Predicting Individual Changes in Terminal Half-Life After Switching to Extended Half-Life Concentrates in Patients With Severe Hemophilia
Supplemental digital content is available for this article.
Abstract
Predicting individual effects of switching from standard half-life (SHL) to extended half-life (EHL) FVIII/FIX concentrates is pivotal in clinical care, but large-scale individual data are scarce. The aim of this study was to assess individual changes in terminal half-life (THL) after switching to EHL concentrates and identifying determinants of a clinically relevant THL extension in people with severe hemophilia. Data from participants with pharmacokinetic studies on both SHL and EHL were extracted from the Web-Accessible Population Pharmacokinetics Service (WAPPS) database and stratified according to hemophilia type and age groups (children/adults). A 30% increase in THL was considered clinically relevant. Predictors of a relevant increase were identified using logistic regression. Data from 688 persons with severe hemophilia (2174 infusions) were included: 89% hemophilia A; median age: 21.7 (interquartile range [IQR]: 11.5–37.7); positive inhibitor history: 11.7%. THL increased by 38% (IQR: 17%–67%) and 212% (139%–367%) for hemophilia A and B, respectively. All EHL-FIX concentrate users showed clinically relevant THL extension. However, 40% (242/612) of people with hemophilia A showed limited extension or decrease in THL after switching. Relevant FVIII-THL extension was predicted by short baseline THL and blood group non-O in both children and adults. In conclusion, clinically relevant THL extension was observed in all 75/76 participants switching to EHL-FIX, and in 60% of 612 switching to EHL-FVIII. Short THL on SHL-FVIII and blood group non-O were identified as predictors for a relevant THL increase after switching to EHL-FVIII. Individualized pharmacokinetic assessment may guide clinical decision-making when switching from SHL to EHL-FVIII.
INTRODUCTION
Hemophilia is a congenital hematological condition, which is characterized by lower levels or complete absence of coagulation factor VIII (FVIII, hemophilia A) or IX (FIX, hemophilia B).1 People with hemophilia (PWH) are at an increased risk of spontaneous (joint) bleeds and impaired joint function. Although nonreplacement and gene therapy were recently introduced, the current standard treatment consists of prophylactic replacement therapy with regular self-administered intravenous infusions of FVIII or FIX.1 Terminal half-life (THL) for naïve coagulation factor concentrates is relatively short: 8–12 hours for FVIII2 and 17–33 hours for FIX,3-7 requiring a high-infusion frequency to maintain adequate trough levels.8 This high-infusion frequency poses a burden on PWH that could lead to reduced adherence to treatment.9
Longer acting concentrates have been developed in recent years. These concentrates are referred to as extended half-life concentrates (EHL, as opposed to conventionally defined standard half-life [SHL]). For the proposed definition of EHL, concentrates had to be designed to extend circulating biological half-life, have an increase of the area under the receiver operating characteristics (ROC) curve (AUC) of at least 25% and a THL increase of at least 30%.10, 11 Implementing longer acting clotting factor concentrates could lead to lower infusion frequencies or higher trough levels while reporting at least similar annualized bleeding rate12-15 and lower patient or caregiver burden.16
When aiming to maintain minimum trough levels of FVIII/IX to prevent bleeding, THL is one of the parameters defining infusion frequency. Consequently, THL is an important parameter in pharmacokinetics of hemophilia treatment.17 Previous studies have reported THL average increases of 1.5- to 2-fold in FVIII EHL products and 4- to 6-fold in FIX EHL products.3, 13, 18, 19 These data are from registration and phase III studies (range: 7–118 subjects), and have recently been confirmed in a large, multicenter dataset spanning across all concentrates.20 However, all these studies assessed and reported THL and its increase at group level. In the era of individualized medicine,21 the individual effects of switching to EHL concentrates seem particularly interesting from a clinical perspective. From that perspective, it is important to assess how many people benefit from switching to EHL concentrates, quantify the (relative) increase in THL, and try to predict the probability of a clinically relevant increase in THL after switching to EHL concentrates.
Therefore, the primary aim of this current study was to assess individual differences in THL after switching from SHL concentrates to EHL concentrates according to hemophilia type across all factor concentrates in a real-world setting. A secondary aim was to quantify predictors for a clinical relevant increase in THL.
