Direct and Indirect Evidence Suggests Continuous Presence of Viral Hemorrhagic Septicemia Virus (Genotype IVb) in Budd Lake, Michigan: Management Implications
Abstract
In May 2007, viral hemorrhagic septicemia virus genotype IVb (VHSV IVb) was isolated from several fish species in Budd Lake, a 71-ha inland lake in central Michigan. Because of the virulence and pathogenicity of VHSV IVb, follow-up testing was conducted between 2007 and 2010. This testing found no further evidence of VHSV IVb presence. Similar patterns (i.e., initial mortality events followed by no evidence of additional infections) have been observed in other waterbodies, which leads to questions regarding the implications that these patterns could have for fisheries management (e.g., could stocking naive fish lead to additional epizootic occurrences?). This study was conducted to determine whether VHSV IVb was still present in Budd Lake by intensively sampling across multiple seasons, locations, and size-groups of Muskellunge Esox masquinongy, Northern Pike Esox lucius, and Largemouth Bass Micropterus salmoides. Individuals were tested for both active VHSV IVb infection and antibody production. We found that VHSV IVb was still present in Budd Lake, as active infections were detected from samples in spring, summer, and fall 2011, with highest infection prevalence in spring 2011. Spring 2011 infection prevalence was 17% for esocids and 33% for Largemouth Bass. Between 74% and 80% of collected esocids produced antibodies, depending on the sampling season. Antibody production in Largemouth Bass ranged from 12% in spring and fall 2011 to 20% in spring 2012. Based on these results, we recommend sustained vigilance in the management of VHSV IVb–positive systems to limit the risk of virus spread as it is perhaps questionable whether systems that have experienced an outbreak will ever be virus-free.
Received September 16, 2014; accepted February 7, 2015
A novel sublineage of the viral hemorrhagic septicemia virus (VHSV) genotype IVb (VHSV IVb) emerged in the Laurentian Great Lakes region of North America in 2003, causing large-scale fish kills (Elsayed et al. 2006; Groocock et al. 2007; Lumsden et al. 2007; Faisal et al. 2012). Fish infected with VHSV may exhibit varying levels of internal and external hemorrhaging, exophthalmia, anemia, ascites, and abnormal swimming behavior (OIE 2009) depending on the course of infection, which can take acute, subacute, or chronic forms (Kim and Faisal 2010a). The disease was first reported in Europe in the 1930s (Schäperclaus 1938), where it spread to both fresh and marine waters, and was detected in the Pacific Northwest of North America and Asia in the late 1980s (Hopper 1989; Eaton et al. 1991; Meyers et al. 1992). Since 2000, the disease has emerged in the Atlantic coast and Great Lakes region of North America. In North America, there are three recognized sublineages of VHSV genotype IV: lineage IVa existing in the Pacific Northwest, IVb in the Great Lakes basin, and IVc in the North Atlantic (Pierce and Stepien 2012). Although VHSV IVb was first identified in Lake St. Clair, Michigan, it has since spread to all five of the Great Lakes, the St. Lawrence River, and several small inland lakes in New York, Ontario, Wisconsin, Michigan, and Ohio (Faisal et al. 2012).
Based on testing conducted to date, VHSV IVb appears more virulent in coolwater species, which is similar to the pathogenicity of VHSV IVa (Winton et al. 1991; Follett et al. 1997; Kocan et al. 1997; Arkush et al. 2006; Hershberger et al. 2007). Kim and Faisal (2010a) found that Muskellunge Esox masquinongy and Largemouth Bass Micropterus salmoides experienced the highest mortality rates from VHSV IVb infection, followed by Yellow Perch Perca flavescens and salmonids. In terms of VHSV IVb antibody production, Millard and Faisal (2012) found that Muskellunge had the highest overall antibody prevalence and mean antibody levels, followed by Northern Pike Esox lucius, Freshwater Drum Aplodinotus grunniens, and Smallmouth Bass Micropterus dolomieu. Temperature has been found to affect host response and disease course, with VHSV IVb having an optimum temperature range for fish mortality of 9–12°C, with an upper limit of 18–20°C, which is similar to the temperature profile of VHSV genotype I (OIE 2009; Goodwin and Merry 2011a).
