Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction
Abstract
Aims
In patients with systolic heart failure (SHF) a high prevalence of sleep-disordered breathing (SDB) has been documented. The purpose of this study was to investigate the prevalence and type of SDB in patients with heart failure with normal left ventricular ejection fraction (HFNEF).
Methods and results
Two hundred and forty-four consecutive patients (87 women, aged 65.3 ± 1.4 years) with HFNEF underwent capillary blood gas analysis, measurement of NT-proBNP concentrations, echocardiography, cardiopulmonary exercise testing (CPX), cardiorespiratory polygraphy, and simultaneous right and left heart catheterization. Sleep-disordered breathing was defined as an apnoea–hypopnoea-index (AHI) ≥5/h. Sleep-disordered breathing was documented in 69.3% of all patients, 97 patients (39.8%) presented with OSA and 72 patients (29.5%) with CSA. With an increasing impairment of diastolic function the proportion of SDB, and CSA in particular, increased. Patients with SDB performed worse on CPX and six-minute walk test. Partial pressure of CO2 was lower in CSA, whereas AHI, left atrial diameter, NT-proBNP, LVEDP, PAP, and PCWP were higher.
Conclusion
There is a high prevalence of SDB in HFNEF. In parallel to SHF, CSA patients in particular are characterized by a more impaired cardiopulmonary function. Whether SDB is of prognostic relevance in HFNEF needs to be determined.
Introduction
Congestive heart failure (CHF) is a major public health problem in many countries.1 Patients who meet the criteria for clinical diagnosis of heart failure are heterogeneous, with an increasing proportion of heart failure with normal left ventricular ejection fraction (HFNEF).2 By the seventh decade of life the prevalence of HFNEF approaches, and by the eighth decade of life it exceeds that of systolic heart failure (SHF).3 Several authors and guidelines have presented a definition for HFNEF, all propose a classification scheme including the following criteria: clinical evidence of CHF, normal or mildly abnormal LV systolic function expressed as ejection fraction (EF), and evidence of abnormal LV relaxation, filling, or diastolic stiffness.4–6 Survival of patients with HFNEF is similar to that of patients with SHF.7
The association between sleep-disordered breathing (SDB) and SHF is well known. In large-scale studies including up to 700 patients, a high prevalence of SDB among patients with SHF had been documented.8–11 Obstructive sleep apnoea (OSA) is considered an independent risk factor for hypertension, CHF, and pulmonary hypertension.12–14 Hypertensive patients with heart failure often present with preserved left ventricular systolic function but compromised diastolic function and comprise a substantial proportion of the patients with HFNEF. On the other hand, Cheyne–Stokes-Respiration (CSR) and central sleep apnoea (CSA) are highly prevalent in patients with pulmonary hypertension,15 one possible consequence of diastolic dysfunction.
The purpose of this study was to investigate the prevalence and type of SDB in 244 patients with HFNEF and to evaluate subjective and objective clinical, echocardiographic, and haemodynamic parameters in these patients.
Methods
Patients
Between January 2006 and March 2008, a total of 878 patients with symptoms of heart failure, admitted to our cardiology facility were screened for presence and type of SDB. Of these, 244 patients with HFNEF, defined according to the guidelines mentioned previously,4–6 were eligible according to the inclusion and exclusion criteria, and were enrolled into the study. In summary, patients had to be in a stable clinical condition, in NYHA class II–IV, with normal systolic function according to echocardiographic and invasive criteria (LVEF >55%) with an end-diastolic volume index (LVEDVI) <97 mL/m2.2 Diagnostic evidence of diastolic dysfunction was obtained invasively [LV end-diastolic pressure (LVEDP) >16 mmHg or mean pulmonary wedge pressure (PCWP) >12 mmHg]. In addition patients had to be on stable medication for at least 4 weeks. The majority of the remaining 634 patients were excluded from the trial due to compromised LV systolic function (n = 511). Other reasons for exclusion were significant valvular heart disease (any valve insufficiency or stenosis >first degree), a history of SDB or ongoing treatment of SDB, evidence of pulmonary disease, hypercapnia (pCO2 >45 mmHg) in capillary blood gas samples, pregnancy, acute coronary syndrome, or acute cardiac decompensation. This was a prospective observational study that used cardiorespiratory polygraphy as an approved diagnostic procedure. The study flow-chart is summarized in Figure 1. Sleep studies as well as all other investigations were performed during the patients’ primary admission to the cardiology department of our hospital.
