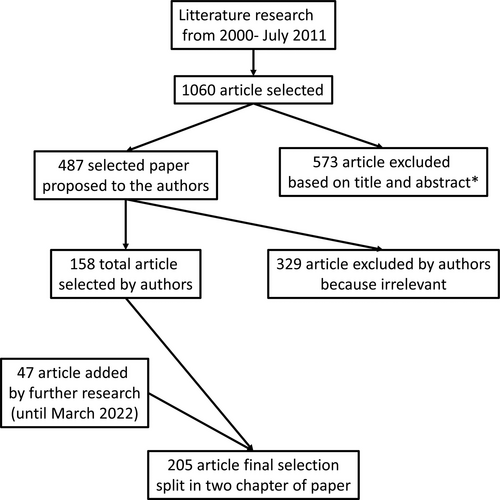
Flow diagram of article selection. *Based on title and type of article (exclusion of case report, case series, and review and duplicates).
EARLY MANAGEMENT AFTER INTESTINAL RESECTION
The specific protocol proposed in this section refers to all patients with a small intestinal resection, which could then be at risk of evolving into SBS-IF requiring HPN support.
Intestinal trauma induces a metabolic stress response that correlates with the duration and the severity of the injury insult and alters energy needs and the metabolism of CHO, protein, and fat (91.).
As outlined in a recent position paper from the Committee of Nutrition of ESPGHAN, the metabolic stress response after intestinal resection can be differentiated in 3 phases: early acute (the first 24–48 hours), late acute or intermediate postoperative (until the first week after surgery), and recovery phase or late postoperative (from about 7 days after surgery until discharge from intensive care) (91.). However, there is no strict time limit to those phases and may be adapted according to the age of the patient at the time of surgical intervention and the individual metabolic response.
Parenteral Nutrition Support
Early Acute Phase (0–48 Hours)
Perioperative fluid management should aim at maintaining tissue perfusion and prevent hypovolemia or fluid overload, hyponatremia, and hyperglycemia (92.). The primary aim of nutritional management is fluid resuscitation, including maintenance of fluid volume through management of losses through the gut, intestinal stomas, fistulas, or drainage tubes (93.).
This early acute phase is characterized by vital organ support, hyper-catabolism, and skeletal muscle breakdown. Fluid overload and acute renal failure are common in neonates after surgery for extensive NEC and are associated with increased morbidity and mortality (94.).
In the early acute phase, the focus of nutritional support is to maintain or re-establish the infant’s normal physiological state, not to promote growth (91.,95.).
Postoperatively, it is recommended that neonates receive nutritional support to cover basic metabolic macro- and micronutrient needs (91.). Furthermore, based on results of the multicenter RCT in pediatric intensive care unit, PN (96.) may be withheld at this early stage and could be replaced by glucose infusion especially for term infants or older children (91.,97.-99.). Benefits and risk of glucose supply must accurately be taken into consideration at this stage. On one hand, during surgery and in the early postoperative phase a rise in blood glucose level is common; this led to the introduction of glucose-free or low glucose-containing isotonic electrolyte solutions for perioperative fluid therapy in children and adults (100.). However, suboptimal glucose supply in neonates seems to increase the risk of rebound hyperglycemia during the postoperative phase and to induce acidosis in neonates (101.); suggesting a glucose need of 3.5–7 mg/kg/min to prevent catabolism.
Intermediate Postoperative Phase (48 hours–7 days)
The beginning of this phase is determined by the first passage of stool and the cardiovascular stability of the patient. If an enterostomy is in place patients with high fluid losses are at increased risk of hyponatremia and dehydration. Thus, patient hydration status (including weight) and plasma sodium and urinary sodium should initially be monitored daily and losses should be compensated using parenteral sodium-containing fluids. According to the clinical experience of the expert of the panel, neonates with stomas should undergo daily urinary sodium level monitoring in this initial phase; this is not true for neonates receiving diuretics which modify urinary electrolytes excretion. Target urinary sodium should be greater than 20 mmol/L and exceed the level of urinary potassium (NaU/KU > 1). High urine sodium output in preterm neonates should however not be considered as a sign of sodium overload because of the tubulopathy of prematurity (92.). In this phase if an adequate hydration state cannot be guaranteed with PN infusion separate fluid infusions should be considered.
