Long-term prognosis of medically treated patients with functional mitral regurgitation and left ventricular dysfunction
Abstract
Aims
To assess long-term prognosis in patients with functional mitral regurgitation (FMR) and left ventricular (LV) dysfunction, receiving current standard pharmacological therapy.
Methods and results
We prospectively enrolled 404 consecutive patients (mean age 70.2 ± 10 years) with ischaemic (76.5%) and non-ischaemic (23.5%) LV dysfunction (ejection fraction 34.4 ± 10.8%) and at least mild MR. Results are reported at 4 years' follow-up. Survival free of all-cause mortality was 53% and cardiac death was 74%. Survival free of all-cause mortality was 50% (95% CI 35–72) for patients with moderate MR, 49% (95% CI 27–65) for severe MR, and 64% (95% CI 47–78) for mild MR (P = 0.03). Survival free of cardiac death was 57% (95% CI 38–74) for patients with moderate MR, 55% (95% CI 30–77) for severe MR, and 94% (95% CI 59–98) for mild MR (P = 0.003). Moderate-to-severe MR [relative risk (RR) 2.7, 95% CI 1.2–6.1, P = 0.003] was an independent predictor of cardiac death but not of all-cause mortality. Survival free of heart failure (HF) was 32%. Survival free of HF was 20% (95% CI 17–35) for patients with moderate MR, 18% (95% CI 15–32) for severe MR, and 62% (95% CI 45–72) for mild MR (P = 0.0001). Moderate-to-severe MR (RR 3.2, 95% CI 1.9–5.2, P = 0.0001) was an independent predictor of HF.
Conclusion
The mortality and morbidity of patients with LV dysfunction and FMR remain high despite current standard pharmacological therapy. Moderate-to-severe MR is an independent predictor of cardiac death and HF.
Introduction
Functional mitral regurgitation (FMR) is a common complication in patients with ischaemic and non-ischaemic cardiomyopathies occurring as a consequence of left ventricular (LV) dysfunction and remodelling.1 FMR conveys an adverse prognosis in both the chronic post-myocardial infarction (MI) phase and in non-ischaemic dilated cardiomyopathy.2–5
The prognostic implications of FMR in the chronic post-MI phase have been demonstrated in several previous studies which have shown that it is associated with a more than three-fold risk of heart failure (HF) and a more than two-fold risk of death at 5 years.2–4 This association was independent of the degree of LV systolic function but there was a graded positive relation between FMR severity and the risk of death and HF.2,3 However, these prognostic data come from early studies in which, for obvious reasons, patients did not receive the currently accepted standard pharmacological therapy for HF and LV dysfunction.
Therefore, the aims of this study were to assess long-term prognosis in patients with FMR and LV dysfunction receiving currently accepted standard pharmacological therapy and to identify the clinical and echocardiographic predictors of mortality and morbidity.
Methods
Eligibility
Patients were prospectively enrolled in the study following an initial echocardiographic and clinical evaluation. Inclusion criteria were: (i) ischaemic or non-ischaemic LV systolic dysfunction defined as LV ejection fraction (EF) <50% measured by 2D echocardiography; (ii) FMR at least mild in degree [effective regurgitant orifice area (EROA) ≥0.10 cm2 and/or vena contracta (VC) width ≥0.2 cm], as MR that occurs with a structurally normal valve. Exclusion criteria were: (i) clinical or echocardiographic evidence of other cardiac diseases, such as organic valvular, pericardial, congenital, or infiltrative heart disease; (ii) organic mitral lesions, such as a prolapsed valve, rheumatic disease, or papillary muscle rupture; (iii) more than trace aortic regurgitation; (iv) acute MI; (v) suboptimal echocardiographic windows, leading to incomplete quantification of FMR or anatomic assessment.