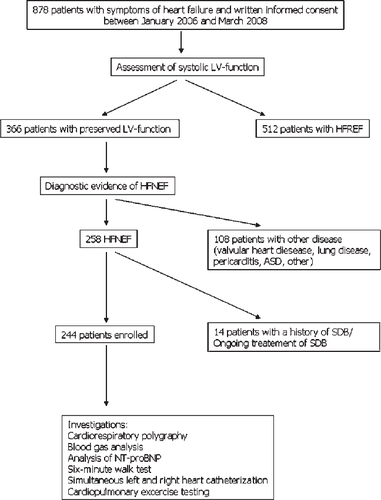
All patients gave written informed consent to the clinical procedures. The study was retrospectively approved by the Ethical Review Board of the University of Ruhr, Bochum.
Cardiorespiratory polygraphy
Sleep studies were performed by in-hospital unattended cardiorespiratory polygraphy (Embletta, Embla, The Netherlands). Nasal air flow, chest and abdominal effort, pulse oximetry, and body position were recorded continuously. The temporary loss of not more than one channel, except for nasal air flow, was allowed. Analyses were performed using Somnologica for Embletta™ software (Medcare, Embla, The Netherlands) and reviewed by two independent SDB specialists not involved in the patients’ treatment. Hypopnoea was defined as a ≥30% reduction in airflow in combination with an oxygen desaturation of at least 3%. Apnoea was defined as a cessation of airflow for ≥10 s, and in case of CSA without any thoracic or abdominal breathing effort.
If the apnoea–hypopnoea-index (AHI) was <5/h patients were considered to have no SDB (noSDB, AHI <5), patients with an AHI ≥5/h, were classified as central (CSA) or OSA according to the majority of events. An AHI 5–14/h was considered to indicate mild, an AHI 15–29/h moderate, and an AHI ≥30/h severe, SDB.
Echocardiography
Diameters and dimensions of the left atrium (LA) and left ventricle were measured by M-Mode and corrected by 2D echocardiography following American Society of Echocardiography guidelines.16 Left ventricular ejection fraction was measured according to the modified Simpsońs method using apical four and two chamber views. Blood flow Doppler as well as tissue Doppler were used to assess diastolic function. Peak early (E) and peak atrial (A) Doppler mitral valve flow velocity, ratio of peak early and peak atrial Doppler mitral valve flow velocity (E/A), and deceleration time (DT) of early Doppler mitral valve flow velocity were documented. Left ventricular filling patterns were classified as normal, impaired relaxation, pseudonormal, or restrictive, taking into account early diastolic (E′) lengthening velocities and the ratio of early mitral valve flow velocity and early diastolic lengthening velocities (E/E′). Normal pattern was defined by an E/A ratio >1, a DT between 150, and 220 ms and E/E′ <10. Impaired relaxation was determined by E/A <1, DT >220 ms, and E/E′ >10. Pseudonormal pattern was identified by E/A between 1 and 2, DT between 150 and 220 ms, and E/E′ >10. Finally, restrictive pattern was defined by E/A >2, DT <150 ms, and E/E′>10.
Left and right heart catheterization
Left heart angiography was performed with a 5–7F pigtail catheter (Cordis, Langenfeld, Germany), which was introduced retrogradely into the LV and connected to a cardiac functional laboratory for acquisition of left ventricular end-diastolic pressure (LVEDP). Pulmonary artery pressure (PAP) and pulmonary capillary wedge pressure (PCWP) were recorded using a balloon-tipped flotation thermodilution catheter (7F Arrow, Arrow International) via the right femoral vein.
Six-minute walk test
A six-minute walk test was performed according to guidelines issued by the American Thoracic Society.17
Capillary blood gas analysis
Partial pressure of carbon dioxide (pCO2), partial pressure of oxygen (pO2), and capillary oxygen saturation were measured using ABL 330 (Radiometer, Copenhagen, Denmark).
N-terminal pro-brain natriuretic peptide
N-terminal pro-brain natriuretic peptide (NT-proBNP) was used as an additional marker of the severity of HFNEF. Analyses were performed using the Elecsys 2010 analyzer (Roche, Basel, Switzerland).
