Implantable defibrillator therapy for ventricular tachyarrhythmia in left ventricular assist device patients
Abstract
Aims
Ventricular arrhythmias (VA) occur frequently after permanent left ventricular assist device (LVAD) implantation in end stage heart failure. Left ventricular assist device patients require rhythm control in contrast to patients with biventricular support. However, the rationale for implantable cardioverter-defibrillator (ICD) utilization in LVAD patients remains unclear. This study investigated the safety and efficacy of primary prevention ICD therapy and the rate of appropriate ICD interventions in LVAD patients.
Methods and results
We prospectively collected data from patients receiving LVADs. Patients without previous ICD received an ICD after LVAD implantation for primary prevention. Sixty-one patients with LVAD and ICD were followed prospectively for 365 ± 321 days. Nine patients died from thromboembolism or haemorrhage. Overall, the rate of appropriate ICD interventions was 34%, mostly for treatment of monomorphic VT in 52%, polymorphic VT in 13%, and VF in 35%. Seventy-one percent of VA were terminated by overdrive pacing, 29% by shock. Patients with a history of VA before LVAD implantation had a significantly higher 1-year rate for ICD therapy compared with LVAD patients with a primary prevention ICD indication LVAD patients (50 vs. 24%). Similarly, patients with non-ischaemic cardiomyopathy had a significantly higher risk for ICD therapy than patients with ischaemic heart disease (50 vs. 22%).
Conclusion
Implantable cardioverter-defibrillator therapy is safe and effective in LVAD patients. Ventricular arrhythmias leading to ICD intervention occur frequently in 34% of LVAD patients after 1 year, with large variations depending on the underlying cardiac disease and previous arrhythmia history. Primary prevention ICD indication after LVAD implantation yields high rates of ICD intervention. A history of previous VA strongly predicts future use of ICD treatment after LVAD implantation.
Introduction
Mechanical left ventricular assist devices (LVAD) have emerged as an effective treatment option besides heart transplantation in patients with advanced stage heart failure, improving survival and functional status as well as restoring quality of life in most cases.1,2 While first generation LVAD devices used pulsatile flow, pulmonary perfusion was maintained even during rapid ventricular arrhythmia (VA) and cardiac arrest was well tolerated without syncope. Delayed termination of ventricular fibrillation or flutter was reported to be safe and feasible in this setting.3 A large retrospective observational study reported a high VA incidence of 30% following pulsatile LVAD implantation for advanced heart failure in particular during the early post-operative period.4 With the growing demand for smaller LVAD devices, second generation LVADs are reduced in size and now use continuous flow, thereby relying on active left ventricular filling for adequate LVAD inflow. These second generation LVADs have recently been reported to effectively improve terminal heart failure.5 Continuous flow devices are not only smaller, but have improved mechanical stability compared with first generation pulsatile devices. Therefore, they are not only used as a bridge to heart transplantation, but with increasing frequency as a chronic therapy.5,6
The pro-arrhythmogenicity of LVAD implantation is a controversial subject and remains under discussion. Recurrent and frequent arrhythmias have been described for the early perioperative period following LVAD implantation and these have been attributed to acute left ventricular unloading,7 altered ventricular repolarization,8,9 mechanoelectrical feedback, and alterations in calcium handling gene expression10,11 during LVAD support. Mechanical irritation of the left ventricular apex at the inflow cannulation site may be another explanation for recurrent VA during LVAD therapy. We observed this phenomenon in an LVAD patient with ischaemic cardiomyopathy, who could reproduce non-sustained VT during Valsalva manoeuvres as shown in Figure Figure 1. This patient frequently experienced dizziness and fainting when bending down, which was attributable to haemodynamically relevant ventricular tachycardia during Valsalva. Previous observations of VA in LVAD patients have used heterogeneous methods for VA detection. Standard ECG during visits as well as implantable cardioverter-defibrillator (ICD) readouts have both been used, although ICDs allow more reliable and continuous monitoring of VA. In addition, these previous reports have mainly included secondary prevention ICD indication in LVAD patients and only a few patients with an ICD for a primary prevention indication, thereby biasing results. The incidence of VA in patients with an implanted LVAD leading to ICD interventions in an unselected LVAD population is unknown. A retrospective study investigating only 23 patients, including 10 patients with ICD implantation after LVAD implantation, proposed a high incidence of VA occurrence (52%).12
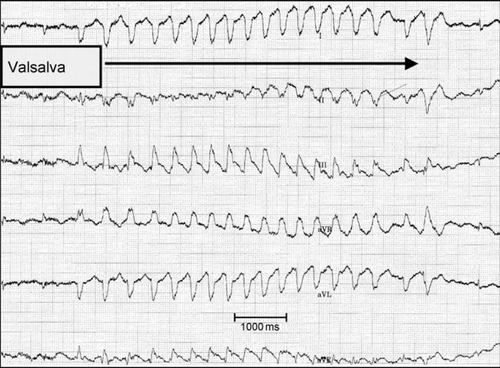
The present prospective observational study was performed to investigate the incidence and prevalence of VA, defined as ICD interventions, in patients with continuous flow LVAD devices. Furthermore, pre-specified subgroups for primary vs. secondary prophylaxis indications as well as ischaemic vs. non-ischaemic cardiomyopathy were studied.