Current guidelines on nutritional management of critically ill preterm infants, neonates, and children recommend to consider careful initiation of nutritional support after 48–72 hours with energy requirements estimated between 60 and 80 kcal/kg/day for preterms and 45–70 kcal/kg/day for term babies (91.,95.,99.,102.). PN is recommended only if adequate enteral nutrition is not feasible. In most patients with extensive bowel resection, PN is indispensable. When providing PN, enteral intakes also need to be considered to optimize nutrient supply while stimulating intestinal adaptation. A minimum protein intake (parenteral AAs) of 1.3–1.5 g/kg/day should be given to ensure a positive nitrogen balance (91.,95.,99.,102.). Fatty acids are an important energy source during illness (103.,104.) and intravenous lipid emulsions (IVLEs) should thus be provided as an integral part of any PN (105.). Patients with SBS are at increased risk of IFALD; Cochrane reports in preterm infants and term infants (106.,107.) did not find any conclusive evidence on the role fish oil containing IVLE in the prevention and treatment of PN-associated cholestasis in neonates. However, more recent publications seem to demonstrate a protective and therapeutic role of fish oil-based IVLE (108.,109.) and thus, if liver disease is established, it is recommended to reduce the IVLE dosage and/or consider the use of a fish-oil containing IVLE along with other measures to reduce other risk factors. Furthermore, due to the less balanced n6/n3 provision provided by pure soybean oil IVLE, it is recommended to use composite IVLEs containing fish oil for PN lasting longer than a few days (105.). Furthermore, those IVLE guarantee a well-balanced essential fatty acid status (110.).
Late Postoperative Phase
When the clinical state and the inflammatory response resolve, nutrient intakes should gradually be advanced (90–120 kcal/kg/day for preterm and 75–85 kcal/kg/day for term babies). Furthermore, nutritional supply in the upper range of recommendations is often needed to cover cumulative deficits, promote tissue repair, and catch-up growth (91.,111.).
In this phase PN cycling should be considered as soon as possible, usually for stable babies over 4-kg body weight which has less risk of hypoglycemia during the off-PN time, to minimize hepatic damage (112.,113.).
Knowledge of the extent of tissue resection and the remaining intestine is essential so that micronutrient supplementation, particularly zinc, iron, copper, manganese, and vitamins (eg, fat-soluble, B12), may be managed appropriately (114.). Patients with stomas are at increased risk of hypomagnesemia and zinc deficiency which can however be prevented with higher parenteral intake (115.). In those patients Zn, Fe, Cu, Se, and Mn status must be assessed in the postoperative period (114.).
Due to the previously mentioned gastric acid hypersecretion, the use of acid-reducing medication may be needed (116.), and periodically reassessed after enteral feeds are established.
CONCLUSIONS
-
Children after surgery for intestinal resection undergo major metabolic stress and should be carefully assessed to determine the different postoperative phases.
R1
-
In the early acute phase, fluid management and resuscitation are crucial; energy requirements are essential to maintain physiological state but not to promote growth.
-
Parenteral energy in the early acute phase is provided essentially by glucose under tight glycemic monitoring.
-
Lipids as energy supply should be introduced as an integral part of PN in the intermediate postoperative phase. Composite IVLE, containing fish oil, should be considered for all patients with longer needs of PN.
-
In the late acute phase, a gradual increase in energy supply is needed taking into consideration enteral intake and intestinal malabsorption due to intestinal resection.
-
During the early management after intestinal resection, calculation of fluid and electrolyte requirements should take into consideration residual intestinal anatomy, stoma presence, and levels of intestinal losses. Regular monitoring should include weight and urinary sodium assessment.
-
Nutritional requirements in the late postoperative phase need to take into account cumulative deficits, tissue repair, and catch-up growth.
-
Cycling PN should be started, under tight glycemic monitoring, in all stable babies over 4-kg body weight.
PP1
-
During hospital stay, urinary sodium levels should be monitored daily in the late acute phase and weekly afterwards especially in children with a distal stoma.
-
Urine sodium levels should be maintained >20 mmol/L and exceed urinary potassium (NaU/KU > 1) in all SBS children if diuretics are not used.
