North American Society for Pediatric Gastroenterology, Hepatology and Nutrition and the Society for Pediatric Radiology Joint Position Paper on Noninvasive Imaging of Pediatric Pancreatitis
Literature Summary and Recommendations
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal's Web site (www.jpgn.org).
This article has been developed as a Journal CME and MOC Part II Activity by NASPGHAN. Visit https://learnonline.naspghan.org/ to view instructions, documentation, and the complete necessary steps to receive CME and MOC credits for reading this article.
Disclaimers: The NASPGHAN practice guidelines and position papers are evidence-based decision-making tools for managing health conditions. They are authorized by the NASPGHAN Executive Council, peer reviewed, and periodically updated. They are not to be construed as standards of care and should not be construed as establishing a legal standard of care or as encouraging, advocating, requiring, or discouraging any particular treatment. All decisions regarding the care of a patient should be made by the health care team, patient, and family in consideration of all aspects of the individual patient's specific medical circumstances. While NASPGHAN makes every effort to present accurate and reliable information, these guidelines are provided “as is” without any warranty of accuracy, reliability, or otherwise, either express or implied. NASPGHAN does not guarantee, warrant, or endorse the products or services of any firm, organization, or person. Neither NASPGHAN nor its officers, directors, members, employees, or agents will be liable for any loss, damage, or claim with respect to any liabilities, including direct, special, indirect, nor consequential damages, incurred in connection with the guidelines or reliance on the information presented. The Society for Pediatric Radiology (SPR) endorses the content of, and recommendations made within, this white paper, which are guidelines for the diagnosis and imaging of health conditions. The recommendations have been vetted by the Abdominal Imaging Committee of the SPR, which is constituted of pediatric radiologists with expertise in abdominal imaging in the pediatric patient. The recommendations are neither intended nor should they be used, to establish a legal standard of care or as encouraging, advocating, requiring, or discouraging any particular treatment. An approach that differs from the recommendations in this white paper does not necessarily imply that the approach is below the standard of care. All decisions regarding the care of a patient should be made by the health care team, patient, and family in consideration of all aspects of the individual patient's specific medical circumstances. A practitioner may responsibly adopt a different course of action based on such circumstances.
A.T.T. has received funding from Canon Medical systems and in-kind research support from ChiRho Clin, Inc. S.Z.H., owns equity in Prevcon. The other authors report no conflicts of interest.
ABSTRACT
The reported incidence of pediatric pancreatitis is increasing. Noninvasive imaging, including ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI), play important roles in the diagnosis, staging, follow-up, and management of pancreatitis in children. In this position paper, generated by members of the Pancreas Committee of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the Abdominal Imaging Committee of The Society for Pediatric Radiology (SPR), we review the roles of noninvasive imaging in pediatric acute, acute recurrent, and chronic pancreatitis. We discuss available evidence related to noninvasive imaging, highlighting evidence specific to pediatric populations, and we make joint recommendations for use of noninvasive imaging. Further, we highlight the need for research to define the performance and role of noninvasive imaging in pediatric pancreatitis.
An infographic is available for this article at: http://links.lww.com/MPG/C9.
What Is Known
- The reported incidence of pancreatitis in children is increasing, currently estimated to be 1 in 10,000 for acute pancreatitis and 2 in 100,000 for chronic pancreatitis.
- The roles of imaging in acute pancreatitis are to: identify findings of acute pancreatitis at diagnosis; assess for local complications; identify potential etiologies of acute pancreatitis; monitor the evolution of local complications; and plan and guide interventions.
- The roles of imaging in chronic pancreatitis are to: contribute to/establish the initial diagnosis of chronic pancreatitis; stage and monitor disease, including complications; assess for superimposed acute pancreatitis; identify potential etiologies of chronic pancreatitis; characterize secretory (exocrine) function; and plan for surgical intervention.
What Is New
- Little information is available regarding the optimal imaging strategy for pediatric pancreatitis.
- Current methods to prognosticate and predict pancreatitis severity and disease progression are inadequate.
- It is currently not possible to identify minimal change or early chronic pancreatitis in pediatric patients.
Recognition and the reported incidence of pancreatitis in children are increasing, with incidence currently estimated to be 1 in 10,000 for acute pancreatitis (AP) and 2 in 100,000 for chronic pancreatitis (CP) (1.-3.). Given that data related to imaging of pediatric pancreatitis are sparse, pediatric recommendations for imaging are largely based on adult data. The purposes of this document, which is jointly endorsed by the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and The Society for Pediatric Radiology (SPR), are to: summarize existing literature and experience regarding imaging of pediatric AP and CP; provide recommendations for the role of imaging in the diagnosis and management of pediatric pancreatitis; and identify knowledge gaps and areas for future study. This document focuses on noninvasive imaging and will not emphasize endoscopic ultrasound (EUS) or endoscopic retrograde cholangiopancreatography (ERCP), which also contribute to the diagnosis and management of these diseases (4.-6.).
METHODS
This document was generated through collaboration between members of the NASPGHAN Pancreas Committee and The SPR Abdominal Imaging Committee with concept approval by the boards of both organizations before generation of the document. Members of each committee volunteered to participate with 8 gastroenterologists and 3 radiologists contributing to drafting the document, grading available evidence, and generating and voting on recommendations. One radiologist (S.A.A., J.H.S., or A.T.T.) and 2 gastroenterologists (A.J.F, J.A.M-F., J.A.M., V.D.M., K.R.P., or U.S.) were primarily responsible for each section of the text. One radiologist (A.T.T.) and 1 gastroenterologist (M.A-E-H) led the project, providing global oversight and structure. Although a systematic literature review was not performed, contributing authors reviewed pertinent literature through April 2019 for their respective section(s). Project leads confirmed inclusion of relevant pediatric articles by performing a PubMed search in July 2019 for the following MeSH terms: “Pancreatitis/diagnostic imaging,” “Tomography, X-Ray Computed/methods,” “Ultrasonography/methods,” “Magnetic Resonance Imaging/methods,” limited by the PubMed “Child: birth-18 years” filter.
