Beyond the cardiorenal anaemia syndrome: recognizing the role of iron deficiency
Abstract
Growing awareness that heart failure, renal impairment, and anaemia are frequent co-morbidities which can exacerbate one another in a vicious circle of clinical deterioration has led to the concept of the cardiorenal anaemia syndrome (CRAS). The role of iron deficiency within this complex interplay has been less well examined. Scrutiny of data from the recent FAIR-HF trial raises a new hypothesis: is it time for ‘CRAS’ to be supplemented with new acronyms such as CRIDS (cardiorenal–iron deficiency syndrome) or even CRAIDS (cardiorenal–anaemia–iron deficiency syndrome)? Iron deficiency occurs frequently in heart failure patients with or without anaemia. It not only impairs oxygen transport through reduced erythropoiesis, but adversely affects oxidative metabolism, cellular energetics, and immune mechanisms, and the synthesis and degradation of complex molecules such as DNA. One large observational study in patients with heart failure found iron deficiency to be an independent predictor of death or urgent heart transplantation (hazard ratio 1.58, 95% confidence interval 1.14–2.17, P = 0.005). In the FAIR-HF trial, i.v. iron therapy was associated with significant improvements in physical functioning in iron-deficient patients with heart failure, even in non-anaemic patients in whom haemoglobin levels did not change following i.v. iron administration. Key questions regarding the use of i.v. iron supplementation in the setting of heart failure merit exploration and could readily be answered by appropriately designed clinical trials. It is to be hoped that these important clinical trials are conducted, to permit a more subtle characterization of the patient's pathological condition and interventional requirements.
The cardiorenal anaemia syndrome and iron deficiency
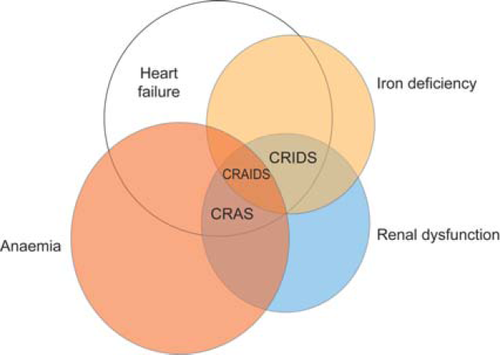
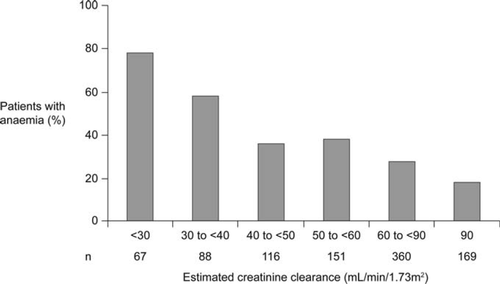
Cardiorenal syndrome is a term used to describe the well-known interdependency of cardiac and renal dysfunction in heart failure (HF).1 More recently, growing awareness that HF, renal impairment, and anaemia are frequent co-morbidities which can exacerbate one another in a vicious circle of clinical deterioration has led to the concept of the cardiorenal anaemia syndrome (CRAS) (Figure 1).2 Renal dysfunction affects up to half of all patients with HF,3,4 caused by haemodynamic derangement, primary renal disorders, or cardiovascular medications such as diuretics and angiotensin-converting enzyme inhibitors. Renal impairment, in turn, is associated with a marked decrease in haemoglobin levels, primarily due to blunted erythropoietin production,5 and thus compounds the inherent predisposition of HF patients to develop anaemia due to increased levels of circulating inflammatory cytokines (i.e. anaemia of chronic inflammation)6 and other factors. Anaemia may be present in as many as 60% of HF patients,4,6,7 becoming more frequent with increasing severity of concomitant renal dysfunction4,8 (Figure 2), and is an independent predictor of mortality in HF patients.6–9 Renal dysfunction in patients with HF is also associated with a significantly higher rate of mortality,3,8,9 not only at moderate levels of impairment [estimated glomerular filtration rate (GFR) <60 mL/min]9 but also when only mild functional deterioration has developed (estimated GFR <90 mL/min).3 Notably, however, the lowest survival rates are seen in patients with concurrent anaemia and renal dysfunction.8,9 Moreover, it has been proposed that the combination of HF, chronic kidney disease, and anaemia—CRAS—may also be associated with an increased risk of cancer.10 The effect of erythropoiesis-stimulating agents (ESAs) in this setting will be addressed conclusively by the ongoing RED-HF trial in which ∼2600 anaemic patients with HF were randomized to darbepoetin alfa or placebo, with the primary endpoint being death from any cause or first hospitalization for worsening HF.11
The role of iron deficiency within the complex interplay of HF, anaemia, and renal dysfunction has been less well examined.12 Classically, the major biological function of iron was believed to be in the formation of haem, the oxygen-carrying component of haemoglobin, but two recent developments have led to a paradigm shift in our understanding of iron physiology. The first was discovery of the peptide hormone hepcidin and elucidation of its role as the master regulator of iron availability in the body. Secondly, there has been a realization that iron has many other metabolic functions13 beyond that of haemoglobin synthesis and the maintenance of exercise capacity, and the impact of iron deficiency on certain other disease states has been the source of much discussion and investigation.
