Ropeginterferon Alfa-2b: Efficacy and Safety in Different Age Groups
Trial registration: EudraCT: 2012-005259-18; 2014-001357-17.
The study was funded by AOP Orphan Pharmaceuticals AG.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
Dear Editor, Interferon alpha (IFNa) and hydroxyurea (HU) are recommended for cytoreductive treatment of polycythaemia vera (PV),1-3 a Philadelphia chromosome-negative myeloproliferative neoplasm characterized by deregulated hematopoiesis driven by mutated constitutive-active JAK2.1, 4, 5 In addition to its ability to induce hematologic responses, IFNa has disease-modifying properties as shown by the high rate of reduction of the mutant JAK2V617F allele burden.6-11 In elderly patients with PV, however, a higher number of toxicity-related dose reductions or discontinuations and lower hematological response rates have been reported among IFNa-treated patients compared to HU.12
Ropeginterferon (Besremi®) is a novel mono-pegylated IFNa-2b with reduced dosing frequency and improved tolerability compared to other pegylated IFNs, and is approved in Europe as first-line therapy for treatment of PV in patients of all ages.10, 13 Here we report the efficacy and safety after 24 months of ropeginterferon treatment compared to HU in the PROUD-PV/CONTINUATION-PV phase III trials (EudraCT: 2012–005259–18; 2014–001357–17), analyzed post-hoc in two age cohorts (<60 years and ≥60 years) in PV patients aiming to evaluate the benefit-risk ratio of ropeginterferon therapy in the elderly.
The full trial methodology of the PROUD-PV/CONTINUATION-PV phase III trials was previously published.10 Briefly, eligible patients aged ≥18 years were diagnosed with PV according to the World Health Organization 2008 criteria. Patients with PV were randomly assigned 1:1 to receive either ropeginterferon (starting dose 50–100 μg) every two weeks or HU (starting dose 500 mg) daily. Patients provided written informed consent in accordance with the Declaration of Helsinki. Study protocols were approved by the institutional review board or independent ethics committees at each site.
Efficacy assessment included the rate of complete hematological response (CHR, defined as hematocrit < 45% with no phlebotomy in the last three months, platelet count < 400 x 109/L and leukocyte count < 10 x 109/L), rate of CHR and symptom improvement (disease-related signs including clinically significant splenomegaly and PV-related symptoms), evolution of JAK2V617F allelic burden, and molecular response rate. Efficacy and safety were assessed in the age categories < 60 years and ≥60 years including all 171 patients who entered the CONTINUATION-PV study. An additional analysis of the age categories < 70 years and ≥70 years was conducted but is considered exploratory due to the small number of patients in the ≥70 year age group (9 in the ropeginterferon arm and 12 in the control treatment arm).
Baseline characteristics were balanced between the treatment groups and indicated an early PV population. The median age was 58.0 years in the ropeginterferon arm and 59.0 years in the HU arm. In both treatment arms, patients aged ≥60 years displayed characteristics of more severe disease than younger patients, indicated by the presence of disease-related symptoms and prior thromboembolic events, and a higher mean JAK2V617F allele burden (Table 1).
In PROUD-PV, 127 patients received ropeginterferon and 127 received HU. After 12 months of treatment 89.6% of ropeginterferon and 68.5% HU treated patients continued in the extension study CONTINUATION-PV; patients who had received HU in PROUD-PV continued to receive standard therapy (98.4% remained on HU).10 Regarding enrolment in the extension study, no selection bias was identified (Supplementary Digital Content, Table 4, http://links.lww.com/HS/A101).10 At month 24, the median dose of ropeginterferon was higher in the cohort ≥60 compared to < 60 years (500 μg vs 350 μg, respectively) administered every 2 to 4 weeks, whereas both age cohorts in the HU arm received a median dose of 1000 mg daily (Supplementary Digital Content, Table 5, http://links.lww.com/HS/A101).