Ischaemic patients were enrolled if they had an MI more than 16 days before the baseline assessment and/or one or more LV segmental wall motion abnormalities and significant coronary disease in a territory supplying the wall motion abnormalities.1,6
NYHA class, mitral valve procedures, and co-morbidities such as hypertension, diabetes mellitus, smoking, atrial fibrillation, renal insufficiency, and chronic obstructive pulmonary disease were also recorded. Renal insufficiency was defined as an estimated glomerular filtration rate <60 mL/min.7
Echocardiographic study
After the exclusion of patients with organic MR, the severity of MR, the degree of LV remodelling and dysfunction, diastolic function, and the degree of mitral valve apparatus deformation were evaluated. The severity of MR was graded with quantitative measurements using at least one of the following methods: (i) proximal isovelocity surface area analysed by the proximal flow convergence; (ii) VC width. MR was classified as mild (EROA ≥0.10 < 0.20 cm2, VC width ≥0.2 < 0.3 cm), moderate (EROA ≥0.20 ≤ 0.39 cm2, VC width ≥0.3 < 0.69 cm), or severe (EROA ≥0.40 cm2, VC width ≥0.69 cm).8 If these two methods gave discordant results for MR severity, EROA was used as the reference value. LV remodelling was evaluated from LV end-diastolic and systolic volumes. LV function was assessed from ejection fraction (EF) calculated using the biplane Simpson method; EF was visually estimated in 7% of patients. Diastolic function was evaluated from the deceleration time of the early mitral inflow. The inter-observer relation coefficients were r = 0.90 and r = 0.93 for EROA and VC width, respectively (all P = 0.005).
Follow-up
Follow-up data were obtained from inpatient and outpatient medical records and from telephone interviews. Patients were evaluated at our outpatient clinic or contacted by telephone every 3 months. Beta-blockers and ACE-inhibitors were up-titrated in all patients until the evidence-based target dose or maximum tolerated dose was reached. Dosages were adjusted during follow-up visits according to the clinical status of the patient, renal function, and serum electrolytes. Diuretics were used in patients with clinical signs or symptoms of congestion, and the dosage was adjusted on the basis of these symptoms using the lowest achievable dose. In patients with persistent moderate-to-severe symptoms despite optimal doses of beta-blockers and ACE-inhibitors, an aldosterone antagonist was introduced unless contraindicated or not tolerated. All HF events and deaths were identified, including outpatient cases. HF events during follow-up were defined as follows: acute exacerbation of chronic HF requiring hospitalization or acute cardiogenic dyspnoea characterized by signs of pulmonary congestion including pulmonary oedema using validated criteria.9 Cardiac deaths were ascertained by reviewing death certificates or autopsy records when available. Cardiac death was defined as due to sudden death, fatal MI, acute pulmonary oedema, and refractory HF. Only incident episodes of HF occurring after the index echocardiographic examination were considered in the analysis of the association between MR and HF after enrolment.
Statistical analysis
Data are expressed as mean ± SD or percentage. Group comparisons used t-test or χ2 test, as appropriate. The endpoints were all-cause mortality, cardiac death, and HF events. The impact of FMR on outcome was analysed, with the presence of mild, moderate, or severe MR at baseline used as the categorical determinant of survival. Predictors of survival were identified by Cox proportional hazards analysis. Variables with <0.10 were tested in multivariate modelling. Results were summarized as relative risk (RR) and 95% CI. Survival and event-free survival of patients with mild FMR and with moderate-to-severe FMR were estimated by the Kaplan–Meier method and compared by long-rank test. Analysis was performed by censoring follow-up at the time of last follow-up or at the time of mitral valve surgery if eventually performed. A P < 0.05 was considered significant.
