Effectiveness of Switching to Subcutaneous Infliximab in Pediatric Inflammatory Bowel Disease Patients on Intravenous Maintenance Therapy
R.K.R. has received consultation fees, research grants, royalties, or honorarium from Janssen, Pfizer, Nestle Health Sciences, AbbVie, Celltrion, Lilly, and Pharmacosmos. D.C.W. has received speaker fees and/or consultancy from Celltrion, AbbVie, and Nestle Health Sciences. The remaining authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
Disclosure of Funding: L.G. fellowship is supported by University of Milan.
Abstract
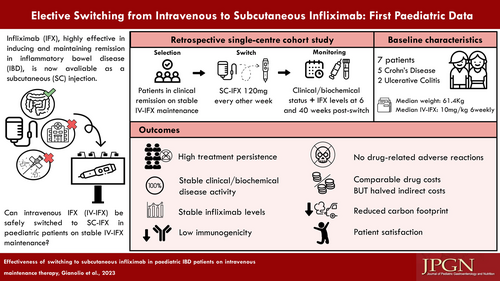
No real-world data are available on subcutaneous infliximab (SC-IFX) in pediatric inflammatory bowel disease (PIBD). We report a single-center cohort experience of an elective switching program from biosimilar intravenous infliximab to SC-IFX, 120 mg fortnightly, as maintenance. Clinical and laboratory data were collected for 7 patients with infliximab trough levels collected prior and at 6 and 40 weeks after the switch. High treatment persistence was registered with a single patient discontinuing the treatment due to high IFX antibodies, already present before switching. All patients remained in clinical remission with no significant changes in laboratory markers and median infliximab trough levels (12.3 µg/mL at baseline; 13.9 and 14.0 µg/mL at 6 and 40 weeks respectively). No newly-developed IFX antibodies were detected and no adverse reactions or rescue therapies were recorded. Our real-world data support the feasibility of an elective switch to SC-IFX in PIBD as maintenance with potential advantages concerning medical resources and patient satisfaction.