Despite the extensive research conducted on VHSV IVb, there are still aspects of the virus that are not well understood. One major area of uncertainty concerns the persistence of the virus in systems where VHSV IVb outbreaks have previously occurred. As is common with endemic or enzootic diseases, VHSV is often detectable for several years within a system after an initial outbreak (Faisal et al. 2012; Garver et al. 2013). Yet in other waterbodies (e.g., Budd and Baseline lakes, Michigan), follow-up testing returned negative results after initial mortality events (Michigan State University, Aquatic Animal Health Laboratory, unpublished data). This raises questions as to what the persistence of the virus is in enzootic regions where mortality events associated with VHSV IVb have not occurred for several consecutive years. As well, it makes it difficult for managers to determine when an area can be considered virus-free. From a fisheries management standpoint, the uncertainty as to whether there is still virus in a lake despite the inability to detect it raises numerous questions. Can management activities such as stocking proceed as normal or do managers need to be concerned that stocking could lead to further outbreaks due to either stressing the system or introducing naive individuals with supposedly limited capacity to resist infection? Do managers still need to be concerned about the spread of the virus from a previously infected system?
In the present study, we aimed to clarify some issues regarding the persistence of the virus by intensively studying a lake (Budd Lake) that experienced a VHSV IVb–related mortality event in May 2007 and then tested negative for the virus from June 2007 to 2010 (details on the outbreak and follow-up testing are described in the Methods). To gain a better understanding of the current status of VHSV IVb in this lake, we assessed the infection status of Muskellunge, Northern Pike, and Largemouth Bass through cell culture and reverse transcription polymerase chain reaction (RT-PCR) testing. Additionally, we evaluated evidence for past infection by testing for specialized antibodies using a serological assay. If VHSV IVb was truly absent from Budd Lake, negative virus and antibody results from all fish would be expected. If there was an active infection in the lake, we would anticipate finding tissues or blood containing viable virus. If a recent infection had occurred, we would anticipate finding VHSV IVb–specific antibodies in sera of surviving fish.
METHODS
Study site.
Budd Lake is a 71-ha inland lake located in Clare County, Michigan, which is in the central region of the state's Lower Peninsula. The lake is within the watershed for Lake Huron and is a locally popular recreational fishery that has been regularly stocked with Muskellunge by the Michigan Department of Natural Resources (MDNR 2012). It is a morainic, calcareous seepage lake (Coffey and McNabb 1974), with the main water sources consisting of precipitation and runoff with groundwater supplementation.
The initial VHSV IVb outbreak in Budd Lake occurred in May 2007. Immediately following a massive mortality event involving Black Crappie Pomoxis nigromaculatus, Bluegill Lepomis macrochirus, Pumpkinseed Lepomis gibbosus, Largemouth Bass, and Muskellunge, 78 specimens (62 Black Crappies, 10 Bluegills, 4 Pumpkinseeds, 1 Largemouth Bass, and 1 Golden Shiner Notemigonus crysoleucas) were collected from the lake, stored whole on ice, and shipped to the Michigan State University Aquatic Animal Health Laboratory for testing. Species in which the virus was isolated by cell culture methods and that were confirmed positive for VHSV IVb by RT-PCR were Black Crappie, Bluegill, Pumpkinseed, and Largemouth Bass (Faisal et al. 2012). In early June 2007, an additional 309 specimens (60 Bluegills, 60 Largemouth Bass, 60 Pumpkinseeds, 60 Bluntnose Minnows Pimephales notatus, 60 Sand Shiners Notropis stramineus, and 9 Golden Shiners) were collected, stored whole on ice, and again shipped to the Michigan State University Aquatic Animal Health Laboratory for testing. Despite employing the same protocol, staff, and cell lines as the previous isolation, VHSV IVb was not isolated from any of these fish. In mid-April 2008, 180 specimens (60 Bluegills, 60 Black Crappies, and 60 Pumpkinseeds) were collected and tested for VHSV IVb using the same methodologies as before. Again, the virus was not isolated from any of these fish. In early May 2010, 150 specimens (30 Yellow Perch, 60 Bluegills, and 60 Pumpkinseeds) were collected and tested for VHSV IVb using the same methodologies; VHSV IVb was again not isolated from any of the tested individuals.