On the basis of a complete draft of the document, project leads generated or extracted specific recommendations from the text. Project leads also assigned classifications for the recommendations based on a modified version of the GRADE system, applying the criteria for studies on diagnostic accuracy (7.). Grades incorporated a score of recommendation strength (1 = Strong, 2 = Weak) and evidence quality (A = high quality, B = moderate quality, C = low quality). The criteria for studies of diagnostic accuracy consider cross sectional or cohort studies with comparison to an appropriate reference standard to reflect high-quality evidence in lieu of randomized controlled trials. Of note, the process of applying the modified GRADE system for this document did not utilize independent evaluators to review the recommendations and supporting evidence, and a formal GRADE report of the literature was not created.
Grades assigned by project leads were preliminarily affirmed by the authors of each manuscript section. The full draft manuscript was then reviewed and approved by all members of the project team who provided suggested edits and commented on the proposed recommendations and GRADE classifications. All members of the project team had reviewed the modified GRADE methodology before affirming and commenting on GRADE classifications. After final edits, all members of the project team voted on the recommendations via a survey built in REDCap, assigning a 5-point Likert score (5, strongly agree; 4, agree; 3, neutral; 2, disagree; 1, strongly disagree) to each recommendation (8., 9.). Voting results were submitted to a research coordinator at Cincinnati Children's Hospital who was not involved in generation of this document or the recommendations. A priori, a minimum 75% frequency of “strongly agree” or “agree” ratings was defined as the threshold required to be considered consensus. Other references that have used this system include a position paper on Nutritional Considerations in Pediatric Pancreatitis by Abu-El-Haija et al (10.).
Although high-quality literature to support and direct the use of specific imaging modalities in pediatric pancreatitis is limited, recommendations are based on expert opinion informed by adult literature and the pediatric literature that exists. Comments are included wherever needed to explain recommendations.
Subsequent to study team affirmation of recommendations, a final version of the document was submitted to committees of NASPGHAN and The SPR for review and comment. Comments provided by the reviewing organizations were reviewed by the project leads and incorporated as appropriate in the final document, which was approved by the NASPGHAN Council and The SPR Board.
BACKGROUND
Acute Pancreatitis
The diagnosis of AP in children has been defined as the presence of at least 2 of the following: abdominal symptoms consistent with AP; serum amylase or lipase values ≥3 times the upper normal level; and imaging findings consistent with AP (11.). Biliary causes, anatomic causes, and genetic pancreatitis represent the most common etiologies of AP in children, but up to 20% of cases remain idiopathic (Table 1) (12.-14.). Severity staging of AP has only recently been defined for pediatrics (Table 2), and is structured to classify which children are most at risk of complicated courses (15., 16.). Mild AP is defined as AP without organ failure or local or systemic complication, and usually resolves within 1 week. Moderately severe AP is defined as either the presence of transient (≤48 hours) organ failure, the presence of local complications, or the exacerbation of comorbid disease. Severe AP (SAP) is defined by organ failure lasting longer than 48 hours. Moderately severe or SAP have been reported to occur in approximately 13% to 30% of children with AP (15., 17.). To date, pediatric-specific risk factors for SAP remain unclear.
In general terms, the roles of imaging in AP are to: identify findings of AP at diagnosis; assess for local complications; identify potential etiologies of AP; monitor the evolution of local complications, and plan and guide interventions. Largely on the basis of the lack of ionizing radiation, transabdominal ultrasound is favored as the initial imaging modality for the diagnosis of AP in children, whereas computed tomography (CT) and/or magnetic resonance imaging (MRI) are reserved for more complicated cases or to answer specific clinical questions (4.).
Acute Recurrent Pancreatitis
Acute recurrent pancreatitis (ARP) has been defined as 2 distinct attacks of AP with more than 1 month pain-free interval between attacks, or with normalization of pancreatic enzymes and complete resolution of pain regardless of interval between episodes (11.). ARP is believed to develop in 15% to 35% of pediatric patients who suffer from an initial event of AP (3., 17., 18.). In 1 study, the majority of patients who developed ARP had a second attack within 5 months after their initial episode (19.). Genetic mutations represent the most common risk factor for the development of ARP with almost 50% of patients carrying a mutation in CFTR, PRSS1, CTRC or SPINK1 in 1 series. Additionally, approximately 1/3 had a pancreatic duct obstructive risk factor, such as pancreas divisum (3., 18.). It should be noted, however, that pancreas divisum alone does not necessarily cause pancreatitis.
In general terms, the roles of imaging in ARP are to: confirm attacks of AP; assess for local complications; identify potential etiologies of ARP; monitor the evolution of complications; plan and guide interventions; and assess for imaging findings suggestive of progression to CP. There are no robust data to define an optimal imaging modality or strategy for ARP though most favor MRI because of its ability to optimally assess both parenchyma and duct (11.).
Chronic Pancreatitis
CP results from progressive inflammation that results in fibrotic replacement of pancreatic parenchyma, and eventually, exocrine and endocrine dysfunction. CP in children has been defined as imaging findings of CP combined with at least 1 of the following: abdominal pain consistent with pancreatic origin; exocrine pancreatic insufficiency (EPI); or pancreatic endocrine insufficiency (11.). Less frequently, surgical or biopsy specimens consistent with CP are obtained. In children, genetic factors (seen in up to 73% of patients) are the most prevalent etiology of CP followed by obstructive causes, similar to those seen in ARP (3., 18.).
In general terms, the roles of imaging in CP are to: contribute to/establish the initial diagnosis of CP; stage and monitor disease, including complications; assess for superimposed AP; identify potential etiologies of CP; identify findings that might herald endocrine or exocrine dysfunction; characterize secretory (exocrine) function; and plan for intervention. Although findings of CP may be identified on ultrasound or CT, MRI/magnetic resonance cholangiopancreatography (MRI/MRCP) is favored for the diagnosis and characterization of CP given its superiority in visualizing parenchymal and duct changes (20.).
IMAGING TECHNIQUES AND GENERALITIES
Transabdominal Ultrasound
Current consensus recommendations favor transabdominal ultrasound as the initial imaging examination to evaluate suspected AP as it is widely available, the examination can be performed with minimal patient preparation, and the examination does not use sedation, contrast material, or ionizing radiation (4., 21.-23.). The major advantages of ultrasound over other imaging modalities are availability and portability. The latter allows bedside imaging of critically ill and difficult-to-transport patients. Ultrasound can, however, be limited in patients with large body habitus, or with excessive bowel gas. In addition, ultrasound may underestimate or poorly delineate the extent of extrapancreatic sequelae of AP (24.).