Cardiopulmonary exercise testing
In order to evaluate exercise tolerance, peak oxygen consumption (VO2-peak), oxygen consumption at the individual aerobic-anaerobic threshold (VO2-AT), and relationship of minute ventilation and carbon dioxide production (VE/VCO2), a symptom-limited bicycle exercise test was performed. Starting at 10 W, workload was increased by 10 W every minute. Maximum workload and total exercise time were recorded; predicted VO2-peak was calculated automatically taking patients gender and age into account.
Statistics
Statistical analyses were performed using SPSS (version 10.2.6) software (SPSS Inc. Chicago, IL, USA). Differences between groups were compared by ANOVA test. Correlation analysis was performed using Spearman rank correlation. A value of P < 0.05 was considered significant for all comparisons.
Results
Patients
A total of 244 patients with HFNEF were enrolled into the study, of these 96 patients presented with an impaired left ventricular relaxation filling pattern, 104 with a pseudonormal left ventricular filling pattern, and 44 patients with a restrictive left ventricular filling pattern. The cause of heart failure was hypertensive heart disease in 108 patients (44%), coronary artery disease in 80 patients (33%), and cardiomyopathies (hypertrophic, restrictive) in 56 patients (23%). Baseline demographic and clinical data are summarized in Table 1. The proportion of male patients was highest in patients with CSA, and lowest in patients with noSDB. Patients with SDB were significantly older, presented with a higher NYHA-class and were more obese than patients with noSDB. A history of hypertension was also more frequent in these two subgroups. The percentage of patients with diabetes was significantly higher in patients with OSA than in those with CSA or noSDB. Compared with patients without SDB, more patients with central sleep apnoea had atrial fibrillation.
| Variable | CSA | OSA | noSDB |
|---|---|---|---|
| n (%) | 72 (29.5) | 97 (39.8) | 75 (30.7) |
| Male, n (%) | 60 (83.3)‡∫ | 61 (63.0)† | 36 (48.0) |
| Age, years | 66.9 ± 2.4‡ | 66.8 ± 1.9† | 61.6 ± 3.3 |
| Height, cm | 174.9 ± 1.9‡∫ | 170.1 ± 2 | 171.3 ± 2.4 |
| Weight, kg | 89.8 ± 3.4‡∫ | 84.9 ± 3.2† | 77.9 ± 3.7 |
| BMI | 29.3 ± 0.9‡ | 29.3 ± 1.1† | 26.42 ± 1 |
| NYHA class | 2.5 ± 0.2‡ | 2.4 ± 0.24† | 2.2 ± 0.33 |
| Hypertension, n (%) | 67 (87.0)‡ | 88 (88.0)† | 60 (80.0) |
| Diabetes, n (%) | 22 (28.6) | 45 (45.0)†∫ | 11 (14.7) |
| Atrial fibrillation, n (%) | 17 (23.6)‡ | 19 (19.6) | 13 (17.3) |
| Cerebrovascular disease, n (%) | 9 (12.5) | 14 (14.4) | 10 (13.3) |
| Medication, n (%) | |||
| Beta-blockers | 64 (83.1) | 78 (78.0) | 64 (85.3)† |
| ACE/AT1-inhibitors | 66 (85.7) | 76 (76.0) | 56 (74.6) |
| Calcium-channel blockers | 30 (39.0) | 46 (46.0) | 29 (38.7) |
| Nitrates | 16 (20.8) | 24 (24.0) | 12 (16.0) |
| Digitalis glycosides | 9 (11.7) | 9 (9.0) | 3 (4.0) |
| Diuretics | 53 (68.8) | 57(57.0) | 41 (54.7) |
| Other | 76 (98.7)‡ | 97(97.0)† | 67 (89.3) |
| Capillary blood gas analysis | |||
| pCO2 (mmHg) | 34.9 ± 0.8‡∫ | 39.4 ± 0.7† | 37.2 ± 0.8 |
| pO2 (mmHg) | 76.0 ± 2.1 | 80.6 ± 15.4 | 79.9 ± 2.5 |
- † P < 0.05 OSA vs. noSDB.
- ‡ P < 0.05 CSA vs. noSDB.
- ∫ P < 0.05 OSA vs. CSA.