Methods
Consecutive patients with drug refractory highly symptomatic congestive heart failure and successful implantation of a continuous flow LVAD between July 2005 and October 2008 were included. After the acute perioperative period, all patients without an ICD underwent ICD implantation. In these patients, ICDs were implanted on average 17 ± 15 days after LVAD implantation. Two different LVAD devices, each with a left ventricular apex inflow cannula, were implanted at our institution during the study period: the Heart Mate II (Thoratec, Pleasanton, CA, USA) and the Heartware (Heartware, Sydney, Australia). Left ventricular assist device selection was not pre-specified and was left to the discretion of the implanting surgeon. During LVAD implantation, previously implanted ICDs were deactivated to prevent inappropriate ICD discharge during surgery. The ICD was reactivated before patients were dismissed from the intensive care unit in order to prevent possible LVAD and ICD telemetry interactions as previously described.13
Patients receiving the ICD after LVAD implantation as well as those with previous ICD for primary prophylaxis without any previous ICD interventions were classified as primary prevention ICD recipients. Patients with previous appropriate ICD interventions and those with previous ICD implantation for documented VA or survived sudden arrhythmic death were classified as secondary prevention ICDs.
Defibrillator settings were intentionally adapted with a long detection duration for VA in order to reduce the frequency of ICD interventions for self-terminating VA. Thirty consecutive intervals (or 10 s) were intended to be programmed for the VT detection criteria and 18 of 24 beats (or 3 s) for the detection of ventricular fibrillation. A lowest detection interval of 340 ms was programmed for primary prophylactic devices, whereas those with previous VA were programmed to index VA cycle length plus 50 ms. For standardization, detection duration was calculated as the longest detection time divided by the lowest detection interval for those devices with programmable duration in seconds instead of intervals. Anti-tachycardia therapy was programmed to three bursts and three ramps and subsequently shocks of maximum energy (30–40 J) in VT zone and one burst and maximum energy shocks (30–40 J) in the VF zone. Pacing was programmed to VVI 40 b.p.m. backup stimulation, unless an anti-bradycardic pacing indication existed. Cardiac resynchronization therapy devices were kept on biventricular DDD pacing mode.
Of the 61 patients included in the study, the majority (n = 43, 71%) received amiodarone empirically during the perioperative period after LVAD implantation for suppression of self-terminating VA and rapid atrial tachyarrhythmia in order to prevent frequent ICD interventions. Amiodarone was discontinued during follow-up, when patients remained free of appropriate ICD interventions for VA at 6 months.
Outpatient routine follow-up was performed at 3-month intervals. During routine follow-up, VA occurrence was diagnosed by standard 12-lead ECG during visits and ICD memory readout. In case of stored episodes of sustained VA, two independent experienced electrophysiologists (H.O. and G.K.) judged them to be appropriate ICD intervention for VA or inappropriate ICD intervention for rapid supraventricular arrhythmia. The number of ICD interventions by shock delivery against ventricular fibrillation as well as overdrive pacing intervention episodes for ventricular tachycardia was recorded. The number of days until the first appropriate ICD intervention for VA after LVAD implantation was recorded.
Statistical analysis was performed using the SPSS 14.0 (SPSS, Chicago, IL, USA) software with Kaplan–Meier and log-rank calculations for VA occurrence including multivariate analysis for baseline criteria (age, gender, medication, and LVAD type). Values represent mean ± SD. Numeric values were tested with a two-sided independent t-test.