-
In late postoperative phase screening for micronutrients deficiency is mandatory.
How to Deliver Enteral Feeds After Surgery?
Prospective randomized trials on when to start EF and the speed of increment of enteral feeds in high-risk patients are not currently available. Therefore, recommendations are primarily based on physiology and clinical experience. Management should be adapted and tailored on a case-by-case basis (117.). As stated earlier enteral nutrition plays a pivotal role in intestinal adaptation, prevention of cholestasis, and maintenance of a healthy microbiota in children with high risk (118.). This is true also for preterm newborns (119.). In particular 2 metanalyses have been performed on refeeding practices after NEC (120.,121.). These found that EF within 5 days of NEC diagnosis did not seem to be associated with adverse outcomes, including NEC recurrence.
Even after emergency GI surgery, early feeding may be feasible in patients without severe shock or bowel anastomosis instability. Early feeding was defined as initiation of EF within 48 hours after surgery (122.). For all those reasons, even if based on low quality evidence, starting EF as soon as possible within 48 hours after surgery has been recommended (92.). Most centers start feeding as soon as stool was passed (123.). Gastric output in this specific context may not reflect appropriately enteral nutrition tolerance since gastric hypersecretion is part of the intestinal adaptation process after resection (56.).
The resting and adaptation phase progression/alteration of feeds depends on the anatomy of the residual small intestine and the presence of the colon and should be guided by GI tolerance (stool volume, lack of nappy rash/perianal excoriation, abdominal comfort, etc) and PN weaned while allowing for growth and biochemical stability (12.).
There is very little evidence regarding the route of enteral nutrition administration (oral or artificial feeding device) and the mode of administration (continuous vs intermittent) (Table 4). Oral feeds are more physiological and should be offered whenever possible, while aggressive weaning and gut overload syndrome should be avoided (8.,12.,124.). Oral feeding promotes the release of epidermal growth factor (EGF) from salivary glands, increases GI secretion of trophic factors, and helps prevent feeding disorders (125.). For the aforementioned reasons, in the attempt of pursuing a more physiological mode of feeding, oral feeding should be the preferred route whenever possible after surgery. If the oral route is not accessible (eg, mechanical ventilation, clinical deterioration, immaturity) it is of utmost importance to promote complementary oral stimulation as soon as possible in order to prevent oral aversion and feeding disorders (11.). In some selected cases mucous fistula refeeding might be considered even if an international consensus is still lacking (126.).
A recent survey from the European Reference Network of Intestinal Congenital Abnormalities (ERNICA) consortium outlined the various clinical practices regarding early feeding in intestinal rehabilitation (123.) such as intermittent feeding use in half of the screened centers and the rest divided into continuous or a combination of both, when the oral route is not possible. On one hand, continuous provision of enteral nutrition may increase mucosal contact and improve receptor saturation, therefore, potentially improving absorption per unit of length of intestine (127.). However, continuous tube feeding eliminates fasting periods and may suppress neuromuscular function with a decrease of intestinal motility and thus, increasing the risk of SIBO and IFALD (12.).
There is no evidence on how to progress with EF but in clinical practice stool output is used as a proxy for intestinal adaptation and enteral nutrition tolerance. Some experts recommend that the upper acceptable limit of stool output is 40–50 mL/kg/day (10 bowel movements) (128.) while other experts recommend an upper limit of 20 mL/kg/day (6 bowel movements) (129.). Even without strong references, stool output should be interpreted according to the anatomy of the remnant intestine and SBS type. The ERNICA questionnaire (36 questions) about strategies used to introduce enteral nutrition postoperatively and start complementary food/solids in infants with SBS associated IF was developed and sent to 24 centers in 15 countries. There was wide variation in early EF practices in infants with SBS. Ten of 24 centers in the ERNICA survey (41.7%) increased EF by 10–20 mL/kg/day.
Recently Channabasappa et al (130.) published an algorithm for the increments of feeds considering stool output and stool frequency which was included in Figure 4.