A right upper quadrant ultrasound examination typically does not provide full imaging of the pancreas and will not evaluate other areas of the abdomen for potential pancreatitis complications. A complete abdominal ultrasound allows evaluation of both upper quadrants and the pancreas, and may include assessment of the lower quadrants for fluid.
Ultrasound examinations are ideally performed fasting (≥4 hours) to reduce bowel gas that can obscure the pancreas and to distend the gallbladder. When the patient is able, drinking water immediately prior to the examination to distend the stomach with fluid and displace gastric air may provide an improved acoustic window for imaging the pancreas. A complete ultrasound examination should assess pancreatic size, contour, echogenicity, pancreatic duct diameter (and for duct filling defects), and should assess for peripancreatic edema and pancreatic or peripancreatic fluid collections (25.). In addition, the gallbladder should be assessed for calculi (an etiology of pancreatitis), and the biliary tree should be assessed for dilation and calculi (4., 22., 26.). Color Doppler along with gray-scale imaging can evaluate the peripancreatic vascular structures for complications, such as splenic vein or portal vein thrombosis and can assess vascularity of the pancreas.
Contrast-enhanced ultrasound (CEUS), which involves the intravenous administration of contrast material consisting of microbubbles, has been explored for assessment of both AP and CP but is not yet accepted as standard of care, so its role in pediatric pancreatitis remains to be defined (27., 28.).
Computed Tomography
The major advantage of CT versus other noninvasive imaging modalities is that the examinations are short and can generally be achieved without sedation or anesthesia. For this reason, CT is the modality of choice for acute assessment of traumatic injury of the pancreas. Body habitus and air-filled bowel loops are not limiting factors for CT (compared with ultrasound).
CT for pediatric pancreatitis should utilize intravenous (IV) contrast material, which optimizes assessment of the solid organs and vasculature. CT with IV contrast material can be performed as a single (arterial or portal venous phase) or multiphase examination. When performed as a single-phase examination for pancreatic indications, portal venous phase imaging is most common. Intravenous iodinated contrast material carries a very low risk of allergic-like reactions. Risk of exacerbation of renal dysfunction in children with estimated glomerular filtration rate less than 60 mL /min/1.73 m2 should be balanced with the potential benefit of the examination (29.).
Use of oral contrast material for CT in pancreatitis is inconsistent; specific recommendations do not exist for children. In adults, oral water is recommended for imaging of pancreatitis (20.). A potential benefit of positive oral contrast, which is high in attenuation, is distinguishing fluid collections from bowel loops but oral contrast material can be difficult for pediatric or acutely ill patients to consume and prolongs the preparatory phase of the CT examination.
Limiting CT to the abdomen only is discouraged. CT limited to the abdomen allows assessment of the pancreas, adjacent vessels, and surrounding structures but does not allow assessment for extension of complications (eg, fluid collections) into the lower abdomen and pelvis. CT of the abdomen and pelvis allows assessment of the pancreas and the full extent of associated complications.
Magnetic Resonance Imaging and Magnetic Resonance Cholangiopancreatography
MRI has the best soft tissue contrast of available cross-sectional imaging modalities. This soft tissue contrast optimizes parenchymal characterization and visualization and characterization of ducts and fluid collections. MRCP, which is sometimes arbitrarily distinguished from other MRI examinations, simply reflects a type of MRI sequence that utilizes heavy T2-weighting to accentuate fluid-filled structures including the pancreatic and biliary ducts. Like CT, body habitus and air-filled bowel loops are not limitations for MRI. Potential need for sedation or general anesthesia in the pediatric population to accomplish relatively long examinations is the primary disadvantage of MRI.
Given its superior soft tissue contrast, MRI is the preferred imaging modality for ARP, CP, and autoimmune pancreatitis where characterization of both the pancreatic parenchyma and duct is important (30.). Protocol guidelines exist for adults but have not been formalized for children (20.). An MRCP sequence should be included in most pancreatic MRI examinations. IV gadolinium-based contrast material can be useful for characterization of the vasculature and for diagnosis of autoimmune pancreatitis but is not required for routine assessment of CP. IV contrast material is not required to acquire an MRCP sequence and hepatobiliary contrast material can compromise MRCP sequences.
Use of intravenous secretin as an adjunctive medication may improve visualization of the pancreatic duct and allows assessment of exocrine function (31.-39.). According to adult studies, secretin improves visualization of the pancreatic duct by MRCP with a higher diagnostic accuracy of detecting pancreas divisum (34.-37.). The reported sensitivity and specificity of diagnosing pancreas divisum with secretin-MRCP falls in the range of 73% to 100% and 97% to 100%, respectively (40.).
Timing of Imaging
Optimal timing of imaging, particularly relative to an attack of AP, depends on the unique patient situation. Few studies have published pediatric data specific to this question, but adult data suggest that imaging within the first 48 hours of an attack of AP infrequently alters management and poorly predicts the severity of organ failure (41.). However, if imaging is necessary to make a diagnosis or to manage a patient, it should not be deferred. In patients with suspected or known CP where imaging confirmation of findings of CP is needed, imaging when the patient does not have superimposed AP is preferred to prevent obscuration of findings by acute inflammation.
General Imaging Technique Summary Statements and Recommendations
-
CT should be performed with intravenous contrast material as a single portal venous phase examination unless specific arterial detail is needed (Table 3).
GRADE: 1C, agreement 100% (11/11; 6, strongly agree; 5, agree; average score = 4.5)
-
When imaging with MRI, intravenous contrast material is not always needed but contributes to the diagnosis and definition of necrosis, assessment of the vasculature, and the diagnosis of autoimmune pancreatitis.
GRADE: 2C, agreement 100% (11/11; 3, strongly agree; 8, agree; average score = 4.3)
IMAGING OF ACUTE PANCREATITIS
Purpose/ Indication/Rationale for Imaging in Acute Pancreatitis
Imaging in the context of suspected or known AP serves the multiple purposes previously described. At diagnosis, it is particularly important to identify gallstones or biliary obstruction (usually because of choledocholithiasis) as etiologies of AP. These entities can be urgently addressed with endoscopic and/or surgical intervention (4., 42.-44.). Identification of local complications of AP including necrosis, acute fluid collections, venous stenosis/thrombosis or arterial aneurysms, and hemorrhage has relevance for clinical staging of attack severity (15., 45.). In adults, severity scoring can help prognosticate and triage appropriate management but there is currently no consensus imaging severity staging/scoring system in children. In a clinical report, the Pancreas Committee of NASPGHAN has proposed stratifying AP in children as mild, moderately severe, or severe utilizing a combination of clinical and imaging criteria (Table 2) (15.).