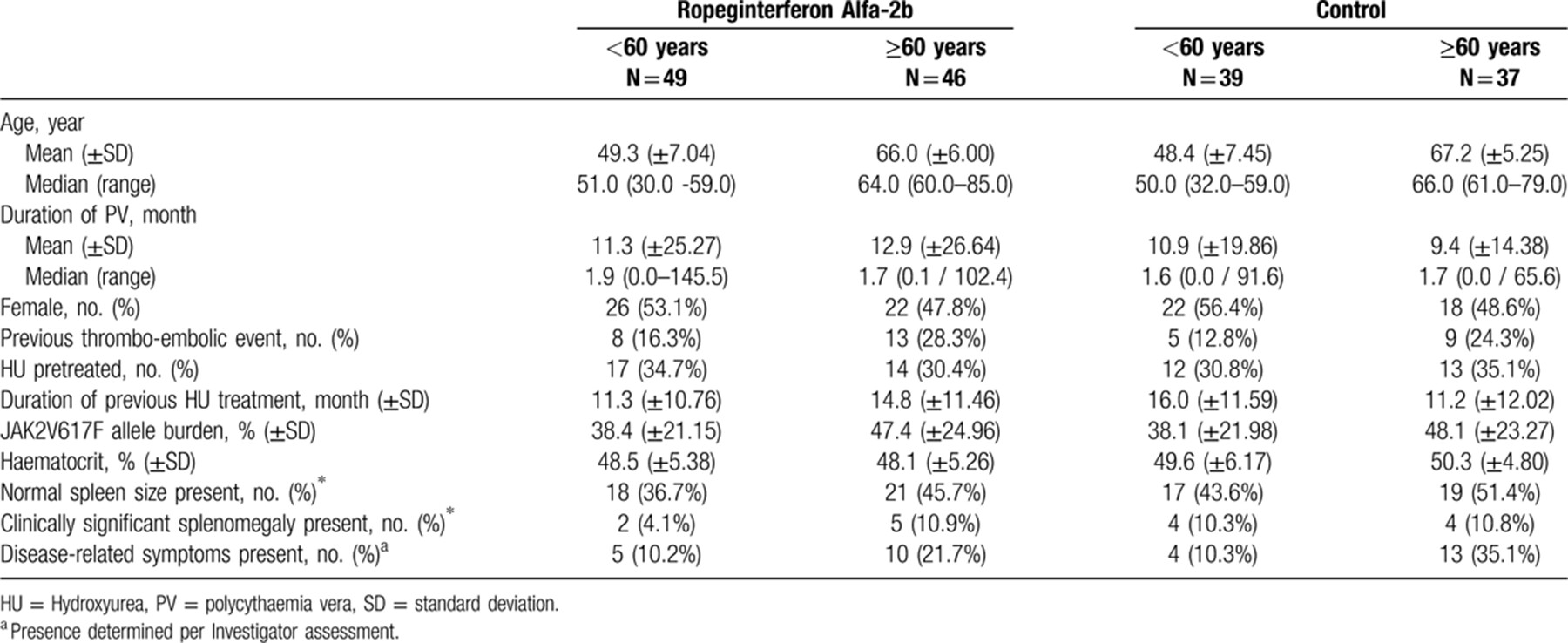
Regardless of age group, response rates were higher among patients treated with ropeginterferon than in those who received HU. Significantly higher CHR rates were observed in the ropeginterferon arm compared to HU at month 24 (70.5% vs 49.3%, respectively; RR: 1.42 [95% CI: 1.09 to 1.87], p = 0.0101) (Supplementary Digital Content, Table 6, http://links.lww.com/HS/A101). Analysis of CHR by age group also showed higher response rates among patients treated with ropeginterferon compared with HU (77.6% vs 55.9%; respectively; RR: 1.39 [95% CI: 1.00 to 1.94], p = 0.0525 in patients aged < 60 years and 63.0% vs 42.4%; RR 1.48 [95% CI: 1.48 [0.94 to 2.34], p = 0.0906 in those aged ≥60 years; Fig. 1Ai; Supplementary Digital Content, Table 7, http://links.lww.com/HS/A101). An exploratory analysis including only patients aged ≥70 years showed a similar trend to that seen in the ≥60 year age group: 44.4% (4/9) patients in the ropeginterferon arm and 41.7% (5/12) patients in the control treatment arm aged ≥70 years achieved a CHR at 24 months (data not shown). Among the 10 patients aged 75 years and over, CHR was achieved at month 24 by all 3 patients in the ropeginterferon arm who had available response data at this time point, and 2 of the 5 patients in the HU arm who had available response data.