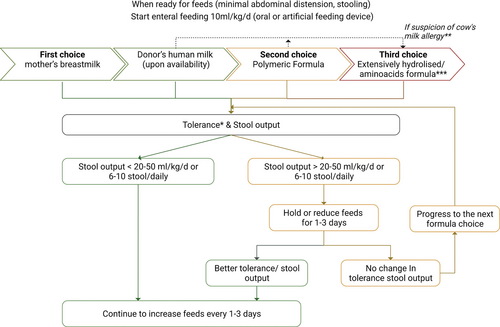
Enteral feeding algorithm after intestinal resection. The algorithm is valuable for term babies or preterm above 36 corrected gestational weeks. * Multifactorial evaluation taking into account abdominal distension, vomiting, and stool output. ** Clinical symptoms for allergy suspicion: vomiting, dermatitis, bloody stools, and enterocolitis recurrence. *** When stability and growth are established, a step back to a polymeric should be considered.
In rare cases of children developing SBS-IF at an older age the diet regime applied before intestinal resection should be resumed as soon as possible after surgery taking into consideration the new intestinal anatomy and feeding tolerance.
Choice of Feeds
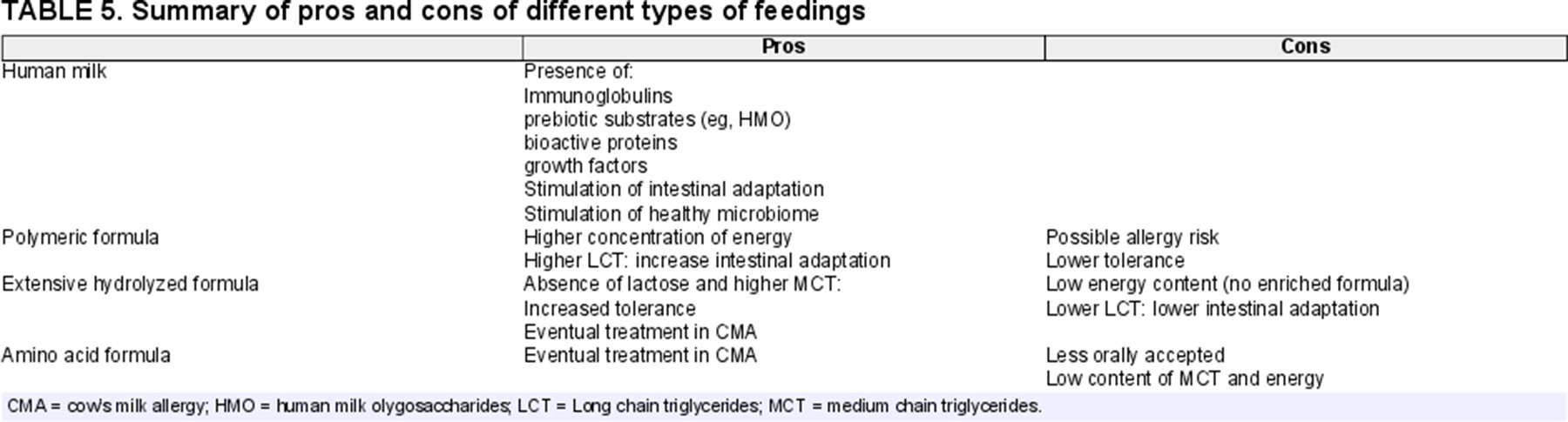
The decision of what type of enteral feed to initiate is controversial in pediatric SBS patients and depends on several factors. The age of the child, the cause and type of SBS (Fig. 1), as well as the intestinal motility are relevant issues. Although data are limited, HM (breast milk or donor milk) is recommended as a first-line enteral regimen for infants with SBS-IF, as it contains immunoglobulins, prebiotic substrates such as HM oligosaccharides, bioactive proteins, and growth factors (growth hormone and EGF) that together promote a healthy microbiome and intestinal adaptation (92.,130.). In addition, it has been hypothesized that the use of HM may result in fewer days of dependence on PN (131.) and may reduce the risk of IF-associated liver disease (132.). In the already mentioned ERNICA survey, maternal breast milk was the first choice in 23 of 24 centers, while donor breast milk, standard preterm/term formula, extensively hydrolyzed, or amino-acid based feed were the second choice. Furthermore 22 of 24 centers introduced weaning foods by 6 months of age. However, what type of complementary foods were used varied significantly among the units and seem to reflect cultural differences amongst European countries. Fifteen (62.5%) centers in the ERNICA survey used nutritional fortifiers.