Results
Study population
Our study population consisted of 61 patients, who underwent LVAD implantation at our institution for refractory congestive heart failure. Baseline characteristics are presented in Table Table 1. Ischaemic and non-ischaemic cardiomyopathy as underlying cardiac disease were equally distributed among the predominantly male (95%) patients. The Heart Mate II was implanted in 44 cases and 17 patients received the Heartware LVAD system. The majority of patients (n = 46, 75%) underwent ICD implantation for primary prophylaxis of VA. In fact, 40 patients received ICD implantation for primary prevention following successful LVAD implantation. The remaining six patients had previously received an ICD for primary prevention without any pre-LVAD ICD interventions. Patient medication is presented in Table Table 1.
| Variable | n (%) |
|---|---|
| Total patients | 61 |
| Age (years) | 50 ± 12 (range 17–75) |
| Male sex | 58 (95) |
| Heart disease | |
| ICM | 30 (49) |
| NICM | 31 (51) |
| Medication | |
| Beta-blockers | 42 (69) |
| ACE-inhibitors | 41 (67) |
| Aldosterone antagonists | 25 (41) |
| Diuretics | 47 (77) |
| Calcium channel blockers | 5 (8) |
| Oral anticoagulation | 61 (100) |
| Platelet inhibitors | 11 (18) |
| Amiodarone | 43 (71) |
| Digitalis | 12 (20) |
| LVAD | |
| Heart Mate II | 44 (72) |
| Heartware | 17 (28) |
- a ICM, ischaemic; NICM, non-ischaemic; LVAD, left ventricular assist device.
Baseline implantable cardioverter-defibrillator properties
Routine defibrillation testing threshold was not performed, because the majority of our patients received high-energy devices with a dual-coil defibrillator lead. Furthermore, it has recently been shown that the gain of information from testing primary prophylactic ICD patients is low and risk is not negligible.14,15 Overall, 18 patients underwent ICD testing by VF induction without complications during the initial phase of the study. Two successful defibrillations at a 10 J safety margin below maximum device shock capacity were called appropriate defibrillation threshold (DFT) during testing (e.g. DFT was 20 J in a patient with two successful 20 J defibrillations in an ICD with maximum energy of 30 J). Shock energy was increased by 5 J if unsuccessful defibrillation was observed, until maximum device shock energy. No step down testing was performed. Defibrillation threshold of 25.7 ± 1.3 J was elevated (all left pectoral device with dual-coil defibrillator lead) compared with what is usually observed in patients with primary or secondary prophylactic ICDs.15,16 In all patients, induced VF was successfully terminated with a 30 J shock. Interestingly, dual-coil lead impedance was slightly higher at 43.6 ± 4.0 Ω compared with single-coil lead shock impedance of 34.5 ± 1.5 Ω. All devices were programmed to deliver only maximum shock energy and no unsuccessful shocks for spontaneous VA were documented during follow-up.
Mean ICD detection interval was 345 ± 31 ms for the lowest VT detection zone with long detection duration of 24 ± 1 intervals, while VF detection was programmed to 286 ± 13 ms with a VF detection counter of 24 ± 1 (Table Table 2).
| ICD devices and settings | n (%) |
|---|---|
| Primary prevention ICD | 46 (75) |
| Secondary prevention ICD | 15 (25) |
| VVI-ICD | 43 (71) |
| DDD-ICD | 5 (8) |
| CRTD | 13 (21) |
| VT-zone interval (ms) | 345 ± 31 |
| VT detection duration (intervals) | 24 ± 1 |
| VF-zone interval (ms) | 286 ± 13 |
| VF detection duration (intervals) | 24 ± 1 |
Patient safety and outcome
During a median follow-up of 12 months of ICD protection during ongoing LVAD support (range 13–1167 days), we observed 9 deaths (15%), which were attributed entirely to thrombo-embolic events and haemorrhage, in particular stroke (Table Table 3). Non-lethal cerebrovascular events were observed in three additional patients. Heart transplantation including LVAD and ICD removal was performed in seven patients (Table Table 3). Two patients, both with progression of non-ischaemic dilated cardiomyopathy, were urgently transplanted due to a combination of worsening heat failure and recurrent and painful ICD intervention for rapid VA despite administration of a combination of amiodarone and mexiletine. Frequent VA could not be suppressed by medication in either case.