The most common complication of AP is the development of acute peripancreatic fluid collections and pseudocysts (13%–15%) (46., 47.). The frequency of these fluid collections secondary to AP has been reported in pediatric studies to be between 8% and 41% (48.). Pancreatic and peripancreatic necrosis (sterile or infected) occur less commonly (47., 49.). As an attack of AP progresses, imaging serves to assess the evolution and maturity of fluid collections (necrotic or simple) to help define the timing of interventions. Fluid collections generally need to have a well-defined wall to be amenable to intervention, particularly endoscopic intervention.
Imaging Findings of Acute Pancreatitis
Two forms of AP are distinguishable by imaging: interstitial edematous and necrotizing pancreatitis, with the former more common (Fig. 1). The imaging features of acute interstitial pancreatitis are similar on ultrasound, CT, and MRI (Table 4). In the very early stages of AP, ultrasound and CT may show no abnormal findings, as laboratory abnormalities often precede imaging findings of AP (22.). This does not, however, seem to apply to MRI, which has higher soft tissue contrast resolution (50.).
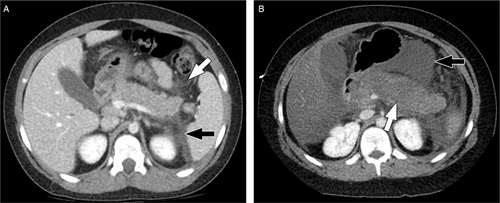
Examples of interstitial edematous acute pancreatitis and necrotizing acute pancreatitis in 2 different patients. (A) Axial image from a CT performed with intravenous contrast material in a 10-year-old boy with interstitial edematous pancreas shows a swollen but homogenously enhancing pancreas with peripancreatic stranding (white arrow) and with an acute peripancreatic fluid collection. (B) Axial image from a CT performed with intravenous contrast material in a 10-year-old boy with necrotizing pancreatitis shows a swollen pancreas with a large area of absent enhancement (white arrow) indicative of necrosis. There is more normal enhancement of the pancreatic tail. An acute necrotic collection is also present in the lesser sac (black arrow). CT = computed tomography.
Findings of necrotizing pancreatitis depend on the stage of necrosis. Early in the course of necrotizing pancreatitis, there is decreased or absent vascularity/perfusion of the gland with hypoenhancement following contrast administration. As necrosis evolves, the gland and peripancreatic tissues may be replaced by necrotic collections. By ultrasound and MRI, these collections will contain debris. By CT, the collections may deceptively appear simpler. Superinfection of these collections may occur, with air in the collection(s) being a specific, but not sensitive, finding (50.).
Fluid collections associated with AP have specific definitions, which were updated in the 2012 Revised Atlanta Classification (Table 5) (41.). The definitions apply to adults but have been extrapolated to children. One important nuance of the Revised Atlanta Criteria is that if acute necrotic collections organize, these are by definition walled off necrosis (not pseudocysts) regardless of how simple they appear. Pseudocysts occur only in the context of acute interstitial edematous pancreatitis, or, rarely, in the case of disconnected duct, due to prior necrosis or intervention.
Diagnostic Performance of Imaging Modalities in Acute Pancreatitis
Little data are available on the diagnostic performance of ultrasound, CT, and MRI for the assessment of AP in children.
Transabdominal Ultrasound
Pediatric-specific data regarding the ability of transabdominal ultrasound to detect gallstones as an etiology for AP are not available. Adult data have shown ultrasound to be approximately 99% sensitive for gallstones in the gallbladder (51.).
The sensitivity of abdominal ultrasound in detecting AP, based on adult data, is reported to be as high as 79% (52.). The sensitivity of ultrasound in diagnosing AP in children has not been well-defined. In a study of 112 children with AP, 75% (n = 84) had an ultrasound performed and, ultrasound was only 52% (95% confidence interval: 41%–63%) sensitive for AP diagnosed based on symptoms and serum enzymes (53.). Prior studies have shown widely variable performance of ultrasound for diagnosis of AP. Benifla and Weizman (46.) reported a diagnosis of AP by ultrasound in 81% of 589 children, but Coffey et al (54.) reported ultrasound findings of AP in only 23% of 77 patients with AP diagnosed by elevated enzymes.
Chao et al and Siegel et al reported the most useful indicator of AP to be a dilated pancreatic duct. The sensitivity and specificity of a dilated pancreatic duct in children on ultrasound range between 78% to 83% and 87% to 92%, respectively, with positive-predictive value (PPV) of 86–91%, and negative-predictive value (NPV) of 75% to 84% (55., 56.).
Computed Tomography
On the basis of adult literature, CT with IV contrast material is considered the imaging reference standard for AP (26., 57., 58.). IV contrast material allows evaluation for necrosis, based on absent parenchymal enhancement, and optimizes identification and assessment of intra- or extra-pancreatic fluid collections. IV contrast material also allows evaluation of the peripancreatic vasculature to ensure patency and assess for pseudoaneurysm formation (22.). Other than for specific assessment of the arteries, adult data suggest that a single-phase portal venous phase examination is sufficient for assessment of AP (59.).
Adult data suggest CT is more sensitive than ultrasound for AP, particularly for severe pancreatitis and acute necrosis (22.). Diagnostic performance has not been specifically defined for children; however, Coffey et al (54.) reported CT to show findings of AP in 62% of 42 patients with AP based on positive enzymes (vs 23% for ultrasound).
Compared with ultrasound, CT with intravenous contrast material provides improved characterization of the location and extent of fluid collections and abscesses, integrity of the splenic vein and portal system, and presence of parenchymal necrosis (43., 52., 60., 61.). A small surgical series (n = 13) in adult patients showed CT to have 100% per patient sensitivity for necrosis (62.). Of note, however, on a per-segment basis, CT was only approximately 64% sensitive, missing additional sites of necrosis in several patients (62.). When infected necrosis is present, gas pockets are more readily visible on CT (Fig. 2) than on ultrasound. CT may, however, underestimate the complexity of fluid collections relative to ultrasound or MRI (Fig. 3) (63., 64.). For serial imaging of children with AP, the ionizing radiation associated with CT should be considered when selecting an imaging modality for follow-up.