When HM is not available various different types of formula have been suggested, ranging from polymeric formulae to extensive hydrolyzed formulas (EHFs) or AA-based types (133.) (Table 5).
Extensive hydrolyzed proteins, which provide dipeptides as well as AA, confer a significant absorptive advantage over AA formulas alone (134.). Most of EHFs do not contain lactose and usually have an increased concentration of MCTs. Components such as MCT can be better absorbed in the case of rapid transit, bacterial overgrowth, and bile acid depletion, which occurs after surgical NEC (135.). For this reason, hydrolyzed protein formulas have been popularized for use in patients in whom absorptive capacity is limited (136.). Nonetheless, the possible disadvantages of EHF should be considered, since they do not satisfy the elevated requirements in preterm infants (137.). Furthermore, studies in animals suggest that intact macronutrients (e.g. long-chain fatty acids) lead to better intestinal adaptation than do medium-chain fatty acids (138.). In this regard, the only randomized study comparing hydrolyzed versus non-hydrolyzed formula in children with SBS is an old trial involving only 10 children and designed to compare intestinal permeability (136.). This trial did not find any significant difference between the 2 groups.
Children with SBS, especially young infants, may have a significant risk of secondary non-IgE-mediated intestinal allergic disease (139.). It is thought that decreased intestinal barrier function, especially caused by SIBO may predispose infants with intestinal failure to allergic gastrointestinal diseases (140.). Children with SBS often have small intestinal dilatation and dysmotility, which results in SIBO. Secondary inflammatory changes in the mucosa may occur which result in permeability to macromolecules, sensitization, and secondary allergic enteropathy. In a retrospective review of 37 children with neonatal SBS over a 10-year period 3 patients (<10%) developed cow’s milk allergy (CMA) (139.).
In this specific setting, when based on clinical parameters an allergy is suspected; EHF and even AA formulas may have a significant therapeutic advantage in the management of SBS in patients younger than 2 years; these advantages are independent of the absorptive differences and are primarily related to the anti-allergic properties of the extensively hydrolyzed proteins or AAs (141.). Many studies involving HFs and AA formulas are small and retrospective, and there is no definitive evidence that one type is better than the other (142.). Capriati et al recently published a review of the enteral nutrition in neonatal SBS. They included 10 studies (822 patients) separated into 3 cohorts: EHF fed patients, EHF + HM-fed patients, and AA + HM-fed patients. There was no difference in the rate of enteral adaptation or PN duration among the groups. They concluded that HM should be used when possible in SBS patients and that HF is just as effective in adaptation as AA-based formulas (143.). However, AA formula does not contain high amounts of MCT as compared with the commercially available EHFs that usually contain an energy supply of 50% lipids of which 40%–60% is MCT.
Currently, there is no evidence to recommend HM fortifiers for SBS children; fortifiers may alter enteral feed osmolarity (144.) possibly inducing increased stool output.
R2
-
Feeding should be initiated as soon as possible after surgery in children with SBS.
-
Oral feeding should be the preferred route of feed administration.
-
If oral feeding is not possible, evidence of advantages of continuous versus intermittent tube feeding are lacking.
-
Enteral feed increase should be based on stoma output and surveillance of feed tolerance in the previous 24 hours.
-
HM (mother’s or donor) is the first option for feeding intestinal surgical patients.
-
In the absence of HM polymeric formula may be attempted as first line option.
-
If HM and polymeric formula are not tolerated an EHF is recommended.
PP2
-
A gradual increase in enteral feeds of 10–20 mL/kg/day may be attempted if stool output is <20–50 mL/kg/day or 6–10 stool/day.
-
Tolerance of enteral nutrition should be assessed using a multifactorial clinical evaluation of abdominal distension, vomiting, and stool output.
-
From clinical experience EHF are well tolerated and have the advantages of providing well-absorbed short peptides.
-
In SBS children, the frequency of CMA may be higher than in the general population. In case of suspected CMA, the diagnostic and therapeutic approach is the same as in the general population.