Sleep-disordered breathing
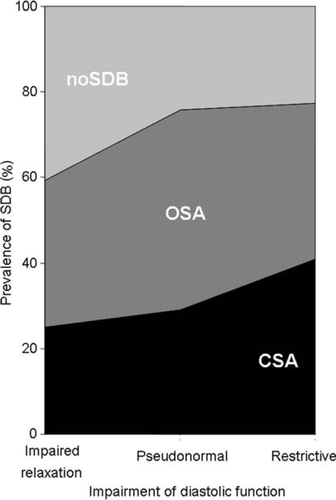
Among the 244 patients with HFNEF, SDB was documented in 169 patients (69.3%), of these 97 patients (39.8%) presented with OSA and 72 patients (29.5%) with CSA. Only 75 patients (30.7%) had no relevant SDB. Severity of SDB was mild in 21 patients with CSA (8.3%) and in 40 patients with OSA (40%), moderate in 15 patients with CSA (6.0%) and 36 patients with OSA (36%), and severe in 41 patients with CSA (16.3%) and 24 patients with OSA (24%). In the group with an impaired left ventricular relaxation filling pattern, 24 patients (25%) presented with CSA, 33 patients (34.4%) with OSA, and 39 patients (40.6%) with noSDB. Of the patients with a pseudonormal left ventricular filling pattern, 30 had a CSA (29.1%), 48 an OSA (46.6%), and 25 patients (24.3%) had noSDB. Of the patients with a restrictive left ventricular filling pattern, 18 had CSA (40.9%), 16 had OSA (36.4%), and 10 had noSDB in 10 (22.7%) (Figure 2). Additional findings are presented in Table 2. The AHI in the restrictive and pseudonormal left ventricular filling pattern group was higher than in the impaired relaxation left ventricular filling pattern group. In addition, patients with a pseudonormal left ventricular filling pattern had longer apnoea and hypopnoea periods compared with the other subgroups.
| Variable | Impaired relaxation | Pseudonormal | Restrictive |
|---|---|---|---|
| n (%) | 96 (39.3) | 104 (42.6) | 44 (18.0) |
| AHI, h−1 | 15.0 ± 3.6 | 20.0 ± 3.3† | 23.4 ± 6.2‡ |
| Mean oxygen saturation, % | 92.6 ± 0.6 | 92.9 ± 0.6 | 92.6 ± 0.9 |
| Maximum oxygen desaturation, % | 84.6 ± 1.0 | 83.6 ± 1.2 | 83.5 ± 1.8 |
| Average oxygen desaturation, % | 4.5 ± 0.4 | 5.2 ± 0.6 | 5.0 ± 0.7 |
| Longest apnoea duration, s | 22.3 ± 4.0 | 30.1 ± 4.0† | 25.4 ± 7.5 |
| Longest hypopnoea duration, s | 32.1 ± 3.0 | 39.1 ± 3.7† | 37.8 ± 6.3 |
- † P < 0.05 pseudonormal vs. impaired relaxation.
- ‡ P < 0.05 restrictive vs. impaired relaxation.
Echocardiographic parameters
Echocardiographic findings are presented in Table 3. Left atrial enlargement was more pronounced in the CSA group than in the OSA and noSDB groups (P < 0.05). Patients with OSA had a larger left atrial diameter compared with patients with noSDB (P < 0.05). E wave was higher in the CSA group than in the noSDB group (P < 0.05), E/A ratio was higher in the CSA group compared with OSA (P < 0.05) and DT was higher in the noSDB group than in the OSA group (P < 0.05).
