Short-term opioids for breathlessness in stable chronic heart failure: a randomized controlled trial
Abstract
Aims
To assess the effect of oral opioids vs. placebo on breathlessness in patients with chronic heart failure (CHF).
Methods and results
Oral morphine (Oramorph), oral oxycodone (Oxynorm), and placebo were studied in an outpatient setting. Once randomized, participants received all three interventions in a controlled double-blind crossover trial for 4 days each, with a 3-day washout between interventions. Patients known to the Hull and East Yorkshire Academic Cardiology department with CHF (New York Heart Association Grade III–IV) were invited to participate. Participants were eligible if they were on standard medical therapy with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, and diuretics.
Participant-rated change in 11-point numerical rating scale (NRS) (average over previous 24 h) breathlessness severity score from baseline (Day 1) to Day 4 of treatment was the primary outcome measure. The study was powered to detect a one-point change in severity.Thirty-nine patients were randomized and 35 completed all three study arms. Breathlessness severity was reduced from baseline with all three interventions. There was no statistically significant difference between active intervention and placebo or between the two types of opioid for the primary endpoint [−1.37 in NRS score for placebo group vs. −0.41 in morphine group (P = 0.13) and −1.29 for oxycodone group (P = 0.90)]. The response to treatment was not affected by aetiology, severity of CHF, or concurrent drug therapy. Opioid administration did not cause detrimental changes in clinical observations and was well tolerated.
Conclusion
We demonstrated no benefit over placebo for the relief of breathlessness with short-term low-dose oral opioids for CHF patients.
Trial registered prior to the recruitment of the first participant with Current Controlled Trials (www.controlled-trials.com; Trial number ISRCTN 85268059).
Introduction
Breathlessness is a cardinal symptom of chronic heart failure (CHF) and forms the basis of the New York Heart Association (NYHA) classification of heart failure severity. It is a cause of patient and carer distress and has a serious impact on quality of life, which is worse than for some patients with incurable cancer.1 The prevalence of CHF is increasing due to the rising age of the population, better survival following acute coronary events,2 and better medical therapy. Symptom control is therefore an issue in a growing number of people.
A cochrane review and meta-analysis of the use of opioids for breathlessness noted a small but statistically significant improvement in breathlessness severity with oral opioids vs. placebo.3 Most of the reviewed studies involved chronic obstructive pulmonary disease (COPD), although one was a single dose trial in CHF. More recently, a randomized controlled trial comparing 4 days of 20 mg/day oral modified release morphine or placebo found a statistically significant, though small, improvement in breathlessness severity in the morphine arm (6.6 mm on a 100 mm visual analogue scale in the morning, and 9.5 mm in the evening).4 The majority (88%) of participants had chronic lung disease. There are only three small studies of CHF exclusively.5 A single dose study looking at the effects of dihydrocodeine on exercise tolerance and chemosensitivity showed an improvement in exercise tolerance, reduced exercise ventilation, and improved breathlessness.6 A single dose study of diamorphine on exercise tolerance demonstrated improved aerobic exercise capacity by reducing ventilatory response to exercise and increasing tidal volume.7 A double-blind, randomized, placebo-controlled cross-over pilot study of 10 patients with NYHA class III/IV heart failure found a fall in median breathlessness score of 23 mm using a 100 mm Visual Analogue Score (P = 0.02) by Day 2 with morphine; the improvement was maintained to Day 4 (end of study period) while there was no significant change in the placebo arm.8
Opioid receptors are found throughout the cardiorespiratory system and endogenous opioids are released as part of the neurohormonal response to CHF.9–11 Sympathetic stimulation and endogenous opioid production are linked. Agonism at the opioid receptor may inhibit sympathetic drive by reducing intracellular cAMP.12,13 Down-regulation of chemoceptors may also reduce ventilatory drive and thus reduce sympathetic outflow.14 Sympathetic activation causes endothelial dysfunction in the peripheral skeletal muscle, leading to enhanced ergoreflex-mediated ventilatory response to exercise.15 Some of these mechanisms might be involved in the genesis of dyspnoea and may thus be modified by exogenous opioids. Oxycodone is an alternative opioid to morphine used in palliative medicine in patients with renal impairment. A study involving healthy volunteers suggested that kappa opioid receptor agonists like oxycodone, and unlike morphine, have greater diuretic properties,16 which might be beneficial for those with CHF. Many physicians are reluctant to use morphine in CHF for fear of adverse events, whereas others are more willing. Guidelines recommend their use in CHF,17 without substantial evidence of effectiveness. We therefore conducted a double-blind, cross-over randomized placebo-controlled trial to compare the effects of morphine and oxycodone for the relief of breathlessness in patients with advanced stable CHF.