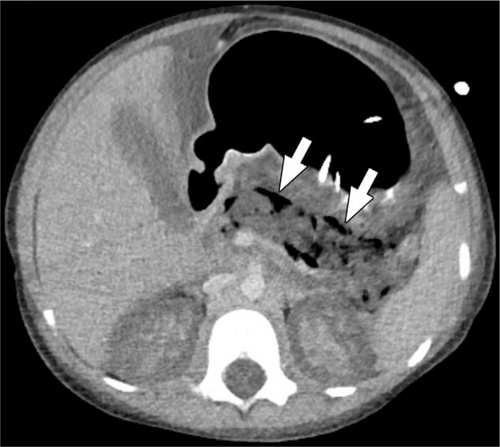
A 5-year-old girl with infected pancreatic necrosis. Axial image from a CT performed with intravenous contrast material shows gas locules in the nonenhancing pancreas (arrows). No normal pancreas is visible and acute fluid is present in the abdomen. The patient also had acute renal cortical necrosis accounting for absent enhancement of the renal cortex. CT = computed tomography.
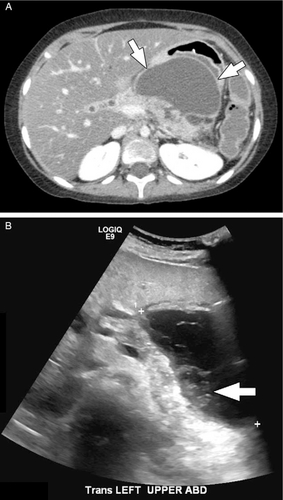
A 10-year-old girl with walled off pancreatic and peripancreatic necrosis. (A) Axial image from a CT performed with intravenous contrast material shows a walled off collection involving the body of the pancreas and the peripancreatic tissues in the lesser sac (arrows). Note how the content of the collection appears relatively simple by CT. (B) Transverse image from a transabdominal ultrasound performed the next day shows the same collection but with layering solid/semi-solid debris (arrow). CT = computed tomography.
CT severity scoring indices (eg, CT severity index [CTSI], Balthazar score) were established in adult populations, but more recently have been applied to children (15., 65.). Similar to adult studies, the CTSI has been shown to be a better predictor of the severity of AP compared with clinical scores in children (65.-67.). A recent pediatric study applying the CTSI scores in 211 children with AP found the sensitivity and specificity of the CTSI in predicting a severe course of AP to be 81% and 76%, respectively, with positive-predictive and negative-predictive values of 62% and 90%, respectively (68.). In this same pediatric cohort, the presence of necrosis in AP was associated with higher rate of major complications (68.).
Magnetic Resonance Imaging/Magnetic Resonance Cholangiopancreatography
Studies are lacking regarding the diagnostic performance of MRI in diagnosing AP in children, particularly compared with other imaging modalities. MRI can be used for assessment of AP, but because of the need for long periods of holding still may not be suitable for children, especially if critically ill. MRI may contribute to confirmation of an attack of AP or to identification/confirmation of acute duct obstruction (see below) but pancreatic edema because of an acute episode of pancreatitis can obscure pancreatic duct anomalies that may be relevant to the cause of pancreatitis.
The greater soft tissue contrast of MRI (vs CT) is advantageous when assessing the pancreatic parenchyma and biliary and pancreatic ducts and when characterizing fluid collections (22.). Adult data suggest that MRI is more sensitive than CT for findings of AP including edema and hemorrhage with up to 15% to 30% of patients with a normal CT showing findings of AP on MRI (69.-71.). Adult data have also shown the diagnostic performance of MRI to be as good as CT for pancreatic necrosis (72.).
MRI, particularly MRCP, has also been shown to be more sensitive than CT for biliary etiologies of pancreatitis (20.). Specifically, in adults, MRCP has up to 100% sensitivity for pancreatic and biliary duct stones greater than 3 mm in size (73.). MRCP can be particularly useful in the evaluation of choledocholithiasis when biliary duct dilatation is found on ultrasound without stone(s) visible in the duct(s) (74.-78.). Structural abnormalities of the pancreatic duct and parenchyma, such as pancreas divisum and an abnormal union of the pancreaticobiliary junction with a long common channel have also been associated with acute pancreatitis (79.), and MRCP is the optimal noninvasive imaging modality for these entities.
MRI can help distinguish acute necrotic collections from acute peripancreatic fluid collections by identifying and characterizing the internal content of these collections (50.). MRI is also superior to CT in detecting hemorrhage, which can be a complication of necrosis (80.) (Fig. 4). In clinical practice, MRI is often used for assessment and monitoring of late complications of AP, such as fluid collections, to time and guide therapeutic interventions (22., 81.). This, in part, not only capitalizes on the high soft tissue contrast but also on the fact that MRI does not involve exposure to ionizing radiation, and is thus, more acceptable for serial examinations.
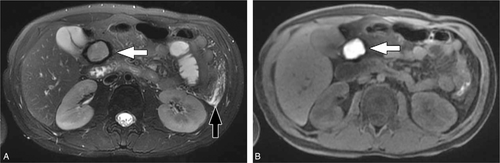
A 9-year-old girl with acute on chronic pancreatitis with a hemorrhagic peripancreatic collection. (A) Axial T2-weighted, fat-saturated MR image shows a fluid collection above the head of the pancreas and adjacent to the gallbladder (white arrow). The collection shows peripheral susceptibility artifact related to evolving blood products. Edema in the left hemiabdomen (black arrow) reflects acute pancreatitis. (B) Axial T1-weighted, fat saturated MR image shows the content of the collection (white arrow) to be T1-weighted hyperintense compatible with blood products.
Acute Pancreatitis Summary Statements and Recommendations
Initial Diagnosis
-
Transabdominal ultrasound is recommended as a first-line noninvasive imaging modality for suspected AP (Table 3).
- This recommendation reflects the availability and portability of ultrasound and the role of ultrasound in identifying biliary causes of AP.