| Outcome | n (%) |
|---|---|
| Patient status | |
| Alive with ongoing LVAD | 44 (72) |
| Alive with explanted LVAD (recovered) | 1 (2) |
| Heart transplantation | 7 (11) |
| Death | 9 (15) |
| Patient safety and complications | |
| Non-lethal cerebrovascular event | 3 (5) |
| LVAD cable infection (total) | 14 (23) |
| Conservatively managed | 13 (21) |
| Requiring surgical revision | 1 (2) |
| Operation for ICD system revision (total) | 14 (23) |
| Heart Mate II and ICD interaction | 4 (7) |
| ICD replacement for battery depletion | 4 (7) |
| Right ventricular lead failure | 3 (5) |
| ICD pocket haematoma revision | 3 (5) |
| VA burden | |
| Patients with appropriate ICD Therapy (total) | 21 (34) |
| With monomorphic VT | (52) |
| With polymorphic VT | (13) |
| With VF | (35) |
| Patients with inappropriate ICD therapy (after 7 days in-hospital blanking period) | 15 (25) |
No death could be related to malignant VA. Local infection of the percutaneous LVAD cable was observed in 14 patients (23%). One patient required surgical revision of the infected cable, whereas the remaining 13 patients were successfully treated by antibiotics and local wound care (Table Table 3). No ICD-related infections were observed. However, 14 patients (23%) required operative ICD revision during the study period (Table Table 3). Four defibrillators implanted before LVAD implantation were replaced due to interactions between the Heart Mate II and ICD telemetry as previously described.13 Implantable cardioverter-defibrillator replacement for regular battery depletion occurred in four patients. Electrode revision for right ventricular lead failure was necessary in three patients. No right ventricular lead failure was related to the LVAD implant procedure. However, one previously implanted left ventricular coronary sinus lead was damaged during LVAD implantation, requiring the deactivation of cardiac resynchronization after LVAD implantation. Wound haematoma within the ICD pocket requiring re-operation occurred in three patients following ICD implantation after previous LVAD implantation.
Only one patient, a 17-year-old male with acute heart failure due to inflammatory cardiomyopathy had his LVAD removed after 250 days since left ventricular function had completely recovered and heart failure symptoms had disappeared. Only very circumscribed apical hypokinaesia could be detected by echocardiography following LVAD removal with normal overall ejection fraction. Interestingly, this patient had five episodes of ventricular fibrillation 1 year after LVAD removal terminated by the ICD. Heart transplantation was performed in seven patients. Two of the transplanted cases received urgent transplantation for a combination of worsening heart failure and arrhythmia burden requiring frequent ICD treatment.
Implantable cardioverter-defibrillator treatment of ventricular arrhythmias during left ventricular assist device support
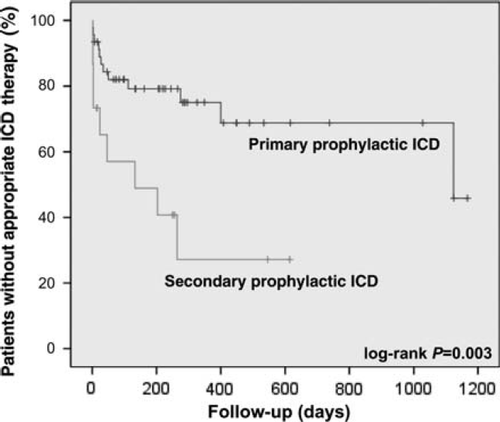
The majority of patients (n = 44, 72%) remained on LVAD treatment without complications (Table Table 3). Ventricular arrhythmia leading to ICD interventions was frequent in patients with LVAD. Thirty-four percent of our study population received appropriate ICD interventions for VA during follow-up. Ventricular arrhythmias were frequent during the early phase of LVAD support but were also observed late during follow-up of up to 3 years (Figure Figure 2). Even after exclusion of the first 7 days after LVAD implantation when patients are exceedingly vulnerable to VA during intensive care unit treatment and close monitoring, there was still a high rate of appropriate ICD interventions (15 patients; 25%) in the later follow-up. Analysis of stored intracardiac electrocardiograms revealed that 52% of all treated patients with VA had monomorphic VT, whereas 35% had ventricular fibrillation and 13% polymorphic VT.