Methods
Patient selection
Patients were identified from a heart failure clinic at Castle Hill Hospital, Hull, UK. Adults with a diagnosis of NYHA III–IV CHF with impairment of left ventricular systolic function (defined as an ejection fraction of <45% on trans-thoracic echocardiography) who were on standard medical therapy (diuretics and an inhibitor of the renin-angiotensin system at stable dose for at least 1 month) were eligible. Beta-blocker, aldosterone antagonist, and digoxin therapy did not form part of the eligibility criteria. Patients with co-existing diagnoses of respiratory disease or a peak expiratory flow rate of <150 L/min were excluded. Patients were excluded if they had known opioid sensitivities, were receiving opioid therapy, or had renal impairment (GFR < 30 mL/min). The first participant was randomized on 18 December 2007 and the last completed the study on 29 May 2009.
Study design
Two orally active opioids [oral morphine (Oramorph) and oral oxycodone (Oxynorm)] were compared with oral placebo. A cross-over design was chosen to reduce the number of study participants needed as it is difficult to recruit to palliative care studies. Equianalgesic doses (British National Formulary) of the two short-acting opioids were used [Oramorph 5 mg four times per day (QDS) and Oxynorm 2.5 mg QDS]. The placebo was designed to have very similar characteristics to the active medications (a clear, colourless liquid with the same viscosity and similar taste). Oxynorm liquid (concentration 5 mg in 5 mL) and Oramorph liquid (concentration 10 mg in 5 mL) allowed equivalent opioid doses for the same volume of liquid (2.5 mL for all three interventions) in order to maintain blinding. Following randomization, participants were assessed at home at baseline (Day 1), then 1 h after the first dose of medication. Participants were asked to take the medication QDS with subsequent assessments made at the same time of day for a total of 4 days in order to allow steady-state levels to be achieved. There was then a 3-day washout before the next intervention. The process was repeated for each of the other two interventions.
The order of interventions for each participant was randomized by the study drug manufacturer (Calderdale and Huddersfield NHS Pharmacy Manufacturing Unit, Huddersfield, UK) using a random number generation program. Block design was not used and there were six possible sequence combinations. The pharmacy dispensed all three medications for use in the required sequence with identical labels except for the treatment order. Hence the investigators and participants remained blinded to the treatment sequence and allocation was conducted distant to the research team. The oral liquid placebo was manufactured to a pre-determined formula and method, according to guidelines (www.mhra.gov.uk/Howweregulate/Medicines/Licencingofmedicines/ClinicalTrials/Legislationandguidancedocuments/index.htm last accessed 07/05/2010) set out by the Medicines and Healthcare Regulatory Authority (MHRA). Trial participants were also given a supply of anti-emetic medications (oral domperidone 10 mg PRN) and laxatives (senna 7.5 mg PRN) at the start of the trial for use in the event of common side effects.
The trial was conducted in accordance with the UK Regulations for Clinical Trials 200418 and was approved by the Leeds East Ethics Committee, Hull and East Yorkshire Research and Development Department, and the MHRA (Eudract number 2006-06718-13). The local National health service Research and Development department audited the study every 3–6 months to ensure adherence to Good Clinical Practice guidelines. The study was registered with the ISRCTN before the start of recruitment (number ISRCTN85268059).