METHODS
Design and setting
This study was multicenter, collaborative project of the University Medical Centre Utrecht (Utrecht, the Netherlands), McMaster University (Hamilton, Ontario, Canada), and the University of Waterloo (Waterloo, Ontario, Canada) and was conducted as part of the activities of the Pharmacokinetics Expert Working Group of the International Prophylaxis Study Group (IPSG). Data were collected as part of the Web-Accessible Population Pharmacokinetic Service—Hemophilia (WAPPS-Hemo). The WAPPS project aims to assemble a database of patient pharmacokinetic data for all existing factor concentrates, develop and validate population pharmacokinetics models, and integrate these models within a Web-based calculator for individualized pharmacokinetic estimation in patients at participating treatment centers.22-24 The dataset included patient characteristics, treatment specific data, and calculated pharmacokinetic data. The WAPPS project was approved by the Institutional Review Board of McMaster University (no. 14-601-D) and University of Waterloo (no. 31977). The approval included the use of the collected data for modeling purposes and for investigating the determinants of factor concentrates pharmacokinetic variability, thus covering the analysis of the present study. All data were anonymized and did not include information on hemophilia treatment centers or date of assessment.
Data collected
Data were downloaded on June 26, 2020. On this date, 298 treatment centers in 47 countries were participating in WAPPS. Patient data were entered in the WAPPS database when the clinician wanted to estimate pharmacokinetic values for their patient. Data of participants with severe hemophilia A or B with at least 1 infusion for both SHL concentrates and EHL concentrates were included in this analysis. No inclusion criteria were formulated regarding minimum time between assessments or a minimum number of assessments. Participants had to provide consent to have their data included in the WAPPS database. Any patient in the participating centers had an equal opportunity to be included in the WAPPS database. Information on the reason for THL assessment was not recorded. However, in case of switching concentrates, it is likely that switching itself was the reason. The distribution of THL on SHL-FVIII was similar to the distribution observed in the entire WAPPS database,20 this suggests no selection bias.
The database included data on patient and disease characteristics (age, disease type and severity, height, weight, inhibitor status, blood group, concentrate) and calculated THL.
The main outcome measure was individual THL, which was defined as the time required for the plasma/blood concentration to decrease by 50% at steady state.25 Individual THL was derived using a Bayesian estimation method leveraging concentrate specific population pharmacokinetic models.22 THL from SHL concentrates was classified as “baseline THL.” Blood group was collected as a proxy for von Willebrand Factor antigen (vWF:Ag). Blood group was more frequently available in the WAPPS database and is not an acute phase protein, unlike vWF:Ag.26 Clotting factor concentrates were classified as SHL or EHL products based on the proposed mechanism of the concentrate, manufacturer data, and publicly available data.27 An increase of ≥30% in THL after switching to EHL concentrates was considered clinically relevant for people with hemophilia A.10, 11 This threshold was specified before the analysis. In the absence of an established threshold for patients with hemophilia B, and given the fact that earlier studies showed much larger increases in FIX_THL than in FVIII (FIX: 4- to 6-fold; FVIII: 1.5- to 2-fold), the authors decided to use an increase in THL of at least 36 hours as clinically relevant as this is expected to lead to a meaningful decrease in infusion frequency. This would mean a doubling of the current median THL.
Statistics
Because of clinically unrealistic THL values in the original dataset, the distribution of THL data were checked and outliers were removed. An outlier in THL was defined according to Tukey's rule. THL values longer than the third quartile (Q3) + (1.5 × interquartile range [IQR: Q1–Q3]) or shorter than the first quartile (Q1) – (1.5 × IQR)28 were considered outliers. Any values beyond these limits were discarded as they were deemed outliers, irrespective of the source of the outlier. Data are presented as median (IQR) or proportion (95% confidence interval [CI]) as appropriate.
Individual differences in THL were presented as the median (IQR) of the individual changes and were compared by means of paired nonparametric testing. The data from each subset (THL, differences in THL, age, weight, body mass index [BMI]) were checked for normality by means of Kolmogorov-Smirnoff testing. Parametric (ANOVA) or nonparametric (Mann-Whitney, Wilcoxon) methods were used for comparisons, as appropriate.
