Evaluation of an Amino Acid−Based Formula in Infants Not Responding to Extensively Hydrolyzed Protein Formula
www.clinicaltrials.gov registration number: NCT01584245.
The study was sponsored by Mead Johnson Nutrition.
J.V. serves as a consultant for MJN. N.M. is an employee of MJN. D.B. has no conflict of interest to report.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.jpgn.org).
ABSTRACT
Nearly 2% to 3% of infants and children younger than 3 years have confirmed cow's milk protein allergy with multiple clinical presentations including atopic dermatitis (AD), diarrhea, and vomiting/spitting up. Although most infants with cow's milk protein allergy experience clinical improvement with the use of an extensively hydrolyzed (EH) formula, highly sensitive infants may require an amino acid−based formula. In this observational, prospective study, 30 infants (1–12 months of age) with a history of weight loss and persistent allergic manifestations while on an EH formula were provided an amino acid−based formula for 12 weeks. Mean weight gain (z score change) improved +0.43 ± 0.28 (mean ± standard deviation) after the 12-week feeding period. Improvement was observed for many allergic symptoms including significant decreases in AD severity (P = 0.02). These results indicate the new amino acid–based infant formula supported healthy weight gain and improvement in allergic manifestations in infants not responding to EH formulas.
What Is Known
- Amino acid−based formulas are recommended for infants with cow's milk protein allergy who continue to exhibit allergic manifestations and poor growth while on an extensively hydrolyzed formula.
- Healthy infants receiving amino acid−based formulas have demonstrated healthy growth and tolerance.
What Is New
- In cow's milk protein allergy−suspected infants not responding to extensively hydrolyzed formulas, the initiation of amino acid−based formula improved growth following a 12-week feeding period.
- Atopic dermatitis and gastrointestinal complications were also significantly reduced after amino acid−based infant formula feeding.
Cow's milk protein allergy (CMPA), the most common food allergy, affects at least 2% to 3% of infants (1., 2.). CMPA can provoke both IgE-mediated (immediate onset) and non−IgE-mediated (delayed onset) reactions, and result in several clinical symptoms including atopic dermatitis (AD), diarrhea, and vomiting (3.-5.). Although 80% of children develop a tolerance to cow's milk protein by 3 years of age, CMPA can significantly reduce infant quality of life and result in poor growth (2., 6.). Current recommendations for CMPA management include complete avoidance of cow's milk protein and the initiation of an extensively hydrolyzed protein (EHP) formula (7.-9.).
Although most of infants with CMPA respond well to EHP formulas and experience adequate growth and reduced allergy symptoms, the formulas may still contain small peptide fragments that can exacerbate allergic reactions in highly sensitive infants (6., 10., 11.). Failure to respond to an EHP formula can result in impaired growth and persistence of symptoms. Therefore, amino acid−based formulas (AAFs) are often recommended for infants with CMPA not responding to EHP formulas (12.). AAFs have demonstrated reduced allergy symptoms and improved growth in infants with CMPA (2., 6., 13.). Furthermore, similar growth, tolerance, and safety outcomes were demonstrated in healthy term infants fed AAF compared to infants receiving a control (EHP) formula (6.). In recent studies, AAFs were well tolerated and supported growth in otherwise healthy infants without CMPA (6.), and in those with CMPA (2.). Furthermore, EHP and AAFs have been shown to improve the gut barrier function (14.).
Although many studies have demonstrated the safety and efficacy of AAFs in healthy infants, few have examined the role of AAFs in CMPA infants not responding to EHP formulas. Therefore, the objective of the present study was to evaluate the efficacy of an AAF in infants between the ages of 1 and 12 months with a history of weight loss and persistent allergy symptoms while on an EH formula.
METHODS
Methods are available online as Supplemental Digital Content (http://links.lww.com/MPG/A759).
RESULTS
Participant Characteristics
Of the 32 infants consented and enrolled in the study, 2 were discontinued, 30 infants completed the 12-week feeding period. Participants who were enrolled but consumed no study formula (n = 1) were not included in subsequent analyses. Of the participants enrolled, 38.7% (12.) were boys and 61.3% (15.) were girls. The mean age of participants was 6.6 (standard deviation [SD] = 3.2) months. IgE testing indicated that 76.7% (23) of infants were IgE-negative, whereas 23.3% (7.) were IgE-positive. AD was present for 41.9% (13.) participants, with a mean scoring atopic dermatitis value of 24.64 (SD = 13.25). At Study Visit 1, 8 infants presented with watery stools. Vomiting/spitting up was reported in 61.3% (15.) participants, with 17 infants presenting with a gastrointestinal (GI) symptom score of >16.