Although it has long been known that iron administration could ameliorate iron deficiency and improve anaemia, the potential effect of correcting iron deficiency in the absence of anaemia has only recently received some attention. Publication of the FAIR-HF study in the New England Journal of Medicine in November 2009 supported this hypothesis14 and drew attention to the importance of diagnosing and treating iron deficiency in patients with HF.15 Improvements in physical functioning were seen following administration of i.v. iron in iron-deficient patients with HF even in those without anaemia and in whom haemoglobin levels did not change following i.v. iron administration. Scrutiny of data from FAIR-HF raises a new hypothesis: is it time for ‘CRAS’ to be supplemented with new acronyms such as CRIDS (cardiorenal–iron deficiency syndrome) or even CRAIDS (cardiorenal–anaemia–iron deficiency syndrome) (Figure 1) to permit a more subtle characterization of the patient's pathological condition and interventional requirements?
Physiological importance of iron beyond erythropoiesis
In addition to its well-established role as the oxygen-attracting atom in the haemoglobin molecule, iron is an essential constituent of the cytoplasmic haemoprotein, myoglobin, which is found in skeletal and cardiac tissue. Structurally and functionally similar to haemoglobin, myoglobin binds oxygen avidly but reversibly. Conventionally, myoglobin was regarded solely as an oxygen storage protein, acting as a local tissue reservoir of oxygen to support locomotor muscle contractions, but other functions for myoglobin have now been identified.16 It buffers intracellular oxygen concentrations during muscular activity, and facilitates intracellular oxygen diffusion from capillary blood in high-oxygen-consuming, mitochondria-rich muscles such as the heart. Myoglobin also appears to scavenge reactive oxygen species by-products of oxidative phosphorylation, including nitric oxide, in oxidative skeletal and cardiac muscle.16
Mitochondrial function is dependent on iron in several ways. Critically, iron is a metal cofactor for haemoproteins involved in the mitochondrial electron transport chain, the ‘engine’ of cellular energy production. Both haem-containing enzymes (cytochromes) and iron-containing non-haem enzymes (NADH dehydrogenase and succinate dehydrogenase) play an essential role in the generation of ATP by oxidative phosphorylation of ADP via the citric acid cycle. Iron deficiency has been shown to impair mitochondrial electron transport in rat hearts.17 Other cytochromes such as the P450 family are essential for metabolism of both biological and pharmaceutical entities, while the haem-containing enzymes catalase and peroxidase act as important scavengers for the reactive oxygen species hydrogen peroxidase. Iron deficiency has been shown to induce ultrastructural abnormalities and release of cytochrome c from the mitochondrial inner membrane, with cardiac myocyte abnormalities.18 The importance of iron for mitochondrial activity has been demonstrated in animal models19,20 and clinically,21 with iron deficiency causing impaired exercise capacity even in the absence of an effect on haemoglobin levels, i.e. through reduced cellular oxidative capacity.22
Immunological responsiveness is also iron dependent, with iron deficiency reducing T-lymphocyte numbers and function, and inhibiting the activity of iron-containing myeloperoxidase, which mediates the bactericidal activity of macrophages.13 Iron is also an essential factor in neuronal myelination23 and an essential cofactor for non-haem enzymes such as ribonucleotide reductase, the limiting enzyme for DNA synthesis.