Backward logistic regression was conducted to predict the probability of at least 30% prolongation in THL after switching to EHL concentrates in people with hemophilia A. Separate models were constructed for children (younger than 18) and adults (18 and older) because of the age-related increase in THL in hemophilia A.20 Before conducting the logistic regression, potential predictors were individually selected by univariable regression. Parameters with a significant result on the univariable regression (P < 0.05) were included in/selected for the multivariable regression model. Age, baseline THL, body weight, BMI, positive inhibitor history, and blood group were included as independent variables. BMI (<25/>25 for adults, age-dependent cutoffs for overweight for children29, 30), positive inhibitor history (yes/no), blood group (O/non-O), and baseline THL (tertiles: short, middle, long) were entered in the model as categorical variables. Baseline THL was divided into 3 equal groups by determining the tertiles, based on the THL distribution for children (short: <8 h; middle: 8–10 h; high: >10 h) and adults (short: <10.5 h; middle: 10.5–13.5 h; high: >13.5 h). Interactions between age and baseline THL, blood group and THL, and between age and body weight were included in the model. The final models were tested for accuracy by means of ROC and AUC. The AUC shows the diagnostic accuracy of the model (range: 0–1, 1 indicates a perfect accuracy). An AUC of <0.5 suggests no discrimination, 0.7–0.8 acceptable and 0.8–0.9 excellent and >0.9 outstanding.31 Multicollinearity between individual predictors was assessed by means of the variance inflation factor (VIF). A VIF <5 was considered to be indicative of moderate multicollinearity that does not need correction.32 The results from the logistic regression and the ROC analysis were used to construct probability tables for a clinically relevant increase in THL (≥30%).10 Finally, a sensitivity analysis was performed to assess the influence of the number of samples entered for the PK estimation on the occurrence of a clinically relevant THL prolongation in people with hemophilia A.
Statistical significance levels were set at 5% (P < 0.05). The statistical analysis was performed using SPSS statistical software, version 26 (IBM corp., Armonk, NY), R (version 3.5.1.) and Rstudio (version 1.1.456).33
Data sharing statement
Original data can be accessed upon request from the original authors. Please contact [email protected].
RESULTS
Participants
Participant demographic and biometric details are shown in Table 1. Data on 688 participants (2174 infusions; SHL: 1073; EHL: 1101) from 121 hemophilia treatment centers in 43 countries were extracted from the WAPPS data base. The dataset consisted of 286 children and 402 adults, all with severe hemophilia. The majority of participants had hemophilia A (children: 91%; adults: 89%). The median age was 9.8 (IQR: 6.0–14.0) for children and 34.6 (26.0–47.5) for adults. The median weight was 36.8 kg (22.0–54.3; BMI: 19.2 [16.1–22.9]) for children and 75.6 kg (66.4–86.5; BMI: 24.6 [22.3–27.7]) for adults. Median time between the SHL and EHL assessments in participants with hemophilia A was 165 (IQR: 49–467) days and 141 (39–441) days in participants with hemophilia B. Although nonsignificant, median time between assessments longer in children than in adults in both hemophilia A (223 [58–428] versus 142 [25–474] d; P = 0.06) and hemophilia B (154 [63–433] versus 126 [18–472] d; P = 0.58). Blood group data was available for 65% of the participants, while inhibitor history and BMI were available for nearly all participants (95% and 96%, respectively). Details on the distribution of clotting factor concentrates are shown in Supplemental Table S4.
| Children (0–17) | Adults (≥18) | ||||
|---|---|---|---|---|---|
| Overall | A | B | A | B | |
| n | 688 (612 A) | 259 (42%) | 27 (36%) | 353 (58%) | 49 (64%) |
| Age (y) | 21.6 (11.5–37.9) | 10.0 (6.0–14.0) | 9.3 (6.6–14.0) | 34.5 (26.0–47.1) | 35.5 (23.8–49.0) |
| Weight (kg) | 66.0 (43.0–80.0) | 37.0 (22.0–53.6) | 30.1 (24.1–57.4) | 75.3 (66.3–85.8) | 79.0 (66.7–91.0) |
| BMIa | 22.5 (18.9–25.4)a | 17.9 (15.9–21.1) | 17.8 (15.7–20.9) | 24.4 (22.2–27.4) | 25.0 (23.6–29.2) |
| Inhibitor historya | 76 (11%)a | 42 (16%) | 3 (11.1%) | 30 (8.5%) | 1 (2.0%) |
| Blood group Oa | 204/444 (46%) | 80/170 (47%) | 8/13 (62%) | 104/243 (43%) | 12/18 (67%) |
| Terminal Half-Life (median [IQR]) | |||||
| SHL concentrate (h) | – | 8.9 (7.6–10.8)b | 33.6 (30.0–38.6) | 11.9 (9.7–14.7)b | 38.7 (32.1–42.6) |
| EHL concentrate (hrs) | – | 13.0 (10.4–15.6)b | 93.3 (71.0–118.5)c | 16.9 (13.4–21.4)b | 117.9 (93.2–131.8)b |
| Time between SHL and EHL assessments (days) | – | 223 (58–428) | 154 (63–433) | 142 (25–474) | 126 (18–472) |
| Absolute increase in THL (hrs) | – | 3.8 (1.9–6.0) | 63.9 (31.8–84.1) | 4.3 (2.0–7.2)c | 80.4 (58.5–99.1)c |
| Relative increase in THL (EHL:SHL) | – | 1.4 (1.2–1.7) | 2.8 (1.8–3.4) | 1.4 (1.2–1.7) | 3.2 (2.5–4.4)d |
- aBMI and inhibitor history were available for 95% and 96% of participants, respectively. Blood group was available for 65% of participants.