The primary outcome was the mean change from baseline to Day 4 in patient-rated severity of average breathlessness over the past 24 h, measured using an 11-point numerical rating scale (NRS): 0 = no breathlessness; 10 = the worst breathlessness imaginable. The NRS is validated in chronic breathlessness19 and increasingly used in studies involving patients with this symptom.20–23 Measurement of ‘average’, ‘worst’ over the past 24 hours and ‘now’ is recommended in chronic breathlessness due to any advanced aetiology by a recent consensus statement and the Borg and NRS recommended by the authors of systematic literature reviews.20,24,25 Secondary outcome measures defined a priori included: change between baseline and Day 4 in worst breathlessness over the past 24 h; breathlessness ‘now’ using the same NRS; the modified Borg scale rated breathlessness severity;26 global impression of change in breathlessness27 from baseline to Day 4 (−7 ‘A very great deal worse’ to +7 ‘A very great deal better’); coping with breathlessness and satisfaction with treatment using the 11-point NRS; change in physical function (Karnofsky performance status28); and quality of life using the SF-12 (acute version 2).29 A daily patient-rated assessment of common adverse events was made using an 11-point NRS for nausea and drowsiness; and a categorical response for constipation, with a daily general enquiry for other adverse effects. Compliance was assessed each day on treatment, with measurement of residual volumes of liquid at trial end. Patients were asked while still blinded to state which week of treatment they preferred for their breathlessness.
Statistical analysis
The sample size was powered to observe a one-point difference between groups in their breathlessness score (80% power, P = 0.05). Allowing for a relatively high drop-out rate of 30%, we needed to randomize 48 patients to have 33 evaluable patients.
The primary analysis was a between-group comparison of the mean change from baseline to Day 4 in NRS average breathlessness over the past 24 h rather than individual group changes, as recommended by Senn30 who states that if the intervention arms are not compared directly one is simply making an observational effect of treatment.
All data were analysed using SPSS version 14 (IBM, Chicago, Illinois, USA) on an intention-to-treat basis. As the results from a crossover trial are not independent, we used paired methods of comparison of outcomes between treatments, for example, the Wilcoxon-signed rank test for continuous data that follow a non-parametric distribution. Paired t-tests were used if the data were normally distributed. McNemar's test was used for any paired dichotomous data. The statistical tests do not allow for period effects, and so the likelihood of such effects was also measured using analysis of variance. No period or sequence effects were identified.
Results
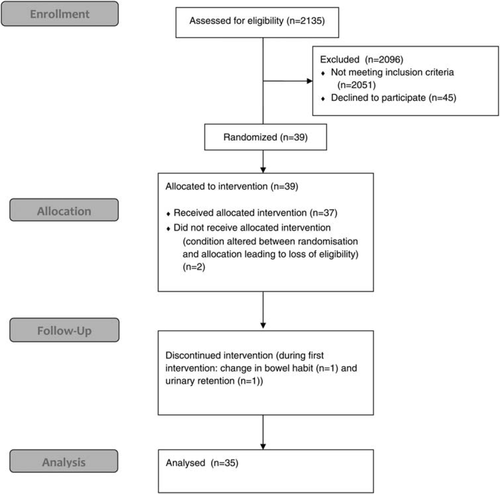
Figure 1 shows the study profile. We randomized 39 participants, and 37 started a study drug. Table 1 details the baseline characteristics of the 35 patients who completed all three study interventions. Two patients withdrew due to adverse events. Both participants had characteristics similar to those that completed the study and both withdrew while on Oramorph in their first week, one due to a change in bowel habit and one due to an episode of urinary retention managed as an outpatient. The patients were predominantly elderly and male, with ischaemic heart disease as the commonest aetiology of heart failure. Most patients were receiving beta-blocker therapy (86%) in addition to angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB) therapy. Performance status and quality-of-life scores were in keeping with heart disease populations.31 A Karnofsky score of 70% represents a level where the participant is able to care for themselves but is unable to carry on with normal activity or do active work.