Thus, iron deficiency not only impairs oxygen transport through reduced erythropoiesis, but also adversely affects oxidative metabolism, cellular energetics, and immune mechanisms, as well as the synthesis and degradation of complex molecules such as DNA.
Rationale for a new terminology
It is important that clinicians understand the interaction of iron deficiency, anaemia, renal dysfunction, and chronic HF, and development of more accurate terminology to describe specific combinations of these adverse phenomena may be one step towards improving awareness. The current term CRAS disregards the potential contribution of iron deficiency, a frequent finding in HF. Estimates of its prevalence, however, vary according to the criteria used and the population studied.24–26 European and US guidelines in non-dialysis patients with chronic kidney disease recommend that serum ferritin be maintained above 100 ng/mL and transferrin saturation above 20%.27,28 One large observational study reported iron deficiency in 32% and 57% of non-anaemic and anaemic patients with systolic HF, respectively, using these cut-off values.26 Using the same definition, Parikh et al.24 reported a prevalence of 61% among community-dwelling HF patients, whereas Okonko et al.,25 who based the definition of iron deficiency on transferrin saturation levels alone, found 43% of patients to have iron deficiency—and 100% of those with New York Heart Association (NYHA) functional class IV. Serum iron markers, however, may be inadequate to detect poor iron status: another trial that used bone marrow aspiration to evaluate iron status identified iron deficiency in 73% of patients with advanced HF and anaemia.29 The presence of concomitant chronic renal dysfunction—an inflammatory condition with increased levels of circulating cytokines—also increases the likelihood of hepcidin-induced iron deficiency.30 In the largest prospective study to investigate iron deficiency in HF, Jankowska et al. found increasing severity of HF symptoms to make iron deficiency more likely [odds ratio 2.92, 95% confidence interval (CI) 1.06–8.03 for NYHA class IV vs. NYHA class I, P = 0.04], although renal deterioration had no independent effect on the risk of iron deficiency.26 In the same study, iron deficiency was an independent predictor of death or urgent heart transplantation (hazard ratio 1.58, 95% CI 1.14–2.17, P = 0.005).26 Interestingly, recent data have also indicated that iron deficiency is associated with increased pulmonary arterial pressure, which in turn adversely affects progression of HF. Iron availability influences the pulmonary vasoconstrictor response to hypoxia and is associated with worse severity of disease and outcomes in patients with pulmonary arterial hypertension.31 Levels of hepcidin, the major regulator of iron in the body, are inappropriately raised in idiopathic pulmonary hypertension32 and would be expected to impair iron absorption from the gut. I.v. iron therapy appears to modulate dramatically both pulmonary arterial pressure and the sensitivity of the pulmonary vasculature to hypoxia.33
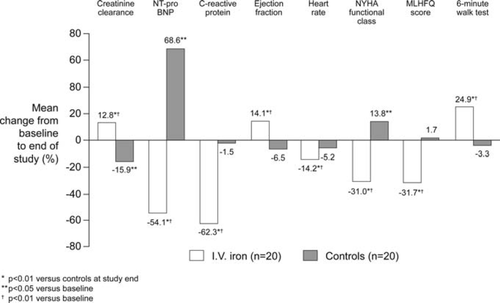
In terms of intervention, i.v. iron avoids the problems of poor absorption, gastrointestinal intolerance, and non-compliance seen frequently with oral iron preparations.34 Interventional studies using i.v. iron supplementation in patients with cardiorenal syndrome and iron deficiency, with or without anaemia, remain relatively limited. To date, three randomized controlled studies of i.v. iron alone in HF populations have been conducted,14,35,36 but only one restricted the study population to patients who had at least mild impaired renal function.35 In this double-blind trial, inclusion criteria were NYHA class II–IV, left ventricular ejection fraction ≤35%, anaemia (haemoglobin <12.5 g/dL for men, <11.5 g/dL for women), iron deficiency (<100 ng/mL and/or transferrin saturation ≤20%), and creatinine clearance ≤90 mL/min (mean creatinine clearance was ∼39 mL/min, i.e. moderate chronic kidney disease). Patients randomized to i.v. iron received iron sucrose 200 mg/week for 5 weeks, while control