Results
Baseline characteristics of patients
During 2002–07, 404 consecutive patients (mean age 70.2 ± 10 years) were enrolled in the study, 314 (77.7%) were males and 90 (22.3%) females. Of these, 309 (76.5%) had ischaemic and 95 (23.5%) had non-ischaemic LV systolic dysfunction, with a mean EF of 34.4 ± 10.8% (range 10–48%). Sixteen per cent of patients underwent mitral valve surgery; 166 patients (41.2%) had mild MR, 195 (48.1%) moderate, and 43 (10.7%) severe. Of the patients with an EF between 40 and 50% (26.5% of patients), 50% had mild MR, 41.6% moderate, and 8.4% severe. Of the patients with an EF between 30 and 40% (41.6% of patients), 38.6% had mild MR, 53% moderate, and 8.4% severe. Of the patients with an EF <30% (32% of patients), 35.2% had mild MR, 50% moderate, and 14.8% severe. Thirty-six per cent of patients were in NYHA class III–IV and 64% in class I–II. The clinical and echocardiographic characteristics of the study population are reported in Table 1.
| Overall population (404) | Survivors (n = 353) | Non-survivors (n = 56) | P-value | |
|---|---|---|---|---|
| Age (years) | 70.2 ± 10 | 69.5 ± 10.7 | 74.3 ± 8.5 | 0.004 |
| Sex (M) (%) | 77.7 | 76 | 83 | ns |
| Moderate-to-severe MR (%) | 58.8 | 56 | 70 | 0.04 |
| EF (%) | 34.4 ± 10.8 | 35.4 ± 10.6 | 32.2 ± 11.2 | 0.04 |
| EDV (mL) | 181 ± 65 | 178.9 ± 64.1 | 196 ± 69 | NS |
| ESV (mL) | 121 ± 54 | 119.9 ± 54.3 | 134.1 ± 55.8 | NS |
| Deceleration time E-wave (ms) | 184 ± 83.3 | 188 ± 85.6 | 160.7 ± 63 | NS |
| NYHA class III–IV (%) | 36 | 30 | 77 | 0.001 |
| Smokers (%) | 52 | 47.8 | 73.2 | 0.007 |
| Atrial fibrillation (%) | 18.3 | 16 | 30 | 0.01 |
| Renal insufficiency (%) | 30 | 26 | 54 | 0.009 |
| Diabetes mellitus (%) | 34 | 33 | 50 | NS |
| COPD (%) | 7.3 | 7.3 | 11 | NS |
| ICD (%) | 19.8 | 19.8 | 9 | 0.04 |
| CRT (%) | 11.8 | 11.8 | 3.5 | NS |
| Beta-blockers (%) | 81 | 81 | 64 | NS |
| ACE-Inhibitors (%) | 87 | 87 | 81 | NS |
| Spironolactone (%) | 48 | 48 | 38 | NS |
| Diuretics (%) | 76 | 76 | 71 | NS |
| Digoxin (%) | 15 | 15 | 26 | NS |
| Revascularization procedures (%) | 66 | 67 | 63 | NS |
- a EF, ejection fraction; EDV, end-diastolic volume; ESV, end-systolic volume; COPD, chronic obstructive pulmonary disease; ICD, implantable cardioverter defibrillator; CRT, cardiac resynchronization therapy; NS, not significant.
Survival analysis
Over 3.3 ± 2.1 years of follow-up, 56 deaths occurred; of these, 31 were cardiac deaths (20 HFs, 8 sudden deaths, and 3 MIs) and 25 non-cardiac deaths (14 cerebrovascular events, 8 cancers, 1 abdominal ischaemia, and 1 sepsis). At 4 years, survival free of all-cause mortality in the total population was 53% (95% CI 44–62) and survival free of cardiac death was 74% (95% CI 51–88). At 4 years, survival free of all-cause mortality was 50% (95% CI 35–72) for patients with moderate MR, 49% (95% CI 27–65) for patients with severe MR, and 64% (95% CI 47–78) for patients with mild MR (P = 0.03, Figure 1A). At 4 years, survival free of cardiac death was 57% (95% CI 38–74) for patients with moderate MR, 55% (95% CI 30–77) for patients with severe MR vs. 94% (95% CI 59–98) for patients with mild MR (P = 0.003, Figure 1B).