| Variable | CSA | OSA | noSDB |
|---|---|---|---|
| Echocardiographic results | |||
| E wave (m/s) | 0.91 ± 0.11‡ | 0.86 ± 0.06 | 0.78 ± 0.06 |
| A wave (m/s) | 0.71 ± 0.08 | 0.77 ± 0.05 | 0.75 ± 0.06 |
| E/A ratio | 1.57 ± 0.28∫ | 1.26 ± 0.15 | 1.27 ± 0.22 |
| DT (ms) | 216 ± 23 | 199 ± 15† | 236 ± 23 |
| LA (mm) | 49.6 ± 1.6∫‡ | 46.1 ± 1.5† | 43 ± 1.7 |
| Cardiopulmonary exercise testing | |||
| VO2 AT (mL/min/kg) | 13.7 ± 1.2‡ | 14.1 ± 1.2 | 19.5 ± 6.5 |
| VO2 peak (mL/min/kg) | 15.9 ± 1.4‡ | 16.3 ± 1.3† | 19.8 ± 1.5 |
| Pred. VO2 peak (%) | 73.5 ± 7.3‡ | 74.8 ± 5.3† | 85.9 ± 5.8 |
| Workload (W) | 89.9 ± 12.6 | 91.1 ± 10.7† | 108.2 ± 13.6 |
| VE/VCO2 slope | 31.6 ± 2.1‡ | 29.6 ± 1.6 | 28.2 ± 1.5 |
| Invasive haemodynamics | |||
| PAP (mmHg) | 31 ± 2.4‡∫ | 27.8 ± 1.8 | 26.4 ± 2.3 |
| PCWP (mmHg) | 21 ± 1.3‡∫ | 19 ± 1.2 | 18.7 ± 1.9 |
| LVEDP (mmHg) | 23.4 ± 1.8‡∫ | 21.1 ± 1.2 | 21.0 ± 1.3 |
- † P < 0.05 OSA vs. no SDB.
- ‡ P < 0.05 CSA vs. noSDB.
- ∫ P < 0.05 OSA vs. CSA.
N-terminal pro-brain natriuretic peptide
A significantly higher level of NT-proBNP was observed in patients with CSA (1237 ± 544 pg/mL) compared with those with OSA (643 ± 378 pg/mL) and those with noSDB (376 ± 192 pg/mL).
Blood gas analysis
pCO2 was lower in patients with CSA than in those with noSDB and those with OSA (P < 0.05). The noSDB group presented with a lower pCO2 compared with the OSA group (P < 0.05, Table 1). There was no significant difference in pO2 among the three groups.
Six-minute walk test
The mean walk distance was 296 ± 68 m in CSA patients, 334 ± 59 m in OSA patients, and 423 ± 63 m in noSDB patients (P < 0.05 noSDB vs. CSA; P < 0.05 noSDB vs. OSA, P < 0.05 OSA vs. CSA).
Cardiopulmonary exercise test
Results from cardiopulmonary exercise testing are presented in Table 3. VO2 at the aerobic/anaerobic threshold in the CSA group was lower than in the noSDB group, whereas patients with CSA and patients with OSA showed a more severely reduced VO2 peak in comparison to patients with noSDB. While the OSA group presented with a lower maximum workload compared with patients with noSDB, the CSA group had a higher VE/VCO2-slope in comparison to patients with OSA or noSDB (P < 0.05).
Invasive haemodynamic measurements
Haemodynamic parameters are summarized in Table 3. Patients with CSA had higher LVDEP, a higher PAP, and a higher PCWP compared with patients with OSA and noSDB (P < 0.05).
Discussion
This is the first large-scale-study to show a high prevalence of SDB in patients with HFNEF, with the proportion rising in parallel to an increased impairment of diastolic function measured by echocardiography. Patients with SDB and especially central sleep apnoea presented with more advanced symptoms and more impaired cardiovascular function.
In patients with CHF and reduced LV-function, a high prevalence of SDB is well documented. In a study of 700 patients with SHF, SDB was documented in 76% of patients, of whom 40% had central sleep apnoea and 36% OSA, using an apnoea–hypopnoea-index cut-off of 5/h.8 Using an AHI cut-off of 15/h, several studies have documented moderate-to-severe SDB in about 50% of heart failure patients with reduced ejection fraction.8–11
In contrast, data on patients with HFNEF are rare. In a small cohort of 20 patients, Chan et al.18 reported that 55% had significant SDB, mainly OSA, which is mostly consistent with our results. Furthermore, they demonstrated that E-wave deceleration time of mitral inflow, an index of diastolic relaxation, was more prolonged in the group with SDB. We could not show analogous results, mainly because our study population included a higher proportion of patients with pseudonormal or restrictive left ventricular filling pattern which produces a faster E-wave deceleration time. However, our finding that with greater impairment of diastolic function the proportion of patients with SDB, and CSA in particular, increases is supported by similar findings for SHF, where a greater impairment of systolic function is linked to a larger incidence of SDB and CSA as well.8
Whether SDB occurs as a consequence of HFNEF or SDB induces diastolic dysfunction has not yet been clarified.