patients received isotonic saline. At the end of the study, the i.v. iron group showed significant improvements not only in haemoglobin and iron status, but also in left ventricular function, heart rate, quality of life, and functional capacity, as well as in renal function and levels of N-terminal pro brain natriuretic peptide (NT-proBNP) and C-reactive protein compared with no change or deterioration in the control arm (Figure 3). The relative risk of hospitalization was also lower in the i.v. iron arm (–2.33 vs. controls, 95% CI 1.59–3.42, P < 0.01). The study population, however, was small (n = 40).35 The other two randomized trials of i.v. iron alone in HF patients, the FAIR-HF14 and FERRIC-HF36 trials, did not specify renal dysfunction as an inclusion criterion. As discussed above, in the FAIR-HF trial, treatment was similarly beneficial in patients with or without anaemia. I.v. iron therapy was also associated with a modest but significant improvement in renal function regardless of the presence of anaemia or renal dysfunction at baseline.37 The FERRIC-HF study (n = 35), while smaller than FAIR-HF (n = 459), also showed that HF patients treated with i.v. iron experienced a significant improvement in exercise capacity and symptoms of HF, with a numerical improvement in renal function.36 Three uncontrolled studies of i.v. iron therapy in HF populations, in addition, have each found a benefit in terms of NYHA functional class.38–40
Questions to address in future clinical trials
- Does i.v. iron supplementation influence hard endpoints such as death, myocardial infarction, and stroke in the presence or absence of anaemia?
- Does i.v. iron supplementation affect the need to hospitalize or re-hospitalize patients in the presence or absence of anaemia?
- Does i.v. iron supplementation influence cardiac function in the presence or absence of anaemia?
- Does i.v. iron supplementation affect renal function in the presence or absence of anaemia?
- Is the absence of safety concerns in the FAIR-HF trial maintained in future trials?
Such studies could usefully be conducted in patients with HF, renal dysfunction, or both conditions. The findings of such trials would determine if the use of new acronyms such as CRAIDS and/or CRIDS is justified. For example, if patients with HF, renal impairment, and iron deficiency were to show clinical improvements following treatment with i.v. iron even in the absence of anaemia, as suggested by the findings of the FAIR-HF study, then the acronym CRIDS would justifiably be introduced into the clinical arena.
It is to be hoped that these important clinical trials are conducted, so that we may receive answers to these pivotal research questions, and thus allow patients with cardiorenal syndrome with/without anaemia and with/without iron deficiency potentially to benefit from modern-day anaemia and i.v. iron therapies.
Acknowledgements
The authors developed the content of this article at a meeting in London, UK, after which I.C.M. prepared the first draft. This was finalized following input from all authors with editorial support from a freelance writer funded by Vifor Pharma.
Conflicts of interest: I.C.M. has received speakers' fees, honoraria, and consultancy fees from several ESA and i.v. iron manufacturers, including Affymax, AMAG, Amgen, Ortho Biotech, Pharmacosmos, Roche, Takeda, and Vifor Pharma. B.C. has received fees for conferences and several grants and/or funding support for clinical trials and scientific studies from Fresenius Medical Care, Baxter, Gambro, Genzyme, Shire, Novartis, Bellco-Sorin, BMS, Amgen, Roche, Janssen-Cilag, Medcomp, and Hemotech. A.L.M.dF. has received speakers' fees from Roche, Amgen, and Vifor Pharma. G.F. has acted as a consultant and has received research support from Vifor. P.P. has received consultancy fees from Amgen and Vifor and speaker's fees from Vifor. D.S. has no conflicts of interest to declare. D.J.V.V. has received consultancy fees from Amgen and Vifor. S.D.A. has acted as a consultant to Vifor, Amgen, Takeda, and Noxxon, and has received research support from Vifor and speaker's fees from Vifor and Amgen.