- bTHL was longer for EHL concentrates than SHL concentrates in both children and adults and was longer in adults than in children in both SHL and EHL (P < 0.01).
- cIncrease in THL is larger in adults than children (A and B).
- dRelative increase in THL is larger in adults than children (B).
- BMI = body mass index; EHL = extended half-life; IQR = interquartile range (Q1–Q3); SHL = standard half-life; THL = terminal half-life.
Terminal half-life according to hemophilia type hemophilia A
The median individual increase of the THL in people with hemophilia A (n = 612) was 4.1 (range: –7 to 21) hours (from 10.6 [IQR: 8.4–13.1] to 14.8 [11.9–18.5] h; P < 0.01) after switching to EHL-FVIII concentrates, indicating a median 1.4-fold (IQR: 1.2–1.7) increase. However, 242/612 (40%) people with hemophilia A showed limited to no improvement of THL after switching to EHL concentrates. THL decreased (median: –1.1 [–2.4 to –0.4] h) in 61/612 (10%) of people with hemophilia A after switching, whereas another 181/612 (30%) reported an increase <30%. Figure 1 shows that prolongation in participants with hemophilia A was dependent on baseline THL: the number of participants with a decrease in THL after switching was higher in participants with a longer baseline THL (short: 9; medium: 16; long: 36; P < 0.01).
Hemophilia B
In contrast to people with hemophilia A, all people with hemophilia B showed an extension of THL after switching to EHL concentrates. The median individual extension of the THL was 74.1 (range: 10–154) hours (from median 35.7 [IQR: 31.3–41.0] to 108.9 [84.1–129.3] h; P < 0.01) after switching to EHL concentrates, which was a median 3.1-fold (2.4–3.7) increase. The majority (65/76; 86%) of participants with hemophilia B showed an increase of >36 hours.
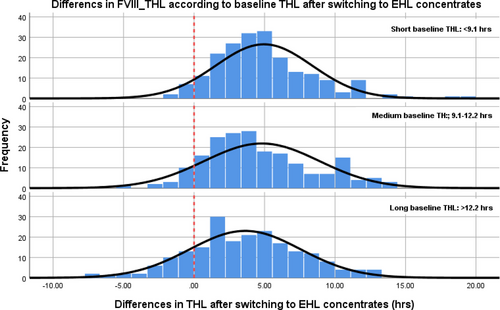
Distribution of differences in THL after switching to EHL concentrates in participants with hemophilia A. The dotted line indicates no change in THL after switching to EHL concentrates. The number of participants with hemophilia A reporting a decrease was greater in participants with a long baseline THL (>12.2 h). EHL = extended half-life; THL = terminal half-life.
Individual changes in THL according to age groups
Table 1 shows changes in THL after switching from SHL concentrates to EHL concentrates for children and adults. THL in children with hemophilia A increased by a median of 3.8 (IQR: 1.9–6.0) hours after switching from SHL concentrates to EHL concentrates (from 8.9 [7.6–10.8] to 13.0 [10.4–15.6]; P < 0.01). THL in adults with hemophilia A increased by 4.3 (2.0–7.2) hours (from 12.0 (9.8–15.2) on SHL concentrates to 16.3 (13.4–21.4) on EHL concentrates; P < 0.01). The relative increase in THL was similar in children and adults with hemophilia A, with a median 1.4-fold (1.2–1.7) increase in both children and adults.
People with hemophilia B showed an age-related increase in THL: children reported a median increase of 63.9 (31.8–84.1) hours (from median 33.6 [30.0–38.6] to 93.3 [71.0–118.5]). This was smaller than in adults, who reported a median increase of 80.4 (58.5–99.1) hours (from 38.7 [32.1–42.6] to 117.9 [93.2–131.8]; P < 0.01). This indicates a smaller, although nonsignificant, relative increase in children than in adults (median 2.8 [1.8–3.4] versus 3.2 [2.5–4.4] fold; P = 0.05).