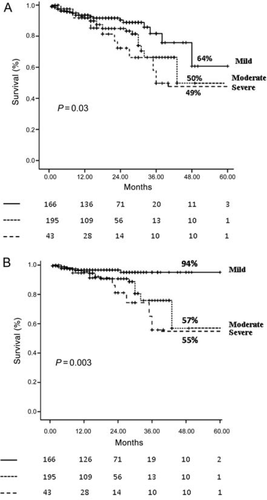
Patients who died were older and had lower EF, a higher prevalence of moderate-to-severe MR, renal insufficiency, atrial fibrillation, NYHA class III–IV, and a less prevalence of ICD therapy (Table 1). In the ischaemic group, there was no difference in the prevalence of revascularization procedures between survivors and non-survivors. At multivariate proportional hazards regression analysis, the independent predictors of all-cause of death were age (RR 2.2, 95% CI 1.1–4.9, P = 0.01), NYHA class III–IV (RR 2.9, 95% CI 1.1–8.1, P = 0.01), and renal insufficiency (RR 3.3, 95% CI 1.2–8.7, P = 0.009).
Comparing the baseline characteristics of survivors and non-survivors from cardiac death, the non-survivors were older (73.8 ± 8.5 vs. 69.9 ± 10.6 years, P = 0.04) and had lower EF (27.8 ± 5.4 vs. 35.5 ± 10.9%, P = 0.0001) and deceleration time of transmitral Doppler E-wave (144.8 ± 57.4 vs. 187.4 ± 84.4 ms, P = 0.01), a higher prevalence of moderate-to-severe MR (83.8 vs. 56%, P = 0.003), renal insufficiency (76 vs. 36%, P = 0.0001), atrial fibrillation (45 vs. 16%, P = 0.0002) and NYHA class III–IV (88 vs. 61.6, P = 0.001). At multivariate proportional hazards regression analysis, the presence of moderate-to-severe MR (RR 2.7, 95% CI 1.2–6.1, P = 0.003), NYHA class III–IV (RR 3.1, 95% CI 2.0–6.4, P = 0.001), EF (RR 0.8, 95% CI 0.89–0.97, P = 0.01), renal failure (RR 3.2, 95% CI 1.1–8.6, P = 0.001), age (RR 1.7, 95% CI 1.1–4.3, P = 0.02), an EF < 30% by moderate MR interaction (RR 2.1, 95% CI 1.3–7.5, P = 0.003) and an EF < 30% by severe MR interaction (RR 4.6, 95% CI 1.9–8.2, P = 0.002) were the independent predictors of cardiac death.
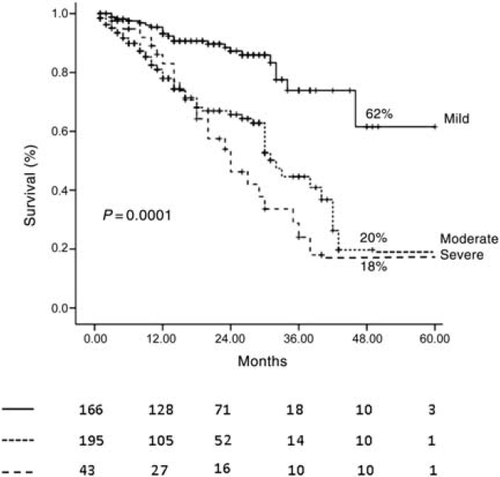
After 3.3 ± 2.1 years of follow-up, 321 episodes of HF occurred in 109 patients, a mean of 0.8 ± 0.9 episodes per patient. At 4 years, survival free of HF in the total population was 32% (95% CI 22–43). At 4 years, survival free of HF was 20% (95% CI 17–35) for patients with moderate MR, 18% (95% CI 15–32) for patients with severe MR, and 62% (95% CI 45–72) for patients with mild MR (P = 0.0001, Figure 2). Patients hospitalized for HF had higher end-diastolic (199.2 ± 64.2 vs. 174.6 ± 64 mL, P = 0.01) and end-systolic (141.3 ± 54.3 vs. 114.7 ± 53 mL, P = 0.0001) volumes, lower EF (31.5 ± 9.3 vs. 36.2 ± 11, P = 0.0001), a higher prevalence of moderate-to-severe MR (77 vs. 51%, P = 0.0001), renal insufficiency (62 vs. 18%, P = 0.0001), atrial fibrillation (35 vs. 12%, P = 0.0001), NYHA class III–IV (35 vs. 12%, P = 0.0001), diuretic treatment (96 vs. 68%, P = 0.0001), and a lower prevalence of beta-blocker therapy (72 vs. 83%, P = 0.03). At multivariate proportional hazards regression analysis, the presence of moderate-to-severe MR, EF, atrial fibrillation, NYHA class III–IV, renal failure, an EF <30% by moderate MR interaction, and an EF <30% by severe MR interaction were the independent predictors of HF (Table 2).