| Age (mean ± SD) | 70.2 years (±11.1 years) |
| Range | 41–89 years |
| Sex | Male 30 (86%); female 5 |
| Aetiology | DCM 3 |
| IHD alone 25 | |
| IHD/hypertension 7 | |
| NYHA Grade | III: 31 (89%) |
| IV: 4 | |
| Ejection fraction: mean ± SD | 34 ± 7%; range 15–44% |
| CG-GFR calculated: (mean ± SD) | 70 mL/min ± 36 mL/min |
| eGFR: (mean ± SD) | 56 mL/min ± 19 mL/min |
| Beta-blocker | 30 (86%) |
| ACEI | 28 (80%) |
| ARB | 26 (74%) |
| Aldosterone antagonist | 21 (60%) |
| Digoxin | 4 (11%) |
| NT-proBNP: mean (±SD) | Mean 1779 ng/L (±2277 ng/L); |
| PEFR: mean (±SD) | Mean 262 L/min (±73 L/min) |
| Mean (SD) Karnofsky score at baseline | 70 (5.9) |
| Mean SF-12 scores at baseline | Physical component 28.2 |
| Mental component 47.2 | |
| Mean (SD) average breathlessness score at baseline | 5.1 (1.8) |
| Mean (SD) worst breathlessness score at baseline | 7.2 (1.5) |
| Mean (SD) average Borg score at baseline | 2.9 (1.4) |
| Mean (SD) worst Borg score at baseline | 4.3 (2.0) |
- a SD, standard deviation; DCM, dilated cardiomyopathy; IHD, ischaemic heart disease; NYHA, New York Heart Association; CG, Cockcroft and Gault; eGFR, estimated glomerular filtration rate; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; NT-proBNP, N-terminal pro-brain natriuretic peptide; PEFR, peak expiratory flow rate
Mean breathlessness scores improved over time in all three study arms (Table 2), but none was superior to any other (Table 3). Comparison of the change in NRS Average breathlessness scores between study arms revealed no statistically significant effect on change in severity [−1.37 in NRS score for placebo group vs. −0.41 in morphine group (P = 0.13) and −1.29 for oxycodone group (P = 0.90)]. Figure 2 shows the change in average breathlessness severity over time for the three interventions. There was an initial reduction in breathlessness scores after the first day that was maintained throughout the treatment course. Similar results were observed for average and worst breathlessness scores on NRS and modified Borg scales.
| Change in estimate from the start to the end of intervention | |||
|---|---|---|---|
| Oramorph (n= 35) | Oxynorm (n= 35) | Placebo (n= 35) | |
| Mean (SD) NRS average breathlessness | 0.41 (2.51) | 1.29 (2.19) | 1.37 (1.86) |
| Mean (SD) NRS worst breathlessness | 0.80 (2.55) | 1.43 (2.70) | 1.91 (2.50) |
| Mean (SD) Borg average breathlessness | –0.01 (1.96) | 0.33 (1.67) | 0.27 (1.66) |
| Mean (SD) Borg worst breathlessness | 0.16 (2.06) | 0.87 (2.31) | 0.80 (1.93) |
| Mean (SD) NRS distress | 0.57 (2.49) | 1.40 (2.43) | 1.77 (2.65) |
| Mean (SD) NRS coping | –0.03 (1.85) | 0.60 (2.16) | 0.43 (2.17) |
| Mean (SD) NRS satisfaction | 0.83 (3.14) | 0.34 (2.94) | 1.37 (2.80) |
| Rating scale | Wilcoxon comparison: placebo vs. oramorph | Wilcoxon comparison: placebo vs. oxynorm |
|---|---|---|
| NRS average breathlessness | Z = −1.52; P= 0.129 | Z = −0.13; P= 0.893 |
| NRS worst breathlessness | Z = −1.69; P= 0.092 | Z = 0.68; P= 0.50 |
| Borg worst breathlessness | Z = −1.41; P= 0.16 | Z = −0.087; P= 0.93 |
| Borg average breathlessness | Z = −0.30; P= 0.76 | Z = −0.34; P= 0.73 |

There was no impact of aetiology, gender, or concurrent drug therapy on response to treatment. Those with more severe CHF (as assessed by NT-proBNP, ejection fraction, or percentage of recommended ACEI/ARB target dose) did not have a greater response to opioids. There was no difference in response for those taking beta-blockers compared with those who were not.