Identifying predictors for clinically relevant prolongation of THL in hemophilia A
Not all participants with hemophilia A reported a clinically relevant increase in THL after switching to EHL concentrates. Table 2 shows the characteristics of participants according to clinically relevant FVIII-THL extension. Participants showing a clinical relevant THL prolongation (≥30%) had blood group non-O more often (60% versus 47%; P = 0.01) and a shorter baseline THL for SHL concentrates (9.5 [7.9–11.9] versus 11.9 [9.9–14.4] h; P < 0.01) than those with a limited prolongation (<30%). Interaction terms for age and baseline THL, blood group and THL, and between age and body weight did not reach significance. Despite blood group non-O and long baseline THL being associated, no indications for multicollinearity were reported (VIF for adults: 1.13, children: 1.05).
| <30% Increase | ≥30% Increase | P | |
|---|---|---|---|
| Median (IQR) or % (95% CI) | |||
| Number | 242 | 370 | |
| Age (y) | 26 (12-40) | 20 (11-35) | 0.06 |
| Children (%) | 39% (33-45) | 45% (40-50) | 0.13 |
| BMIa | 22 (20-25) | 22 (18-25) | 0.13 |
| Weight (kg) | 67 (46-80) | 65 (40-80) | 0.44 |
| Blood Group O (%)a | 53% (45-60) | 40% (34-46) | 0.01 |
| Inhibitor Status (%)a | 14% (10-19) | 11% (8-15) | 0.47 |
| Baseline THL_SHL (h) | 11.9 (9.9-14.4) | 9.5 (7.9-11.9) | <0.01 |
- Bold numbers indicate significant differences (P < 0.05).
- aBMI was available for 95% of participants with hemophilia A, blood group data for 65%, inhibitor status for 96%.
- BMI = body mass index; CI = confidence interval; FVIII-THL = terminal half-life for factor VIII; IQR = interquartile range (Q1–Q3); kg = kilos.
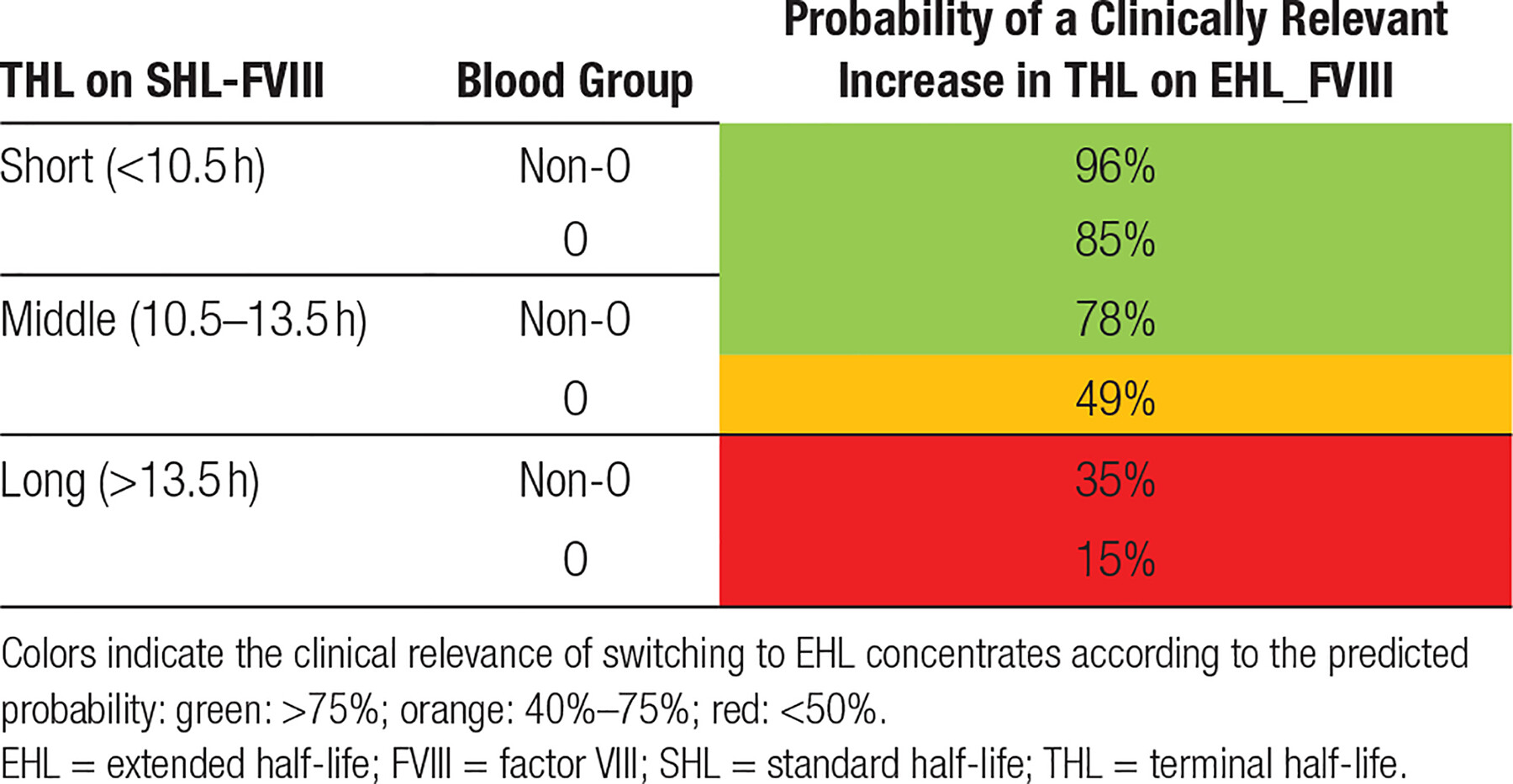
To identify predictors for a clinically relevant prolongation in hemophilia A, separate logistic regression models were generated for children (Suppl. Table S1) and adults (Suppl. Table S2). Baseline THL and blood group O were identified as relevant predictors in children. ROC analysis reported an AUC of 0.74 (95% CI: 0.66-0.82) for children, indicating that the model for children had an acceptable predictive value. In children, the model behaved well on correctly predicting a clinically relevant increase in THL (positive predictive value [PPV]: 97%). However, the performance on predicting nonrelevant prolongation of THL was poor (negative predictive value [NPV]: 22%).
Adults with a short baseline THL showed higher odds of a clinically relevant THL extension. ROC analysis reported an AUC of 0.73 (95% CI: 0.66-0.79) for adults. The model for adults behaved particularly well in predicting a clinically relevant outcome (PPV: 77%), while moderately well in predicting a nonrelevant outcome (NPV: 57%).