| RR | 95% CI | P-value | |
|---|---|---|---|
| Moderate-to-severe MR | 3.2 | 1.9–5.2 | 0.0001 |
| EF | 0.8 | 0.7–0.98 | 0.02 |
| NYHA class III–IV | 1.5 | 1.2–3.5 | 0.03 |
| Atrial fibrillation | 2.4 | 1.0–5.7 | 0.01 |
| Renal insufficiency | 1.3 | 1.1–2.3 | 0.04 |
| EF <30% × moderate MR | 2.1 | 1.1–3.8 | 0.008 |
| EF <30% × severe MR | 2.0 | 1.3–4.8 | 0.01 |
Ischaemic subgroup
At 4 years, survival free of all-cause mortality in the ischaemic subgroup (n = 309) was 53% (95% CI 43–65) and survival free of cardiac death was 73% (95% CI 50–85). Survival free of all-cause mortality was 47% (95% CI 34–62) for ischaemic patients with moderate MR, 36% (95% CI 20–49) for patients with severe MR, and 61% (95% CI 44–75) for patients with mild MR (P = 0.01). Survival free of cardiac death was 52% (95% CI 36–66) for ischaemic patients with moderate MR, 40% (95% CI 25–54) for patients with severe MR, and 96% (95% CI 56–98) for patients with mild MR (P = 0.0001).
The independent predictors of all-cause of death in the ischaemic subgroup were age (RR 2.0, 95% CI 1.3–5.4, P = 0.03), NYHA class III–IV (RR 2.5, 95% CI 2.1–5.4, P = 0.004), and renal insufficiency (RR 2.1, 95% CI 1.2–8.5, P = 0.03). There was no difference in the prevalence of revascularization procedures between survivors and non-survivors. At multivariate proportional hazards regression analysis, the presence of moderate-to-severe MR (RR 2.1, 95% CI 1.2–5.9, P = 0.001), NYHA class III–IV (RR 3.0, 95% CI 1.0–5.8, P = 0.001), EF (RR 0.3, 95% CI 0.12–0.69, P = 0.001), renal failure (RR 2.9, 95% CI 2.4–5.8, P = 0.002), age (RR 1.3, 95% CI 1.1–5.6, P = 0.01), an EF <30% by moderate MR interaction (RR 2.4, 95% CI 1.2–8.0, P = 0.001), and an EF <30% by severe MR interaction (RR 3.9, 95% CI 1.2–7.8, P = 0.01) were the independent predictors of cardiac death.
At 4 years, survival free of HF in the ischaemic subgroup was 36% (95% CI 23–46). Survival free of HF was 26% (95% CI 20–37) for patients with moderate MR, 20% (95% CI 15–35) for patients with severe MR, and 58% (95% CI 41–74) for patients with mild MR (P = 0.0001). The presence of moderate-to-severe MR (RR 3.9, 95% CI 1.8–5.7, P = 0.0001), EF (RR 0.56, 95% CI 0.37–0.84, P = 0.001), atrial fibrillation (RR 1.8, 95% CI 1.2–3.7, P = 0.01), NYHA class III–IV (RR 1.5, 95% CI 1.2–3.8, P = 0.03), renal failure (RR 1.3, 95% CI 1.1–2.7, P = 0.03), an EF <30% by moderate MR interaction (RR 4.2, 95% CI 2.5–7.8, P = 0.001), and an EF < 30% by severe MR interaction (RR 4.4, 95% CI 2.3–8.1, P = 0.001) were the independent predictors of HF. Undergoing a revascularization procedure was a univariate predictor of HF but not an independent predictor at multivariate analysis.