Growth and Allergy Symptoms
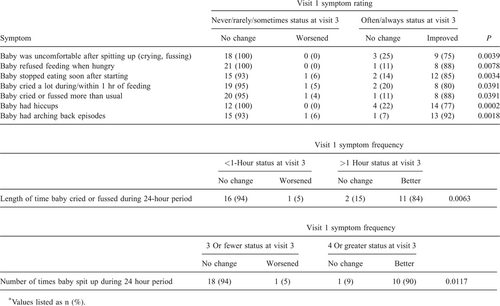
A mean weight z score change of +0.433 (SD = 0.281) was observed after the 12-week feeding period, and was not significantly different from the inclusion criteria z-score change of 0.5 (P = 0.1938). Improvement was observed for all allergic manifestations, both in terms of the number of infants presenting symptoms and symptom intensity. The incidence of AD significantly decreased from 13 participants at visit 1 to 7 participants at visit 3 (Table 1). The mean scoring atopic dermatitis score of the 7 patients still experiencing AD significantly decreased from 32.73 (SD = 10.30) at visit 1 to 9.04 (SD = 5.94) at visit 3 (P = 0.0156). The number of infants experiencing vomiting/spitting up (as defined by a GI symptom score >16) decreased from 17 at visit 1 to 4 at visit 3 (Table 1). A statistically significant decrease was observed in the GI symptom score (P < 0.0001), with a mean change of −10.5 (SD = 1.8) (Table 2).
DISCUSSION
The present study demonstrated improved weight gain and decreased allergic manifestations in suspected CMPA infants receiving an amino acid−based infant formula. Before the initiation of an AAF all study participants were below the 50th percentile of the WHO reference population for weight and were experiencing allergic symptoms while on an EHP formula. Following the 12-week feeding period, there was an increase in weight (+0.433 z score change), relative to the WHO reference population. Although not statistically significant, participant length was increased during the 12-week feeding period. These results indicate that the AAF provided adequate nutrition while managing CMPA symptoms. These findings are in agreement with previous studies, which demonstrate that AAFs provide healthy growth when provided to CMPA infants (6., 11.). In healthy infants, AAFs provided similar weight and growth compared to infants given EHP formula (6.). A similar study demonstrated weight and length gains in infants with CMPA not responding to EHP formulas when fed AAF for 11.4 months (16.).
Recommended management of CMPA includes complete elimination of cow's milk protein and the initiation of a hydrolyzed protein formula (7.). Although 90% of infants exhibit healthy growth and reduced allergic symptoms on an EH formula, highly sensitive infants may require an AAF. Before initiation of an AAF, infants in the present study had not responded to various EHP formulas, including cow's milk based EHP formulas and hydrolyzed rice protein formulas. Although previous studies have demonstrated that hydrolyzed rice protein formulas are well tolerated in CMPA infants unable to tolerate other cow's milk−based EHP formulas, the findings from the present study suggest that infants with severe CMPA may require an AAF (17.-19.). Furthermore, it is possible that infants with non−IgE-mediated allergies, a group highly represented in the study, may be more susceptible to persistent allergic manifestations and unable to tolerate traditional EHP and hydrolyzed rice protein formulas.
Significant improvement was demonstrated in all allergic manifestations in the present study indicating that the AAF properly managed CMPA symptoms. Incidence and severity of AD and vomiting/spitting up were significantly reduced during the 12-week study period. Furthermore, all 8 infants experiencing watery stools at visit 1 had recovered after 12 weeks of receiving study formula. These results are in agreement with the demonstration that short-term feeding of AAF in infants with CMPA reduces the presence and severity of allergic manifestations (2., 6., 15.). In a prospective, controlled study, atopic infants with CMPA receiving an AAF for 6 months demonstrated clinical improvement and growth compared with infants fed an EHP formula (20.). In another study, data suggested that hypoallergenic (AAFs) formulas improved the gut barrier function and minimized gastrointestinal complications in atopic infants (14.). Similarly, when fed an AAF, infants with CMPA with multiple food allergies demonstrated reduced allergic symptoms and normal growth (15., 21., 22.). The results in the present study indicated that longer-term feeding of an AAF in infants with poorly managed CMPA, improved long-term allergy management.
There were some limitations of the present study, with 1 being the observational, nonrandomized nature of the study design. Another limitation was the relatively small sample size of study participants. Despite these limitations, the present study, however, provided support for the use of this new amino acid−based infant formula in infants with suspected CMPA not responding to EH formulas. The results of the present study build on the past literature supporting the efficacy and safety of AAFs for CMPA management. The new amino acid formula in the present study supported healthy weight gain and improvement in allergy symptoms in CMPA infants not responding to EHP formulas.
Acknowledgments
The authors would like to thank all of the participating families. The authors also thank Kaitlin Dehlin, PhD, RD, for assistance in the writing, editing, and submission of the manuscript.