Efficacy and Safety of Tacrolimus or Infliximab Therapy in Children and Young Adults With Acute Severe Colitis
J.M.Z. is a consultant for Takeda. Dr Bousvaros is a consultant for Arena, Takeda, Best Doctors, and Eli Lilly and receives research support from Prometheus, Janssen, Abbvie, Takeda, Buhlmann, Arena, and Eli Lilly. P.A.R. is a consultant for Abbvie, Daiichi Sankyo, and Very Well and receives research support from TechLab and IBD4Cure. The remaining authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
This article has been developed as a Journal CME and MOC Part II Activity by NASPGHAN. Visit https://learnonline.naspghan.org/ to view instructions, documentation, and the complete necessary steps to receive CME and MOC credits for reading this article.
Abstract
Introduction:
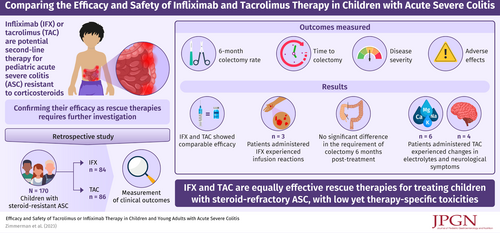
One-third of children and young adults admitted for management of acute severe colitis (ASC) fail intravenous corticosteroids. Infliximab (IFX) or tacrolimus (TAC) is often used to prevent urgent colectomy in these patients. However, no prior studies have reviewed the outcome of pediatric patients with ASC who were treated with either IFX or TAC.
Methods:
We retrospectively identified 170 pediatric patients with ASC admitted to our institution who did not respond to intravenous corticosteroids and were subsequently treated with either IFX or TAC. We compared 6-month colectomy rates, time to colectomy, improvement in disease activity indices, and adverse effects.
Results:
The mean age of patients in the IFX (n = 84) and TAC (n = 86) groups were 14 and 13.8 years, respectively. The median study follow-up time was 23 months. The rate of colectomy 6 months from rescue therapy was similar whether patients received IFX or TAC (22.6% vs 26.7%, respectively, P = 0.53). The mean decline in Pediatric Ulcerative Colitis Activity Index scores from admission to discharge in those treated with IFX (31.9) or TAC (29.8) was similar (P = 0.63). Three patients treated with IFX experienced infusion reactions. Six patients treated with TAC experienced changes in renal function or electrolytes, and 4 patients reported neurologic symptoms.
Conclusions:
There were no significant differences in the likelihood of colectomy 6 months after initiating IFX or TAC rescue therapy. Efficacy of both agents was comparable. The types of adverse effects differed by therapy. These data support the use of either TAC or IFX in children with ASC refractory to intravenous corticosteroids.