PREPARING FOR HOME PN
Family Communication
Despite being a chronic condition, which frequently impacts not only on physical but also on psychosocial and emotional functioning, literature on the role of counseling/information and proper communication is scarce in this setting. A recent cross-sectional study on children and caregivers perspective of SBS demonstrated a lower degree of health care satisfaction, mainly due to lack of information and communication in children older than 5 years (145.).
As highlighted in a retrospective study surgical counseling may be necessary even prenatally because of the frequent association between prenatal diagnosis of congenital intestinal abnormalities and SBS (146.,147.). In other non-congenital conditions such as NEC or volvulus acute counseling may be necessary and should involve information on prognosis (29.,148.,149.) and possible complications (150.-152.).
After intestinal surgery an appropriate discussion with the family should take place to explain SBS diagnosis and include information on prognosis (including expected weaning possibilities) (25.,153.), impact on daily family care [impact daily assistance and quality of life (QoL)] (154.-156.), and possible complications (157.,158.).
For all those reasons, in order to build a sustainable long-term relationship with SBS children and their families, we strongly suggest not to neglect 2 important steps of communication:
-
Counseling with surgeon before surgery whenever possible (surgical procedure should be explained before the procedure).
-
Counseling with a multidisciplinary team composed of a neonatologist, pediatric surgeon, and pediatric gastroenterologist specializing in intestinal failure rehabilitation, after the intervention to explain SBS diagnosis implication and prognosis.
Discharge and Family Education
Retrospective collection of data from children suffering from IF has demonstrated that HPN may be associated with less IF-related complications and improved QoL compared with hospital PN (159.). Furthermore, accessing HPN program seems to improve children’s QoL by allowing them to participate in the same activities as their healthy peers (160.,161.). For those reasons, HPN is now considered the standard of care for patients with prolonged/irreversible IF and should be considered for all children likely to require PN for more than 12 weeks (3.). However, HPN may have some limitations, for example, the ability to perform rapid changes in PN formulation depending on the specific HPN program (159.). Children considered for home discharge should thus have sufficiently stable fluid and electrolyte needs and tolerate cyclical PN while maintaining blood glucose levels.
Retrospective analysis of patients on HPN has demonstrated a 30% rate of hospital readmission within 30 days of discharge both for adults and children (162.,163.), even if only 20% of those readmissions were related to IF complications. Specific studies on HPN children are lacking even if we know from clinical practice the high burden of medical care of those patients.
The positive impact of a structured training program in preventing end stage nutritional failure was demonstrated in a retrospective cohort of children listed for intestinal transplant (164.,165.). This comes from retrospective data and we are not aware of any prospective data from small centers where a structured training program for intestinal rehabilitation is not available (166.). Despite limited evidence, we strongly recommend a structured training program to teach families how to manage HPN, in order to prevent hospital readmissions and complications. At least one (ideally both two) parent(s)/caregivers should have been trained by a nutrition nurse and the home has to be suitable for HPN (3.). Families should be carefully prepared for discharge on HPN, with extensive explanation about safe medical environment, hand hygiene, central venous catheter (CVC) care, HPN preparation, infusion pump usage, and infection control (163.).
Recently, various methods have been described in order to improve CVC and PN management such as online and video simulation which may be used in addition to face to face training for safe, on-demand rehearsal of techniques such as accessing the CVC for blood sampling, connection/disconnection of the PN bag, dressing change of the catheter exit site, and pump operation (167.-169.).
As stated in the ESPGHAN pediatric PN guidelines and confirmed by recent evidence (170.-173.), taurolidine locks should be in use in long-term PN (174.). When taurolidine is not available, ethanol locks may be considered (174.).
R3
-
Appropriate and extensive communication should be scheduled before and after intestinal resection, at the time of SBS diagnosis and periodically during HPN follow-up, by a multidisciplinary team involving a neonatologist, pediatric surgeon, and pediatric gastroenterologists specializing in intestinal rehabilitation.
-
Clinically stable SBS children requiring PN for >12 weeks should be considered for discharge on HPN.
-
One and preferably both 2 parents/caregivers should be trained to manage CVC and PN within a structured home PN training program.
-
Home PN services should include appropriate training on the use of antiseptic locks.
PP3
-
Children considered for HPN should have: (1) secure CVC; (2) stable fluid and electrolyte needs; (3) tolerate a parenteral nutrition infusion break.