- Note: A negative ultrasound does not exclude AP (low-to-moderate sensitivity).
GRADE 1B, agreement 91% (10/11; 9 strongly agree, 1 agree, 1 neutral, average score = 4.7)
-
If ultrasound is negative for AP and an imaging diagnosis of AP is needed, either CT or MRI is recommended.
- This recommendation reflects the only moderate sensitivity of ultrasound and the greater sensitivity of CT and MRI.
GRADE 1B, agreement 100% (11/11, 7 strongly agree, 4 agree, average score = 4.6)
Suspected Complications of Acute Pancreatitis
-
CT or MRI is recommended for identification and assessment of known or suspected complications of AP.
- Note: CT has the potential to underestimate the complexity of fluid collections.
GRADE 1C, agreement 91% (10/11, 6 strongly agree, 4 agree, 1 neutral, average score = 4.5)
Follow-up of Known Complications, With or Without Planning for Intervention
-
Ultrasound can be used to follow known AP fluid collections for resolution or progression (changes in size).
GRADE 2C, agreement 82% (9/11, 6 strongly agree, 3 agree, 1 neutral, 1 disagree, average score = 4.3)
-
CT or MRI should be used to characterize the degree of organization of collections before intervention.
- Note: CT has the potential to underestimate the complexity of fluid collections
GRADE 1C, agreement 100% (11/11, 6 strongly agree, 5 agree, average score = 4.5)
Acute Recurrent Pancreatitis Summary Statements and Recommendations
The recommendations for AP above also apply to assessment of repeated episodes of AP in the child with ARP.
-
MRI is recommended to identify structural or obstructive causes for ARP.
- This recommendation reflects the high soft tissue contrast and ability to assess the pancreatic and biliary ducts afforded by MRI.
GRADE 1B, agreement 100% (11/11, 9 strongly agree, 2 agree, average score = 4.8)
Serial Follow-up for Progression to Chronic Pancreatitis
-
When clinically indicated, MRI is recommended to follow children with ARP and to assess for progression to CP.
- This recommendation reflects the strengths of MRI in monitoring changes in both parenchyma and duct. This also reflects the lack of ionizing radiation associated with MRI
- Note: The need for, and frequency of, serial follow-up in children with ARP as a means for assessing for progression to CP has not been defined.
GRADE 1C, agreement 100% (11/11, 7 strongly agree, 4 agree, average score = 4.6)
-
In a child who requires sedation for imaging, it is reasonable to alternate MRI with ultrasound or CT for serial monitoring of ARP.
GRADE 2C, agreement 82% (9/11, 3 strongly agree, 6 agree, 1 neutral, 1 disagree, average score = 4)
IMAGING OF CHRONIC PANCREATITIS
Purpose/Indication/Rationale for Imaging in Chronic Pancreatitis
Imaging in CP serves multiple purposes. The dominant role of imaging at the time of diagnosis is to identify findings of CP that can be leveraged in combination with other criteria (clinical and histologic whenever available) to make the diagnosis of CP (11.). Accurate diagnosis of CP will lead to altered management as it is now understood that children diagnosed with CP require specific clinical and laboratory follow-up as well as nutritional management (10.).
In addition to diagnosing CP (11.), there are specific imaging features that are of interest to the clinician in the child with known or suspected CP. Signs of acute inflammation or complications that may require therapeutic intervention/drainage, and, certainly, suspected pancreatic masses are relevant to diagnosis and management. One relatively rare but increasingly recognized and important differential diagnosis of a pancreatic “mass” is autoimmune pancreatitis (AIP) (82., 83.). AIP is highly responsive to steroid therapy, and hence its diagnosis will lead to a medical therapeutic intervention. Focal, segmental, or global enlargement of the pancreas with loss of the normal contour can be evidence of AIP, especially with delayed enhancement and the presence of a capsule-like rim, which is uncommon but very specific (Fig. 5) (84., 85.). Focal pancreatic enlargement should be differentiated from tumors as management is completely different (82.).
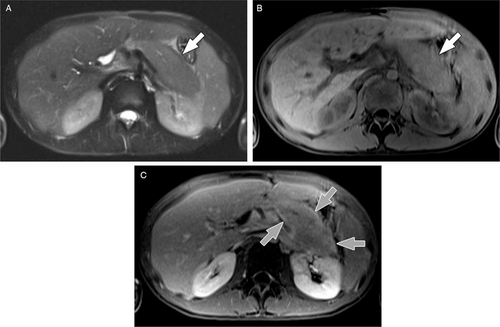
A 15-year-old boy with autoimmune pancreatitis. (A) Axial T2-weighted, fat-saturated MR image shows a diffusely enlarged, mildly T2-weighted hyperintense pancreas (arrow). (B) Axial T1-weighted, fat-saturated MR image shows a diffusely enlarged, T1-weighted hypointense pancreas (arrow). (C) Axial T1-weighted, fat-saturated MR image obtained 5 minutes after administration of intravenous contrast material shows a thin rim of enhancement surrounding the enlarged pancreas (grey arrows). CT = computed tomography; MR = magnetic resonance.
The presence of pancreatic atrophy is helpful in interpretation of biochemical markers of CP and estimating the clinical risk for pancreatic exocrine and endocrine dysfunction. Studies correlating imaging findings and function are, however, lacking (86.). The presence of parenchymal calcifications is a major feature of most criteria (generally adult-focused) for CP, though anecdotally, calcification appears to be less common in pediatrics.
Characterization of biliary and pancreatic duct anatomy is also important, particularly with regard to diagnostic and therapeutic decisions. Congenital anomalies, such as pancreaticobiliary maljunction and pancreas divisum can contribute to the underlying pancreatitis (87., 88.). Duct filling defects/calcifications can be both diagnostic criteria and therapeutic targets. An irregular narrow main pancreatic duct without marked upstream dilation and with smooth tapering of the common bile duct can be suggestive of AIP in the appropriate clinical context (89., 90.).
Imaging plays a critical role in surgical planning for patients with CP. Decompressing surgeries, such as lateral pancreaticojejunostomies, can be utilized in cases of very dilated pancreatic ducts, with or without the presence of intraductal stones (91.). Pancreaticoduodenectomy-type procedures are considered particularly with pancreatic head pathologies (92.). Total pancreatectomy-islet cell autotransplantation (TP-IAT) surgery is considered not only based on symptoms and the underlying etiology of CP but also based on the overall imaging appearance of pancreas, including perceived capacity to retrieve a critical mass of islet cells (93.). As such, characterization of duct abnormalities and the extent of parenchymal change are important.