The subgroup of 21 patients with appropriate ICD intervention after LVAD implantation received a total number of 144 episodes of spontaneous VA, 102 (71%) were treated by overdrive pacing only and 42 (29%) were treated by shock for ventricular fibrillation. Overall, we observed an average of five events per patient (range 1–28) and a median of five ventricular tachycardia episodes (range 0–21) and two (range 0–7) shocks for spontaneous ventricular tachycardia/ventricular fibrillation per patient.
Patients with a secondary prevention indication for ICD implantation, in whom the ICD was implanted before LVAD implantation (12 patients, mean ICD treatment before LVAD 16 ± 16 months), a monthly event rate was calculated (number of spontaneous VA divided by observation time) for the period before LVAD implantation (0.65 ± 1.56 VA events per month) and after LVAD implantation (0.65 ± 1.58 VA events per month). This calculated event rate did not differ for the period of time before vs. after implantation of the LVAD (P = 0.99). Patients with no previous arrhythmia history had an estimated 1-year risk of 24% for appropriate ICD treatment. Patients with a secondary prevention indication had an even higher 1-year risk of 50% (Figure Figure 2).
Patient symptoms were not investigated systematically. However, patients with rapid VA seemed free of symptoms if overdrive pacing terminated the arrhythmia. Implantable cardioverter-defibrillator shock, even for very rapid VA including ventricular fibrillation, was frequently perceived as palpitations without fainting. Left ventricular assist device function seemed to maintain circulation even during rapid VA at least temporarily to a degree, which prevented syncope. Thus, ICD shocks were frequently experienced painfully.
Ventricular arrhythmia event rate was significantly higher in non-ischaemic cardiac failure compared with ischaemic patients. One year risk for VA intervention was 50 and 22%, respectively. Multivariate analysis for other baseline criteria (age, gender, medication, and LVAD type) revealed no further differences.
Inappropriate ICD intervention was observed in 15 (25%) patients following the early 7 day blanking period. Two patients experienced inappropriate shocks due to right ventricular lead failure causing over sensing requiring surgical intervention. The remaining 13 patients had asymptomatic inappropriate overdrive pacing during high ventricular rate atrial fibrillation, no inappropriate shocks were documented.
Discussion
Our study shows that (i) more than one-third of LVAD recipients experience appropriate ICD therapy in the first year, (ii) patients with a secondary prophylactic ICD indication have a two-fold increased risk for appropriate shocks compared with patients with a primary prophylactic ICD indication, and (iii) patients with non-ischaemic cardiomyopathy have an even higher risk of appropriate ICD therapies than patients with ischaemic cardiomyopathy.
Ventricular arrhythmias have been reported to occur frequently in selected groups of LVAD recipients17 but previous data are biased since the detection of VA was performed by standard ECG during follow-up and only occasionally by ICD data readouts. In addition, the distribution of ICD recipients in previous published LVAD studies is likely to be preferentially for secondary prophylactic ICD indications and is mostly not differentially addressed.4,18 Published data on ICD implantation for primary prevention in LVAD patients is limited to a retrospective study which included 23 Heart Mate II patients.12 Our prospective study explicitly describes the incidence and the character of VA in an unselected group of LVAD recipients with ischaemic as well as non-ischaemic cardiomyopathy and differentiates between primary and secondary prophylactic ICD indications. Our data show that patients with a history of previous VA and a secondary prevention indication have a two-fold higher risk of VA recurrence and ICD intervention, supporting the ongoing use of ICD in these patients even after LVAD implantation. The incidence of VA in our study is much higher than previously reported in other mainly secondary prophylactic ICD trials. In previous studies, the incidence of appropriate ICD therapy ranges from 8 to 22% depending on the patient population and also the programming of the device which makes a direct comparison difficult.19–22 However, in our study, the 1-year risk of an appropriate ICD therapy was 50%, which appears higher than these previous reports and might be explained by our LVAD patient population with severe progressive heart failure. Notably, the TOVA study has recently shown that higher NYHA class is an independent predictor of appropriate ICD therapy.20 Furthermore, some degree of pro-arrhythmogenicity of LVAD treatment, in particular the left ventricular apex cannulation, could very well play a role in generating higher event rates in our study population.