Table 4 shows the effect of treatment on pulse, blood pressure, respiratory rate, and resting arterial oxygen saturation. There was no statistically significant change in any variable with either of the two active treatments or placebo.
| 1 h oramorph | 1 h oxynorm | 1 h placebo | Day 4 oramorph | Day 4 oxynorm | Day 4 placebo | |
|---|---|---|---|---|---|---|
| Mean (SE) resting pulse change (b.p.m) | −0.80 (1.19) | −4.29(1.10) | −2.65 (0.95) | 0.03 (1.76) | −5.83 (1.19) | 2.34 (1.14) |
| Mean (SE) resting systolic BP difference (mmHg) | −0.86 (1.77) | −1.26 (2.17) | 0.82 (2.78) | −5.34 (2.34) | −1.31 (2.51) | −0.34 (2.72) |
| Mean (SE) resting diastolic BP difference (mmHg) | −1.40 (1.78) | −0.26 (1.60) | 0.53 (2.07) | −5.74 (2.08) | −2.80 (2.69) | −1.09 (1.85) |
| Mean (SE) resting respiratory rate change (breaths/min) | −1.14 (0.29) | −1.60 (0.27) | −1.11 (0.33) | −0.46 (0.46) | −1.60 (0.42) | −0.86 (0.46) |
| Mean (SE) resting O2 saturations difference (%) | −0.46 (0.29) | −0.20 (0.26) | −0.59 (0.27) | −0.71 (0.33) | −0.49 (0.34) | −0.31 (0.28) |
Table 5 shows adverse effects. The figures represent the number of participants affected rather than the number of episodes, but most participants only experienced a single episode of the adverse event. One serious adverse event occurred at the end of the last day of the placebo therapy in one patient—a recurrence of a transient ischaemic attack, which was thought not to be related to the study. SF-12-rated quality of life and Karnofsky performance status did not alter for any intervention.
| Adverse event | Oramorph (n= 35) | Oxynorm (n= 35) | Placebo (n= 35) |
|---|---|---|---|
| Mean (SD) change in nausea on NRS | −1.57 (2.90) | −0.43 (1.63) | 0.20 (2.18) |
| Mean (SD) change in drowsiness on NRS | −0.40 (2.67) | 0.37 (2.64) | 0.69 (2.55) |
| Constipation on Day 4 | 12 (34%) | 10 (29%) | 4 (11%) |
| Vomiting | 3 (9%) | 2 (6%) | 0 |
| Itch | 3 (9%) | 0 | 0 |
| Light headedness/dizziness | 7 (20%) | 4 (11%) | 1 (3%) |
| Headache | 2 (6%) | 0 | 1 (3%) |
| Abdominal pain | 1 (3%) | 0 | 0 |
| Sweating | 1 (3%) | 1 (3%) | 0 |
| Dry mouth | 2 (6%) | 0 | 0 |
At the end of the study, patients were asked which of the three phases of treatment they preferred. Fourteen preferred the week with oramorph, nine the week with oxynorm, and eleven chose the placebo week. One participant had no preference for any week. Compliance with treatment was good for all three study arms. A residual volume of only 14% of the total dose was left at the end of each of the three study arms, suggesting participants received 86% of the recommended dose.