Tables 3 (children) and 4 (adults) show probability tables for clinical purposes. These tables show the probability of a clinically relevant increase in THL based on the patient characteristics (baseline THL and blood group in both children and adults). Although BMI was identified as a significant predictor in adults as well, the added value turned out to be limited. Adding BMI to the model did not change the final outcome. Therefore, BMI was excluded from the final clinical model.
Irrespective of blood group and BMI, adult participants with a baseline THL on SHL concentrates of <10.5 hours had the highest probability to achieve a clinically relevant increase in THL (predicted probability: 85%–98%). Participants with a baseline THL between 10.5 and 13.5 hours showed medium probabilities (63%–86%), indicating that an individual approach and extensive monitoring is warranted in this group. Participants with a baseline THL of >13:30 hours showed the lowest probability (≤35%), indicating that the majority of participants with a high-baseline THL showed no relevant pharmacokinetic benefit of switching to EHL concentrates. As in adults, children with a short baseline THL (<8 h) showed the highest probabilities of a clinically relevant increase in THL (69%–96%). This was irrespective of type of blood group.
Extension of FVIII-THL in children with a baseline THL of 8–10 hours or >10 hours was highly dependent on blood group. Children with blood group non-O were mostly likely to show a clinically relevant THL increase (8–10 h: non-O: 81% versus O: 27%; >10 h: non-O: 55% versus O: 10%).
Effect of the number of samples per assessments: a sensitivity analysis
Suppl. Table S3 shows the distribution of the number of samples per assessment in participants with hemophilia A. The majority of participants with hemophilia A had 1 or 2 samples available for analysis. A sensitivity analysis showed that adding “number of samples” did not significantly change the outcome (OR: 0.89 [95% CI: 0.75-1.07; P = 0.21]), nor did an additional analysis only including participants with >3 samples per assessment (OR: 0.89 [0.52-1.54]; P = 0.67). Table 5 shows the descriptive results when including all participants or all participants with at least 3 samples per assessments.
| All Assessments | ≥3 Assessments for Both SHL and EHL | |||
|---|---|---|---|---|
| A | B | A | B | |
| N | 612 | 76 | 129 | 17 |
| Median (IQR) or N (%) | ||||
| THL_EHL | 14.8 (11.9–18.5) | 109 (84–129) | 15.5 (12.7–18.3) | 112 (96–130) |
| THL_difference | 4.1 (1.9–6.6) | 74 (53–93) | 4.1 (2.0–6.5) | 77 (68–93) |
| THL_ratio | 1.4 (1.2–1.7) | 3.1 (2.4–3.7) | 1.4 (1.2–1.7) | 3.3 (2.5–4.3) |
| <30% progression THL | 241/612 (39%) | 1/76 (1.3%) | 53/129 (41%) | 0 |
- EHL = extended half-life; IQR = interquartile range (Q1–Q3); SHL = standard half-life; THL = terminal half-life; THL_difference = absolute differences between THL_SHL and THL_EHL; THL_ratio = relative differences between THL_SHL and THL_EHL.