Discussion
The results of this study show that in this contemporary cohort of patients with LV dysfunction and FMR, the mortality rate and the prevalence of HF are relatively high, despite current standard medical treatment in the majority of patients. A moderate-to-severe degree of FMR remains a strong prognostic predictor of cardiac death and HF, both in patients with ischaemic and non-ischaemic LV dysfunction, although other co-morbidities such as age, NYHA class, renal insufficiency, and atrial fibrillation represent independent risk factors for morbidity and mortality. In particular, the combination of a very low EF and moderate or severe MR is a very strong risk factor for cardiac death and HF. Finally, in this study, 45% of deaths had a non-cardiac aetiology, which highlights the underlying role of other clinical risk factors besides the degree of FMR.
The present series of patients had better survival in terms of both all-cause mortality and cardiac death than previously reported ones.2,3 These prognostic differences could be explained by the fact that our patients represent a more contemporary series of HF patients receiving up-to-date medical treatment. Indeed, 87, 80, and 47% of patients received ACE-inhibitors, beta-blockers, and spironolactone, respectively. This high level of treatment combined with the high percentage of revascularized patients in the ischaemic group has been shown to be associated with better outcomes in this group of patients.10 For this reason, we chose not to censor patients who had undergone CABG and/or PTCA. This decision was based on the fact that nowadays the majority of patients with ischaemic FMR undergo revascularization procedures, thus reflecting the real population with FMR. Interestingly, a low percentage of patients in our study received ICD and/or CRT devices. This could be explained by the fact that few patients matched the clinical criteria for device implantation. On the other hand, there was also a low prevalence of sudden cardiac death. Regarding the impact of therapy on HF occurrence and death, we have reached the same conclusions as Grigioni4: pharmacological therapy does not reduce the impact of FMR on HF occurrence and cardiac death. Indeed, in our study, ACE-inhibitors, beta-blockers, and spironolactone were not independent predictors of mortality and HF events. However, we do not know exactly how many patients received beta-blockers and spironolactone in the Grigioni studies.3,4 We do know that 77% of patients in the Grigioni study received ACE-inhibitors compared with 87% of our population,3 which could partially explain the differences in terms of better survival between our studies, but does not modify the role of FMR on prognosis.
The presence of moderate-to-severe MR was not an independent marker of all-cause mortality in our study, whereas age appeared to be the most powerful marker. The relatively advanced age of our patients (mean age 70.2 ± 10 years), similar to other published studies,2–4 exposes the subjects to a high prevalence of HF and co-morbidities such as renal failure, and atrial fibrillation that represent risk factors for mortality due to non-cardiac causes (cerebrovascular accidents, cancers, etc). Non-cardiac death accounted for 45% of all-cause mortality in our study. On the other hand, it is well known that the prevalence of HF with and without associated FMR and other non-cardiac morbidities increases with age.11–16
The risk of cardiac mortality and HF is directly related to the severity of regurgitation assessed quantitatively at rest by Doppler echocardiography. These data underscore the importance of the degree of FMR as a marker of poor cardiac outcome in patients with LV dysfunction. Moreover, FMR is a dynamic lesion, its severity may vary over time, and it is also very sensitive to exercise. Using exercise echocardiography, Lancellotti et al.17,18 demonstrated the strong prognostic importance of the dynamic component of FMR over the degree of MR at rest. A large increase in the degree of MR during exercise was associated with increased mortality risk and HF events. In our study, we included both patients with ischaemic and non-ischaemic LV dysfunction, representing 77.7 and 22.3% of the study population, respectively. Moreover, the majority of published data on the prognostic impact of FMR refer to ischaemic patients (683 patients in total),2–4 whereas only one study has evaluated the impact of FMR in non-ischaemic patients (92 patients).5 Therefore, on the basis of our data and current literature, information about the prognostic value of FMR in non-ischaemic patients is very limited and further investigation is needed to confirm the impact on outcome.