Discussion
There are few interventions for intractable breathlessness, and patients with severe CHF may have considerable distress despite maximal medical therapy. Ours is the first repeat dose, placebo-controlled study of two oral opioids in patients with CHF with 80% power to detect a change in severity of breathlessness of one-point on an NRS scale. We found that neither opioid improved breathlessness more than placebo. Our findings contrast with previous published research in opioids for intractable breathlessness in COPD or lung cancer and do not support current guidance for CHF.17 We did not identify the benefit found in the only previous comparable study (a pilot study8). The benefit found in the placebo-controlled study of sustained release morphine in patients with primarily COPD was equivalent to <1 on an NRS 0–10 scale,4 and our study may have been underpowered to detect such a small difference. Although studies indicate that the minimally clinically important difference (MCID) in acute breathlessness is 2 cm on a visual analogue scale,32–34 the MCID derived from patient rated data for chronic breathlessness in CHF is unknown, although MCID calculated from effect size and standard deviation in COPD suggests that even a difference of 1 cm or a change of one-point on an NRS scale is relevant. Thus, it may be that even a small difference in an intractable symptom is noticed by the patient. This needs to be explored further.
As inhibition of sympathetic outflow is a possible mechanism whereby opioid therapy may affect the perception of breathlessness, it is possible that the treatment period was too short to identify any benefit, and a future study should have a longer treatment period. Future studies should address whether opioid doses should be increased in those patients with good creatinine clearance who did not have a response, and a parallel group design would allow longer follow-up. The dose of opioid reflected doses used in previous studies4,8 to allow comparison. A recent dose titration study in multi-aetiology breathlessness demonstrated that 20 mg morphine per day (equivalent to the doses used in this study) yielded a response rate of 93%.35 In addition, there was no significant correlation between renal function and response in breathlessness measure or adverse event profile in our study (data not shown).
Measurement of chronic breathlessness is difficult. We have used NRS and Borg scores, both recommended by a recent consensus statement,25 but acknowledge that, apart from addressing the distress caused by the breathlessness, other aspects of the experience such as ‘mastery over the breathlessness’ was not assessed. Future work should include multi-variable assessments such as the Chronic Heart Failure Questionnaire36 or the similar Chronic Respiratory Questionnaire37 in addition to the unidimensional measure. One potential problem with the planned analysis is that changes in NRS score from baseline to Day 4 involves two potential measurement errors, rather than just one at the end of therapy. However, post-hoc analysis using end-of-therapy scores did not show a difference between the three study arm interventions (see Figure 2).
Overall, opioids were well tolerated and compliance with treatment was good. The most common unwanted effect of constipation was lower than expected (in the study by Abernethy et al.,4 nearly three quarters experienced at least mild constipation on morphine despite laxative prescription). This may be because only presence or absence was enquired after rather than a grading of none to severe. A recent pharmacovigilance study of low-dose opioids for breathlessness in people with advanced disease showed constipation to be the main drug-related adverse event leading to study withdrawal (6 of 52 patients), followed by drowsiness and nausea (4 patients each). Unsurprisingly for such a short intervention period, in our study, SF-12-rated quality of life and Karnofsky performance status did not alter for any intervention.
We have failed to demonstrate any benefit over placebo in reducing breathlessness although opioids were well tolerated and no patient developed cardio-respiratory compromise. As this study may have missed a delayed benefit, we recommend a subsequent parallel group placebo-controlled study with longer follow-up, powered at 90% to detect a one-point improvement on an NRS scale at 1 month. The study should include a qualitative assessment of patient and carer experience of any effect on breathlessness and a health economic assessment.
Acknowledgements
We thank the staff at the Academic Cardiology Unit, Castle Hill Hospital, Hull, UK for their support and in particular Mandy Walters, research nurse, for her help with screening and recruitment. Data sharing: no additional data available.
Funding
This study was funded with a Clinical Research Fellowship from Hull York Medical School, which remains independent of the research process. The sponsor of the study had no role in the study design, data collection, analysis and interpretation, writing of the report or the decision to submit for publication. All authors had access to the data in the study and take responsibility for its accuracy and integrity.
Conflict of interest: none declared.