DISCUSSION
Main findings
This study with 688 participating people with hemophilia represents the largest study assessing individual changes in THL after switching from SHL to EHL products so far. The majority of participants showed an extended THL after switching to EHL products, although large interindividual differences were observed in patients with both hemophilia A and B. In addition, this study showed that 40% of participants with hemophilia A did not have a clinically relevant improvement of THL (30% no clinically relevant prolongation; 10% with reduction) after switching to EHL concentrates. The relative increase in THL was age-related in people with hemophilia B but not in people with hemophilia A. Baseline THL and blood group were identified as predictors in both adults and children.
Strengths and limitations
The primary strength of this study is its size and its multicenter, multinational nature. In addition, it includes a substantial number of young children (10% under 6, 1% under 2), which is a valuable addition to the information on previous reports, which have mostly relied on hemophilia on adults and children over 6.10, 34-36 Especially, the inclusion of young children allowed for assessment of the effects of age on individual changes in THL over the entire age range. Furthermore, the distribution of THL on SHL-FVIII was similar to the global WAPPS database, suggesting no selection bias was introduced.20
THL and other pharmacokinetic data in this dataset were modeled using concentrate-specific population pharmacokinetic models and Bayesian methods.23, 24 Applying Bayesian methods is an established and widely used practice in population-based pharmacokinetic prediction models to limit patient and economic burdens of traditional pharmacokinetic samping.37-40 These Bayesian models were established with existing, real-life data. All clotting factor activity assessments were performed in local laboratories, inducing interlaboratory variation. These real-world data are representative of the large number of users of the WAPPS model. No correction for laboratories was applied in this large dataset. In addition, the external validity of the present study is expected to be higher than in controlled studies, as many different laboratories and reagents are included in the dataset.
The definition of a clinically relevant increase in THL after switching remains subjective. Therefore, we used the international expert consensus definition for hemophilia A, stating that an increase in THL of 30% was required for a concentrate to be regarded an EHL concentrate.10, 11 This relative value was used as a cutoff for a clinically relevant THL prolongation in this study. Although this relative increase seems most feasible to make comparisons, the increase in absolute hours is also presented to promote clinical application.
The accuracy of the modeled data is associated with the number of samples taken for PK assessment.41, 42 Estimates based on only 1 sample may cause bias. However, a sensitivity analysis suggests no significant effect of adding “number of samples” to the model. Still, future studies should aim to exclusively include patients with multiple assessments to model more accurate data and to avoid inaccurate estimation of true THL.42
THL is known to be lower in blood group O than in non-O for patients with hemophilia A.26, 43 However, this does not directly explain why blood group non-O is a relevant predictor of a higher increase in THL. Besides this, blood group is hypothesized to be associated with vWF. However, vWF is an acute phase protein as well, making blood group potentially a better predictor than measured vWF. However, other than the short THL at baseline, neither previous publications nor pathophysiological reasoning provide an explanation for the observation that blood group O was associated with more extension of THL after switching to EHL-FVIII.
Blood group data were lacking in a substantial number of participants (35%). However, the number of remaining available blood group data was sufficient to be included in the analysis. Furthermore, the fact that the distribution of the included blood group data was similar to the global distribution suggests there was no selection bias.
Comparison with other studies
In the present study, 10% (CI: 5%-20%) of children younger than 6% and 12% (7%-19%) of children age 6–12 reported no prolongation in THL. This is similar to the publication of Young et al reporting no individual prolongation in around 5% of children under 6 (n = 19; CI: 0%-26%) and 12% of children aged 6–12 (n = 27; CI: 7%-19%).44
Our findings are also in line with previous reports on series of intrapatient changes in THL for both hemophilia A and B. For hemophilia A, Mahlangu et al (1.5-fold increase, n = 28, aged > 12) and Traets (1.4-fold increase, n = 15, 9 adults) reported similar increases in THL.13, 45 For hemophilia B, Powell et al reported a 2.4-fold increase(n = 22) and Traets et al reported a 2.6-fold increase (n = 15, 10 adults) in people with hemophilia B.3 Fischer et al46 reported a similar increase in THL (3.7-fold [CI: 2.6-5.5]) in 30 children, with 50% under 6 years, with hemophilia B switching from SHL to EHL concentrates.