Results for CHR with improved disease burden showed a trend towards higher rates in the ropeginterferon arm vs control (55.1% vs 37.1%, respectively; RR: 1.49 [95% CI: 0.91 to 2.43], p = 0.1092 in patients < 60 years; 43.5% vs 36.1%; RR: 1.22 [95% CI: 0.70 to 2.11], p = 0.4774 in those ≥60 years; Fig. 1Bi; Supplementary Digital Content, Table 7, http://links.lww.com/HS/A101). Maintenance of CHR (response maintained from first occurrence to 24 months assessment) was also higher in the ropeginterferon arm compared to control in both age groups (49.0% vs 17.9%, respectively; RR: 2.82 [95% CI: 1.37 to 5.79], p = 0.0048 [<60 years]; 37.0% vs 18.9%, respectively; RR: 1.96 [95% CI: 0.91 to 4.22], p = 0.0845 [≥60 years]) (Fig. 1Aii; Supplementary Digital Content, Table 7, http://links.lww.com/HS/A101). Similarly, analysis of maintenance rate of CHR and symptom improvement indicated higher response rates in the ropeginterferon arm vs HU in both age groups: 32.7% vs 15.4%, respectively; RR: 2.21 [95% CI: 0.96 to 5.06], p = 0.0618 (<60 years), 28.3% vs 18.9% respectively; RR: 1.52 [95% CI: 0.68 to 3.43], p = 0.3113 (≥60 years) (Fig. 1Bii; Supplementary Digital Content, Table 7, http://links.lww.com/HS/A101).
Efficacy assessment of ropeginterferon alfa-2b (ropegIFN) vs control in 2 age cohorts: <60 years and ≥60 years. (A) Complete haematologic response (CHR) at 24 months [i], CHR response maintenance (from first occurrence to 24 months assessment) [ii]. (B) CHR and improvement in disease burden response at 24 months [i], CHR and improvement in disease burden response maintenance (from first occurrence to 24 months assessment) [ii]. (C) Molecular response at 24 months (last observation carried forward [LOCF]). (D) JAK2V617F (%) relative change from baseline (LOCF) at 12 months and 24 months in patients < 60 years (i) and ≥60 years (ii). (Ropeginterferon alfa-2b: n = 49 [<60 years], n = 46 [≥60 years]; control: n = 39 [ < 60 years], n = 37 [≥60 years]).
Molecular response14 at 24 months was significantly higher in the ropeginterferon arm than in HU (69.2% vs 28.6%, respectively; RR: 2.13 [95% CI: 1.26 to 3.59], p = 0.0046) (Supplementary Digital Content, Table 6, http://links.lww.com/HS/A101). After applying the age cut-off, higher molecular response rates were still evident among ropeginterferon treated patients compared to HU (77.1% vs 33.3%, respectively; RR: 2.17 [95% CI: 1.38 to 3.42], p = 0.0008 in patients aged < 60 years and 58.7% vs 36.1%; RR: 1.53 [95% CI: 0.95 to 2.48], p = 0.0822 in patients ≥60 years; Fig. 1C). Molecular responses were also observed in the small group of patients aged 75 years and above (n = 10), in one patient in the ropeginterferon arm and one in the control arm. At month 24 the relative change from baseline in the percentage of JAK2V617F allele burden was higher in the ropeginterferon arm vs control irrespective of age (−54.8% vs −4.5%, respectively; RR: -51.15 [95% CI: −78.92 to −23.37], p = 0.0003 in the < 60 year age group and −35.1% vs -18.4%; RR: −15.85 [95% CI: −32.16 to 0.46], p = 0.0568 in patients aged ≥60 years; Fig. 1Di,ii; Supplementary Digital Content, Table 7, http://links.lww.com/HS/A101).