Fish and sample collection.
Pulsed-DC boat electrofishing was conducted in spring (May 12 and 13), summer (July 11), and fall (September 29 and October 21) 2011. On the days of sampling, water temperature was approximately 15°C in May, 27°C in July, 16°C in September, and 12°C in October. Muskellunge were the primary esocid targeted during sampling, but Northern Pike were also collected if they were seen. Sampling for Largemouth Bass was also conducted on April 13, 2012, based on results from the spring 2011 sampling. Water temperature for the spring 2012 sampling was approximately 11°C. For the purpose of sampling Largemouth Bass, the lake was stratified into two areas (north and south regions) and approximately equal sample sizes from both areas were targeted to help ensure collections were distributed throughout the lake. The lake's entire perimeter was electrofished, as was the shoreline of an island in the middle of the lake. Sampling was generally conducted in the morning and took approximately 2 h to complete. For Largemouth Bass, the target sample size for each sampling event was 60, which is the sample size needed to have a 95% probability of detecting a pathogen given a prevalence of at least 5% for a test with perfect sensitivity (Hnath 1993; USFWS and AFS–FHS 2005; Fenichel et al. 2011). Sampling of esocids was more opportunistic given the lower densities and difficulties associated with sampling Muskellunge and Northern Pike.
For sampling conducted in 2011, collected Largemouth Bass were transported to the Michigan State University Research Containment Facility and held live until further processing. For sampling conducted in 2012, Largemouth Bass samples were collected on site within a few hours of capture. For esocids, tissue and blood samples were obtained nonlethally. Captured esocids were anesthetized with tricaine methanesulfonate (Argent Laboratories, Redmond, Washington) at a concentration of 0.1 g/L of water buffered with 0.2 g/L of sodium bicarbonate. Approximately 5 mL of blood were collected from the caudal vein when possible, although in some cases (four in spring, one in summer, and seven in fall) blood samples were not drawn because of difficulties in locating the vein and concerns about returning fish to the lake as soon as possible. A small gill biopsy, fin biopsy, and mucus sample (<1 g each) were collected from each individual as well and were stored separately at 4°C until processing. Total lengths (TLs) were recorded for all individuals. Fish were then placed into fresh lake water until equilibrium was restored and then they were released.
Largemouth Bass were euthanized with an overdose of tricaine methanesulfonate at a concentration of 0.25 g/L of water buffered with 0.5 g/L of sodium bicarbonate. As with the esocids, blood was collected from the caudal vein when possible (blood was not able to be collected from 4 individuals in spring 2011, 31 in summer 2011, 5 in fall 2011, and 1 in spring 2012). Kidney, spleen, and heart tissues were collected, pooled, and stored in 4-oz bags. All blood and tissue samples were stored at 4°C until processing. Both tissue and serum were collected to more accurately assess the state of infection; logically, if serum was positive for the virus but tissue was not, that could indicate a recent infection and that the fish was viremic. If both tissue and sera were positive for the virus, that could indicate an older infection that had spread to multiple organs (Dua 2012).
Tissue sample processing.
All tissues were processed within 12 h of collection. The weight of each tissue sample was multiplied by 4 to obtain the volume of diluent to be added to each sample. Diluent consisted of primarily Earle's salt-based minimum essential medium and tryptose phosphate broth, in addition to 1 M of tris buffer containing Trizma base and Trizma hydrochloride (Sigma-Aldrich, Saint Louis, Missouri), gentamycin sulfate (Sigma-Aldrich), penicillin and streptomycin (Invitrogen, Carlsbad, California), and 250 μg/mg of Fungizone (Fisher Scientific, Hampton, New Hampshire). Tissues were homogenized with a Stomacher 80 Biomaster laboratory paddle blender (Seward, Worthing, England) for 4 min at full speed. Homogenized samples were centrifuged at 5,000 rpm for 30 min at 4°C, and supernatant was stored for no more than 24 h at 4°C until cell culture inoculation. Blood was centrifuged at 5,000 rpm for 10 min to separate the serum. Serum was collected and stored at −80°C until serology assays were performed.