In patients with a primary prophylactic ICD indication, we also found a higher than expected rate of appropriate ICD therapies, 24% after 1 year. In the SCD-HeFT study, 22.8% of the patients experienced appropriate ICD shocks after 45 months although the device programming was limited to a ventricular fibrillation detection zone only (lowest detection level 320 ms).23 The higher than expected rate of ICD interventions in LVAD patients with a primary prophylactic ICD indication might reflect the larger substrate for VA induction and perpetuation and possibly again mechanical myocardial irritation by the LVAD cannula.
Our data do not in general support the hypothesis of pro-arrhythmogenicity of LVAD implantation, as event rates in the subgroup of patients with long-term ICD use before and after LVAD implantation were similar. Previous reports of increased VA occurrence after LVAD implantation may be explained by the critical condition of patients receiving an LVAD. However, myocardial substrate causing VA remains arrhythmogenic after LVAD implantation, which may limit LVAD use in patients with a known high VA burden. Neither an increase nor a significant reduction in VA event rates was observed in our subgroup with long-term ICD therapy before and after LVAD implantation.
Inappropriate ICD shocks were only observed in two patients with right ventricular lead failure. We hypothesize that the lack of frequent inappropriate ICD shocks during the ambulatory outpatient period observed in our study cohort was achieved by our conservative ICD programming: long VA detection periods, high detection heart rates, and implementation of available discrimination algorithms. Furthermore, strict adherence to medication including amiodarone might have prevented many episodes of inappropriate treatment of supraventricular arrhythmias.
Non-ischaemic cardiomyopathy patients had a significantly higher rate of ICD intervention for VA compared with patients with ischaemic heart disease. The reason for this difference remains unclear, baseline data did not show differences in age, left ventricular dysfunction, NYHA stage, or medication. Interestingly, one patient who received an LVAD for inflammatory cardiomyopathy and who completely recovered under ventricular unloading was explanted 9 months later. However, he experienced five ICD therapies for VF 12 months after LVAD explantation. This case shows that even after full recovery, risk for VA might remain increased either due to the remaining substrate of underlying heart disease or iatrogenic scar at the ventricular apex after LVAD therapy. As we had no 12-lead ECG data for the episodes, we could not localize the ventricular origin of the episodes treated by the ICD.
Given the fact that ICD implantation has been shown to overestimate the risk of malignant VA,24 our study results must be interpreted with caution. Implantable cardioverter-defibrillator interventions do not directly reflect the rate of true sudden death events. However, we tried to reduce overtreatment by using conservative ICD programming with a high number of intervals to detect before ATP or shock delivery. Furthermore, it is unclear how often ventricular fibrillation in LVAD patients leads to cardiogenic shock and how often LVAD support is sufficient to sustain long-term circulation in non-pulsatile LVADs.
Another limitation of our study is the lack of randomization.
Clinical implications
Our study is the first to show prospective data for a large cohort of primary prevention ICD indication patients after LVAD implantation, adding important information to previously published ICD intervention rates in selected patients with implanted defibrillators.18 Our study demonstrates a high prevalence of VA in LVAD recipients, even for those with a primary prevention indication, supporting the use of ICDs in patients after LVAD implantation for primary prevention of sudden arrhythmic death. Furthermore, LVAD patients with a previous ICD implantation should be kept on ICD protection.
For ICD therapy in an LVAD patient, we would recommend implantation of a high-energy ICD device, because we observed an increased DFT in our patients. We hypothesize that this finding is due to end stage heart disease in LVAD recipients on the one hand, and electrical interference of conducting artefacts inside the thorax especially in anterior shock vectors in single-coil defibrillator leads on the other hand. The latter finding is supported by the observation of lower shock impedance in the single-coil system configuration compared with the dual-coil configuration. A general recommendation in favour of ICD implantation in every patient receiving an LVAD cannot be derived from our data and requires a larger prospective investigation.
Conclusion
Implantable cardioverter-defibrillator therapy is safe and effective in LVAD patients. Ventricular arrhythmias leading to ICD intervention occur frequently in LVAD patients over 1 year of follow-up, with large variations depending on the underlying cardiac disease and previous arrhythmia history. A primary prevention ICD indication after LVAD implantation yields high rates of ICD intervention. A history of previous VA strongly predicts future use of ICD treatment after LVAD implantation.
Conflict of interest: none declared.