Imaging Findings of Chronic Pancreatitis
Imaging features of advanced CP have been well characterized and described in the adult literature (Table 6) (21.). Imaging findings of advanced CP specific to pediatrics have not yet been defined, and findings of early or probable CP, when intervention might be attempted to slow disease progression, have not been well-defined for any population (94.). Features that may herald “early” CP include a few ectatic duct side branches, parenchymal volume loss, or mild T1 signal changes but these remain to be validated (95.).
Currently, no standard imaging classification system exists for CP in children. The Cambridge classification, based on pancreatic duct findings on ERCP in adult patients (Table 7), has been adapted to MRCP in adults but this classification does not incorporate parenchymal changes of CP, and this classification has not been validated in children (96., 97.). The Cambridge classification defines CP based on the number of abnormal side branches, cavities, filling defects, or obstruction visualized (20.). The M-ANNHEIM Classification for adult CP published in 2007 relied partially on the Cambridge classification as well as other imaging findings at transabdominal ultrasound, CT, EUS, and/or MRI but has not been validated in children (Table 8) (98.). Recently, the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC) proposed new reporting standards for CT and MRCP in adults with CP (20.). These remain to be validated in adults, and their applicability to pediatrics is unknown.
Given the expected growth and maturation of the pancreas during childhood, knowledge of normal anatomy and gland and duct size based on age is critical to define abnormalities (eg, atrophy and duct dilation) suggestive of CP. Normative values for gland thickness exist for ultrasound and CT (56., 99.). Based on no significant difference between CT measurements and measurements previously reported at ultrasound, Trout et al (99.) suggested that thickness values could likely be extrapolated to MRI, though this remains to be confirmed. Normative values for pancreatic duct diameter also exist for ultrasound and MRI (Table 9) (56., 99.). Although normal values exist, cut-off values for diagnosis of CP have not been defined.
Diagnostic Performance of Imaging Modalities in Chronic Pancreatitis
Data specific to the diagnostic performance of specific imaging modalities for pediatric CP are not available. A meta-analysis of adult literature concluded ultrasound, MRI, and CT all have high diagnostic sensitivity and specificity for CP without significant differences (100.). Reference standards varied across the included studies. Specifically, in 3460 adults, estimated sensitivities were 67% (95% CI: 53%--78%) for ultrasound, 78% (95% CI: 69%--85%) for MRI, and 75% (95% CI: 66%--83%) for CT. Estimates of specificity were 98% (95% CI: 89%--100%) for ultrasound, 96% (95% CI: 90%--98%) for MRI, and 91% (95% CI: 81%--96%) for CT.
Transabdominal Ultrasound
When planning for surgical procedures, ultrasound provides insufficient anatomic assessment, particularly with regard to vascular variants relevant to surgical approach (eg, right hepatic artery or accessory right hepatic artery arising from the superior mesenteric artery).
Computed Tomography
Use of IV contrast material is recommended when performing CT for CP. IV contrast material allows optimal assessment of the pancreatic parenchyma and allows evaluation of the peripancreatic vessels for patency. For adults, multiphase protocols that include an unenhanced phase, parenchymal/arterial phase, and portal venous phase have been recommended (20.). No such recommendations exist for pediatrics but given the relative infrequency of calcifications in pediatric pancreatitis, an unenhanced phase is likely unnecessary. As with AP, a parenchymal/arterial phase can be useful if clinical questions relate to the arteries but a single portal venous phase examination is generally sufficient to characterize CP in children.
CT with IV contrast material provides excellent assessment of the pancreatic parenchyma, allowing identification of features of ARP and CP, particularly calcifications and pancreatic atrophy. CT can also identify congenital anomalies, such as annular pancreas and can assess for superimposed acute pancreatitis and complications of pancreatitis, including established vascular collaterals because of chronic/established thrombosis. CT (and MRI) outperform ultrasound to define vascular anatomy relevant to surgical planning. CT is, however, limited by suboptimal visualization of the pancreatic and biliary ducts.
Magnetic Resonance Imaging/Magnetic Resonance Cholangiopancreatography
MRI and MRCP have the benefits of providing information on both parenchymal and duct changes of CP but are limited in their ability to visualize calcifications. Adult and pediatric data suggest that the sensitivity of MRCP to detect pancreatic duct abnormalities may be improved by the administration of secretin (38.). Theoretically secretin distends the pancreatic duct and may allow for earlier detection of side branch-ectasias and provide information on exocrine function by quantifying duodenal filling (101.-103.).
Although MRI is the favored noninvasive imaging modality for assessment of the pancreatic duct and does well in this capacity, ERCP remains the only modality that allows the pancreatic and biliary ducts to be imaged distended under pressure, maximizing characterization of duct anomalies and abnormalities (104.).
Imaging techniques are becoming increasingly quantitative. Adult data have shown that pancreatic exocrine function can be noninvasively assessed with ultrasound or MRI with reasonable agreement with direct stimulation tests with collection of intra-duodenal fluid (105.). In children, measurement of the volume of fluid secreted by the pancreas in response to secretin has been shown to be highly accurate by MRI (<10% error in measured volume) with high inter-reader reproducibility (106.). Further, normative data for secreted fluid volume measured by MRI in children now exist but diagnostic cut-offs for EPI remain to be defined (107.).
Elastography allows the measurement of tissue stiffness by either ultrasound or MRI, and in other organs, increased stiffness is associated with tissue fibrosis. Although correlation between pancreatic stiffness and fibrosis related to CP remains to be defined, studies have revealed differences in stiffness in patients with CP compared with controls. In an adult study, transabdominal strain ultrasound elastography, when combined with greyscale ultrasound, was shown to correctly diagnose presence of CP in approximately 95% of those confirmed to have CP (108.). Pancreatic stiffness as measured by MR elastography has also been shown in adults to be significantly different between controls and patients with either mild CP or moderate/severe CP (1.21 ± 0.13 vs 1.50 ± 0.15 vs 1.90 ± 0.16 kPa; P < 0.001) (109.). A recent publication including 49 controls and 14 children with pancreatitis (acute or chronic) showed measured stiffness to be lower in those with pancreatitis (normal controls: 1.7 ± 0.3 kPa for both of 2 readers; AP or CP: 0.9 ± 0.2 and 1.1 ± 0.3 kPa) (110.).