Virus isolation.
The epithelioma papillosum cyprini cell line (Fijan et al. 1983) was used throughout this study for virus isolation. Cells were seeded onto flat-bottom 96-well plates (Corning Life Sciences, Corning, New York) supplemented with a media consisting of primarily Earle's salt-based minimum essential medium (Invitrogen) and tryptose phosphate broth (Sigma-Aldrich), in addition to heat-inactivated fetal bovine serum (Gemini Bio-Products, West Sacramento, California), 200 mM of L-glutamine (Invitrogen), 1 M of tris buffer (Sigma-Aldrich), penicillin and streptomycin (Invitrogen), gentamycin sulfate (Sigma-Aldrich), and 250 μg/mg of Fungizone (Fisher Scientific). A confluent monolayer of cells had developed for 24–48 h before inoculation of tissue or serum homogenate. Then 30 μL of homogenate supernatant was dispensed into each well at six replicates per sample. Cytopathic effects were observed at 14 d postinfection. All samples underwent a second passage for an additional 14 d, allowing for a total incubation period of 28 d. Samples were stored at −80°C until RNA extraction. To assess the presence of the virus in sera, samples were diluted 1:10 and 30 μL was inoculated into each well at six replicates per sample. If there was not enough serum for viremia testing on cell culture, samples were saved for serological assays. Results were observed and samples were stored until RNA extraction.
Infected cells were monitored daily for the formation of cytopathic effects; however, RT-PCR was used as the confirmatory test for VHSV IVb RNA on all inoculated cell cultures. The QIAamp Viral RNA Mini Kit (Qiagen, Venlo, Netherlands) was used to extract RNA from all tissue and serum cell culture supernatant by following the manufacturer's instructions. Viral RNA was translated into complementary DNA via reverse transcription using AffinityScript Multiple Temperature Reverse Transcriptase (Agilent Technologies, Santa Clara, California) and by following the manufacturer's instructions. Polymerase chain reaction was performed using a conserved region of the VHSV IVb genome. Primers were used in accordance with recommendations by the Office International des Epizooties (OIE; OIE 2006) and contained the following sequences: 5′ GGG GAC CCC AGA CTG T 3′ (forward) and 5′ TCT CTG TCA CCT TGA TCC 3′ (reverse). The 25-μL reaction consisted of 2.5 μL of each primer, 12.5 μL of GoTaq Green master mix (Promega, Madison, Wisconsin), 5 μL of nuclease-free water (Promega), and 2.5 μL of template. Polymerase chain reaction began with one cycle of polymerase activation at 94°C for 2 min, followed by 35 cycles of annealing at 94°C for 30 s, elongation at 52°C for 30 s, and denaturation at 68°C for 60 s, and it was completed with one cycle of elongation at 68°C for 7 min. Nucleic acid was stained with SYBR Green II (Lonza, Basel, Switzerland) and results were observed through gel electrophoresis with a 1.5% Ultrapure agarose gel (Invitrogen). The size of cDNA fragments were compared with a 2-log (0.1–10.0 kb) ladder (New England BioLabs, Ipswich, Massachusetts). Bands at 811 kbp were considered positive for VHSV IVb.
Antibody production.