The individual risk in these patients to develop HF during long-term follow-up remains high and contributes to the current HF epidemic.4,19 The occurrence of HF is a turning point for outcome and is associated with a considerable excess of cardiac mortality.11,20–22 Thus, the identification of predictors of HF in patients with no or minimal symptoms represents a core approach in the management of patients with LV dysfunction.4 The link between MR and cardiac events involves several mechanisms. FMR is a major determinant of filling pressures and can directly cause HF.23 Volume overload of FMR stimulates LV remodelling,24 leading to long-term mortality. Furthermore, FMR is also a determinant of rapid increase in QRS duration and can subsequently induce permanent electromechanical dyssynchronization,25 accelerating the progression to refractory HF.25,26 Thus, our results in combination with previously published data emphasize the importance of quantitative assessment of FMR in patients with LV dysfunction, and aggressive therapeutic interventions should be considered.3,4 Medical, pacing, and surgical therapies are potential therapeutic options to ameliorate HF symptoms, improve LV remodelling and function, and improve the intermediate/long-term outcome. Cardiac resynchronization therapy has demonstrated beneficial effects in terms of FMR reduction, improving the quality of life beyond that achievable with drug therapy and should be considered a valid therapeutic option.27–29 Moreover, the persistence of a high degree of severity of FMR after device implantation is an independent predictor of long-term survival, suggesting that this factor may predict or mediate the long-term response to this therapy.30 ICD therapy improves survival in HF patients and, therefore, it should be used in appropriate patients for the primary and secondary prevention of arrhythmogenic sudden cardiac death.31–33 Even though there are no randomized trials that compare surgical vs. medical therapy, restricted annuloplasty, which is the most standardized surgical procedure, is associated with low surgical mortality and leads to an improvement in HF symptoms,34,35 but it does not seem to confer a long-term survival advantage compared with medical therapy.10 Indeed, patients with untreated FMR at the time of isolated revascularization have a higher incidence of hospitalization for HF during follow-up.36 Thus, the goal of surgical therapy is not only to improve survival but also to improve functional status and quality of life. Therefore, we can conclude that high-risk perioperative patients with multiple co-morbidities and/or short life expectancy should be treated conservatively, whereas in low-risk perioperative patients with few or no co-morbidities, mitral valve repair seems a valid therapeutic option for symptom control.
Limitations
Potential limitations should be kept in mind in the interpretation of these data. Patients were prospectively enrolled in the study following an initial echocardiographic and clinical evaluation, thus MR may have been present beforehand in some patients. This is a limitation shared by all studies in this area. Echocardiography merely represents a brief snapshot of the severity of MR at the time of the observation. We did not include a control group of patients without FMR. However, our study was an observational follow-up study in medically treated patients with confirmed FMR, in which the long-term prognosis of these patients and the predictors of survival in a more recent era of HF therapy were evaluated. EF was estimated visually in 7% of patients and this could have affected the results, but the limited number of patients in whom this method was used should not have significantly influenced the results. Follow-up was conducted by telephone interview in some patients; however, only HF events that required hospitalization and deaths including outpatient cases were considered, and both HF events and deaths were confirmed by reviewing medical records and death certificates or autopsy records when available. Finally, we did not perform a separate analysis in the two different groups (ischaemic and non-ischaemic patients) for two reasons. First, because that analysis was not the objective of our study, and second because the non-ischaemic group was relatively small with little power to distinguish outcomes and predictors from those of the ischaemic patients.
Conclusions
These data demonstrate that the mortality and morbidity of patients with LV dysfunction and FMR remain high despite treatment with currently accepted standard pharmacological therapy. A high degree of regurgitation is an independent predictor of cardiac death and HF. Moreover, in this population, 45% of deaths had a non-cardiac aetiology, thus several clinical co-morbidities, besides the degree of MR, represent risk factors for death and HF in this group of patients.
Conflict of interest: none declared.