T1 relaxation time, which quantifies the finding of T1 signal loss associated with CP, has been shown in adults to have 77% sensitivity and 83% specificity for CP (109.). Further, Wang et al (109.) showed that T1 relaxation times were significantly different between controls and patients with either mild CP or moderate/severe CP (865 ± 220 vs 1075 ± 22 vs 1350 ± 139 milliseconds; P < 0.001) though AUCs were lower than for pancreatic stiffness. Notably, multiple regression analysis showed that T1 relaxation time and stiffness were independent predictors of mild CP. Pediatric data are just being generated for pancreatic T1 relaxation time; for example, Gilligan et al (111.) reported values in a small cohort of healthy children at 1.5 and 3 T.
Chronic Pancreatitis Summary Statements and Recommendations
Initial Diagnosis
-
MRI is the recommended modality for imaging of suspected CP (Table 3).
- This recommendation reflects inadequate characterization of findings (particularly duct findings) of CP by transabdominal ultrasound. This also reflects the superior soft tissue contrast of MRI, which allows characterization of both parenchyma and duct findings of CP.
GRADE 1C, agreement 91% (10/11, 9 strongly agree, 1 agree, 1 disagree, average score = 4.6)
Assessment for Superimposed Acute Pancreatitis
-
When imaging is needed to assess a suspected or known episode of AP in a child with CP, transabdominal ultrasound is the preferred first-line imaging modality.
- This recommendation reflects the availability and portability of ultrasound and the role of ultrasound in identifying biliary causes of AP.
- Note: A negative ultrasound does not exclude AP (low to moderate sensitivity).
GRADE 1B, agreement 91% (10/11, 6 strongly agree, 4 agree, 1 neutral, average score = 4.5)
-
If ultrasound is negative for AP in a child with CP and an imaging diagnosis of AP is needed, either CT or MRI are recommended.
- This recommendation reflects the only moderate sensitivity of ultrasound and the greater sensitivity of CT and MRI.
GRADE 1B, agreement 100% (11/11, 6 strongly agree, 5 agree, average score = 4.5)
Intervention Planning
-
CT or MRI are recommended for planning of endoscopic or surgical interventions in a patient with known CP.
- This recommendation is based on the large field of view afforded by CT and MRI, optimal characterization of the degree of organization of fluid collections, and optimal characterization of the peripancreatic vasculature.
- Note: When the intervention will target the duct, MRI is favored over CT.
GRADE 2C, agreement 100% (11/11, 6 strongly agree, 5 agree, average score = 4.5)
Serial Monitoring
-
MRI is recommended for clinically indicated serial imaging of CP.
- This recommendation reflects the optimal soft tissue contrast of MRI, no need for intravenous contrast material (in most cases), and the lack of associated ionizing radiation.
- Note: In the child who requires sedation for MRI, risks of sedation must be balanced with the need for serial imaging.
GRADE 1B, agreement 100% (11/11, 9 strongly agree, 2 agree, average score = 4.8)
FUTURE DIRECTIONS/GAPS IN KNOWLEDGE/NECESSARY RESEARCH
Basic Imaging Strategies
A paucity of rigorous research exists on the diagnostic performance of transabdominal ultrasound (including CEUS), CT, and MRI for pediatric pancreatitis. These basic studies are needed to define the optimal imaging modality(ies) for AP, ARP, and CP in children and to inform future diagnostic guidelines. Such research should include exploration of the optimal timing, relative to attack(s) of AP, of imaging aimed at identifying a pancreatic duct cause of pancreatitis. Further, research is needed to define the optimal imaging strategies for pancreatitis that balance diagnostic performance with cost, and the risks of sedation, intravenous contrast material and radiation exposure.
Prognostication and Prediction
Research into imaging (and other diagnostic) methods is needed to prognosticate and predict disease severity in the context of AP and disease progression in the context of AP and ARP. Currently, it is not possible to predict which patients will progress from AP to ARP and from ARP to CP. However, preliminary data are emerging. A recent study in adults showed a decrease in pancreas volume after 3 episodes of AP, with volume loss possibly reflecting an early finding in the transition to CP (112.). Understanding and predicting the progression through various stages of pancreatitis would allow for more effective counseling and optimization of the timing of interventions. Further, research is also needed into the role of imaging in prognostication based on genetic etiologies of pancreatitis.
Identification of Minimal Change Chronic Pancreatitis
Currently the identification of CP is often delayed, with imaging findings only apparent when disease is well established. CP, and attendant pancreatic dysfunction, are sources of significant morbidity, particularly in children, and thus early identification and intervention are critical. As such, research is needed to facilitate identification of minimal change or early CP. Early studies in adult populations suggest quantitative MRI methods, such as T1 mapping and MR elastography may be able to identify early CP (109., 113.).
Noninvasive Pancreatic Function Assessment
Diagnosis of exocrine and, to some degree, endocrine insufficiency remains invasive. Research is needed to identify noninvasive, or minimally invasive techniques to diagnose insufficiency, and more importantly to predict development of insufficiency to allow early intervention. Some data suggest that imaging can noninvasively assess exocrine and endocrine pancreatic function, but these techniques require further study (114., 115.).
CONCLUSIONS
Pancreatitis in the pediatric population, both acute and chronic, is increasingly being recognized. As with adults, imaging plays a role in the diagnosis, staging, and follow-up of both acute and chronic pancreatitis. Pediatric-specific literature informing the use of imaging in pancreatitis is, however, sparse. For this reason, much of what we know and recommend regarding imaging of pediatric pancreatitis is extrapolated from the adult literature. This document provides summaries and recommendations of the literature regarding imaging of the child with pancreatitis that can be used to inform clinical decision-making. Many of the recommendations are largely based on expert opinion. Going forward, dedicated pediatric studies of imaging in pancreatitis are clearly needed. These studies should address not only optimal basic imaging strategies but should also address the more complex problems of identification of early stage disease and prognostication of disease course to enable generation of pediatric-specific evidence-based guidelines.