On the one hand, Fung et al.19 investigated 68 OSA patients for parameters of diastolic dysfunction and stated that more severe SDB was associated with a higher degree of diastolic dysfunction. These results were supported by Otto et al.,20 who compared 23 otherwise healthy patients with OSA to 18 patients without OSA and found an increased left atrial volume index as well as abnormal diastolic filling parameters in the OSA group. Besides an increased left atrial volume index, Romero-Corral et al.21 reported an association between SDB and an impaired myocardial performance index. Correspondingly Sidana et al.22 found a higher prevalence of diastolic dysfunction in moderate-to-severe OSA than in those with mild or no OSA. The main reason for this could be repeated nocturnal hypoxaemias leading to sympathetic nerve activation with a consequent increase in hormonal activation and arterial blood pressure,14 thus predisposing to wall thickening and compromised diastolic function.
On the other hand, a mechanism of central sleep apnoea is said to be based upon pulmonary congestion induced stimulation of pulmonary vagal irritant receptors and enhanced central and peripheral chemosensitivity leading to hyperventilation and respiratory instability.23–27 In HFNEF, pulmonary congestion may arise from a compromised left ventricular filling pattern which supports the theory that SDB, and CSA in particular, could be a consequence of HFNEF. Our findings of a correlation between PCWP and AHI in the entire cohort and in the CSA-group specifically, support this idea as do the findings of Bucca et al.28 that diuretic treatment of HFNEF produces a significant decrease in AHI, possibly due to a reduction in pulmonary congestion.
Finally, the impaired clinical, haemodynamic, and echocardiographic parameters detected in this study suggest that SDB, and central sleep apnoea in particular, may serve as a marker of the severity of HFNEF, a model that has been proposed for heart failure with reduced ejection fraction as well,29 possibly suggesting a worsened prognosis. Therefore event-driven studies are needed to confirm the clinical implications of the data presented here.
There are some limitations to our study that should be mentioned. First, the study population was selected from patients attending a tertiary academic cardiac referral unit, thus it includes patients with a number of different cardiomyopathies who met the inclusion criteria. This might not be representative of patients in outpatient facilities and community hospitals. On the other hand, this study setting allowed deep insights into diastolic heart failure by investigating the whole range from early-stage impaired relaxation to end-stage restrictive filling patterns of the left ventricle. Unlike previous studies18–22 that mainly concentrated on early-stage patients, this study aimed to give an overview of HFNEF, a goal which is difficult to achieve by just investigating patients with coronary artery disease or hypertensive heart disease. Secondly, we evaluated SDB by cardiorespiratory polygraphy rather than polysomnography (PSG) which still represents the gold standard. In a previous study, Embletta™-based cardiorespiratory polygraphy results were compared with PSG. Even though theoretically AHI in unattended cardiorespiratory polygraphy could be underestimated due to shorter actual sleeping time compared with recording time, an extremely close correlation between the results obtained by PSG and Embletta™ screening (ρ = 0.98, P < 0.001), was reported.30 Thirdly, we founded our classification on a one-night-investigation which may not be representative of other nights. However, a recent study in 50 patients with SHF showed that a single night of cardiorespiratory monitoring gave representative results on severity and type of SDB.31
Finally we classified patients as having either CSA or OSA according to the majority of events. Even though ≥10% of events were obstructive events in 13% of our CSA-cohort and ≥10% of events were central events in 7% of our OSA-cohort, the vast majority of patients presented with either CSA or OSA. In addition, there was not a single case with equal proportions of obstructive apnoea/hypopnoea events and central apnoeas/ periodic breathing, which is supported by our daily clinical experience and previous studies.8
In conclusion, we found a high prevalence of SDB in patients with HFNEF, with a correlation between impairment of diastolic function assessed by echocardiography and frequency of SDB. Patients with SDB presented with more advanced symptoms and greater impairment of cardiovascular function. Controlled studies are now required to investigate the prognostic impact of SDB in HFNEF and to evaluate whether treatment of obstructive and central sleep apnoea in patients with heart failure with preserved left ventricular ejection fraction can ameliorate symptoms of heart failure and improve cardiopulmonary exercise tolerance.
Conflict of interest: none declared.