Advanced age did not negatively influence rates of adverse events (AEs) which were comparable in patients aged < 60 years (89.8% [ropeginterferon] vs 92.3% [HU]) and in patients ≥60 years (93.5% [ropeginterferon] vs 91.9% [HU]) in both treatment arms (Supplementary Digital Content, Table 8, http://links.lww.com/HS/A101). Similar rates of adverse events were seen in the subset of patients aged ≥70 years (88.9% [ropeginterferon] vs 100% [HU]; data not shown). In patients below 60 years, comparable numbers of treatment-related AEs were observed for ropeginterferon (77.6%) and HU (74.4%). Interestingly, in patients aged ≥60 years, despite receiving higher doses of ropeginterferon compared to patients aged <60 years, a trend towards fewer treatment-related AEs was seen for ropeginterferon (63.0%) vs HU (89.2%). Serious adverse events (including events that were unrelated to study treatment) occurred more frequently in elderly patients, regardless of treatment group (6.1% [ropeginterferon] vs 10.3% [HU] in patients < 60 years, 21.7% [ropeginterferon] vs 24.3% [HU] in patients ≥60 years). With respect to treatment-related serious AEs, none were reported in the ropeginterferon arm, whereas four events (acute leukemia, anemia, leukopenia and granulocytopenia) were reported in the HU arm (all in patients aged ≥60 years, including one fatal case of acute leukemia). Comparable numbers of AEs of special interest in IFNa therapy (including autoimmune, mood and ocular disorders) were observed in both treatment groups (<60 years: 6.1% [ropeginterferon] vs 2.6% [HU]; ≥60 years: 13.0% [ropeginterferon] vs 10.8% [HU]). No patients aged ≥70 years experienced AEs of special interest in the ropeginterferon arm; in the HU arm, two AEs of special interest were reported in this age group.
Drug-related toxicity led to discontinuation of three patients in the ropeginterferon arm (due to elevated hepatic enzymes, nervousness and depression, and Sjogren's syndrome); one patient was aged ≥70 years and the remaining two were aged ≥60 to < 70 years. In the HU arm, one patient aged ≥60 died due to drug-related toxicity (acute leukemia). Additionally, one PV-related case of acute leukemia led to discontinuation of a patient aged < 60 years in the HU arm.
In summary, after 24 months ropeginterferon induced higher hematological and molecular response rates and higher maintenance of response than HU in PV patients in the age groups < 60 years and ≥60 years, with the latter consisting of a majority of patients between 60 and 70 years of age and only a small number of patients aged over 75 years. In contrast to previous observations with other pegylated IFNs,12 ropeginterferon was efficacious and well tolerated even in the elderly cohort. Moreover, the safety analysis in patients ≥60 years showed a positive trend regarding fewer and less serious treatment-related AEs compared to HU. The divergent observations on efficacy and safety between early generation pegylated IFNs and ropeginterferon are potentially the result of optimized protein-engineering, reducing non-specific pegylation and thus improving the pharmacological properties and tolerability15 of ropeginterferon.
Disclosures
HG reports grants and personal fees from AOP Orphan during the conduct of the studies; grants and personal fees from Novartis and personal fees from PharmaEssentia, MyeloPro Diagnostics and Research, Janssen-Cilag, Roche, and Celgene, outside the submitted work. CK, KK report that they were employees of AOP Orphan during the conduct of the studies. JM reports grants from AOP Orphan during the conduct of the studies. RK reports personal fees from AOP Orphan Pharmaceuticals and PharmaEssentia, during the conduct of the studies; personal fees from Qiagen and Novartis and stock ownership in MyeloPro Diagnostics and Research, outside the submitted work. HH reports Data Monitoring Board honoraria from AOP Orphan during the conduct of the studies; grants from Novartis outside the submitted work. JJK reports grants and personal fees from AOP Orphan during the conduct of the studies; grants and personal fees from Novartis and personal fees from Celgene, outside the submitted work. The remaining authors have nothing to disclose.
Acknowledgements
The authors thank Pavla Kadlecová for statistical analyses performed.