Clinical relevance and future directions
This study showed that 40% of people with hemophilia A did not achieve clinically relevant THL prolongation after switching to EHL concentrates. A short baseline THL and blood group non-O were identified as possible predictors for a clinically relevant increase of THL. Predictive tables were created for adults and children to guide clinicians and patients in their decision-making concerning switching to EHL concentrates in people with hemophilia A.
This study assessed the increase in THL after switching from SHL to EHL concentrates. However, focusing on THL is a limited approach.47 The current dataset did not assess the clinical consequences of switching (eg, annual bleeding rate, annual number of infusion, and through levels). These clinical consequences of switching on these parameters need to be studied as well. A prolonged THL may allow for the use of a lower prophylactic infusion frequency, potentially leading to a lower burden for patients or caregivers. This study showed a median increase in THL of 4 hours for FVIII and 74 hours for FIX. This means the infusion intervals are extended by 20–24 hours (±1 d) for FVIII and 300–350 hours (±12–14 d) for FIX, which may enable a reduction of infusion frequency without dose changes. Concomitantly, EHL extension results in higher trough levels when the same infusion frequency is maintained. Both seem viable options to improve bleeding protection people with hemophilia, the exact rationale for switching to EHL concentrates depends on the patient and his particular circumstances (eg, bleeding phenotype, physical activity levels). This study represents a first step to identify predictors for a clinically relevant increase in THL after switching to EHL concentrates. Future studies should include clinical results and expand on other pharmacokinetic parameters to assess overall individual effects of switching to EHL concentrates.
CONCLUSIONS
All people with hemophilia B and 60% with hemophilia A showed a clinically relevant extension (≥30%) of THL after switching from SHL to EHL concentrates. Clinically relevant FVIII-THL extension was predicted by short baseline THL and blood group non-O. These results support the importance of individualized treatment strategies to guide clinical decision-making when switching from SHL to EHL concentrates. The predictive tables created in this study, can support clinicians and patients to make an appropriate, evidence-based decision regarding the switch to EHL concentrates.
ACKNOWLEDGMENTS
The authors wish to thank the members of the Executive Committee of the International Prophylaxis Study Group (Drs. Lou Aledort, Rolf Ljung, and Victor Blanchette) and of the Pharmacokinetics (PK) Expert Working Group of the International Prophylaxis Study Group (Drs. Massimo Morfini, Guy Young, Victor Blanchette and Savita Rangarajan) for their useful comments regarding this manuscript. Drs. Alfonso Iorio and Kathelijn Fischer are members of the PK Expert Working Group of the IPSG. The IPSG is funded by educational grants to the Hospital for Sick Children (“SickKids”) Foundation from Bayer, Novo Nordisk Health Care AG, Pfizer, Sanofi, Takeda and Spark Therapeutics. The IPSG provided salary support for Olav Versloot, a PhD student working under the supervision of Dr. Kathelijn Fischer at the University Medical Centre Utrecht, the Netherlands, for his work regarding this collaborative project. None of the industry partners of the IPSG were involved with the design or conduct of the work reported in this manuscript and the opinions reported in the manuscript are those of the authors alone on behalf of this IPSG/WAPPS collaboration.
AUTHOR CONTRIBUTIONS
All authors were involved in the design of the study. OV, EI, PC, and KF analyzed the data and wrote the initial version of the report. All authors were involved in data interpretation. All authors reviewed and approved the final version of the paper.
DISCLOSURES
The Van Creveldkliniek has received speaker fees from Novo Nordisk and research support from Bayer for work done by OV. FG has received research funds from Novo Nordisk, Roche, Takeda, Bayer. AE has received speaker's fees from Bayer and Pfizer and research funds via the University of Waterloo from Grifols, Novo Nordisk and Bayer. RS has received research support from CSL-Behring and Sanquin, and is an editor for HemaSphere. AI receives career support via the Mike Gent Chair in HealthCare Research. He has received research funds via McMaster from Bayer, BioMarin, CSL, Freeline, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Spark, Takeda, and Uniqure. The Van Creveldkliniek has received speaker's fees from Bayer, Baxter/Shire, Biotest, CSL-Behring, Octapharma, Pfizer, Novo Nordisk; consultancy fees from Baxter/Shire, Biogen, CSL-Behring, Freeline, NovoNordisk, Pfizer, Roche and SOBI; and research support from Bayer, Pfizer, Baxter/Shire, and Novo Nordisk for work done by KF. All the other authors have no conflicts of interest to disclose.
FUNDING
This study was funded by the International Prophylaxis Steering Group (IPSG).