The competitive enzyme-linked immunosorbent assay (cELISA) detects antibodies that bind to any available proteins on the outside of a virus. A cELISA specialized for VHSV IVb was recently developed and optimized by Millard et al. (2014) and was used for this study to assess VHSV IVb antibody production. For the cELISA, the level of antibody production is measured in terms of percent inhibition. Millard et al. (2014) established a threshold of 14.6% inhibition as an indicator of positive antibody production for Muskellunge based on a sampling of individuals that had never been exposed to VHSV IVb. We used this threshold value for Muskellunge and Northern Pike. For Largemouth Bass, we used a threshold of 9.6% based on the cELISA analysis of 170 Largemouth Bass that did not harbor the virus and were collected from waters known to be free of VHSV IVb and using the same threshold-identification methodology as that of Millard et al. (2014) (M. Faisal, unpublished data).
Data analysis.
The apparent prevalence of active VHSV IVb infections and antibody production by sampling group (esocids, Largemouth Bass) and collection season were determined by dividing the number of positive samples by the total number of samples. Confidence intervals (95%) for the apparent prevalences were calculated using the Clopper and Pearson (1934) approach. Differences in antibody production between and within esocids and Largemouth Bass were compared using odds ratios. Confidence intervals (95%) were calculated for all odds ratios as a means of assessing the uncertainty associated with the measures. Odds ratios were used to compare prevalences rather than statistical tests of significance due to their emphasizing the biological significance of results as opposed to statistical significance (Rutledge and Loh 2004). The odds ratio compares the odds of a species (e.g., esocids) or sample type (serum) testing positive for VHSV IVb versus the odds of another species (e.g., Largemouth Bass) or sample type (visceral tissue). An odds ratio of 1 means the odds of testing positive are the same between the species or sample type, whereas an odds ratio > 1 suggests that one species or sample type (in the above example esocids or serum) has a higher odds of testing positive for VHSV IVb (<1 would indicate a lower odds). Odds ratios and associated confidence intervals were calculated in R (R Development Core Team 2014). Confidence intervals for the apparent prevalences were calculated in R using the PropCIs package (Scherer 2014).
RESULTS
The total available sample sizes for characterizing VSHV IVb infection status and antibody production were 30 Muskellunge (TLs ranging from 47.2 to 112.8 cm), 6 Northern Pike (TLs ranging from 45.8 to 92.7 cm), and 252 Largemouth Bass (TLs ranging from 5.0 to 46.2 cm). Esocid sample sizes by season were 23, 1, and 12 for spring, summer, and fall 2011, respectively. Only one esocid (Northern Pike) was collected in summer 2011. We attribute this low number to warmer temperatures that resulted in Muskellunge and Northern Pike moving to deeper waters and thus not being vulnerable to sampling gear. Largemouth Bass sample sizes by season were 63, 58, 71, and 60 for spring 2011, summer 2011, fall 2011, and spring 2012, respectively. The sample sizes available for testing varied by sample season and test type for each species due to several factors, such as limited sera or the inability to collect sera. In summer 2011, high mortality of Largemouth Bass after transport to the Michigan State University Research Containment Facility resulted in sera being collected from approximately half (n = 27) of sampled individuals.
Infection with VHSV IVb
Based on isolation on epithelioma papillosum cyprini cells and confirmation by RT-PCR testing, in spring 2011 9% (2 positive out of a sample size of 23; 95% CI = 1–28%) of esocids were positive for VHSV IVb in tissue, whereas 12% (2 positive out of 17; 95% CI = 1–36%) of serum samples were positive (Table 1). Combining tissue and serum samples, 17% (4 positive out of 23; 95% CI = 5–39%) of esocids tested positive for VHSV IVb. All positive esocid samples were from Muskellunge. One Muskellunge tested positive for the virus in mucus and gill tissues, one in fin tissue only, and two in serum samples only. As previously indicated, only one esocid (a Northern Pike) was collected in summer 2011. This individual did not test positive for VHSV IVb in tissue samples (serum sample was not collected). In fall 2011, 8% (1 positive out of 12; 95% CI = 0–38%) of collected esocids tested positive for VHSV IVb. This individual was a Muskellunge and tested positive in a fin tissue sample. Across all sampling periods and tissue types, TLs of Muskellunge that tested positive for VHSV IVb ranged from 48.0 to 100.0 cm.
| Species, sample location, and total | Spring 2011 | Summer 2011 | Fall 2011 | Spring 2012 | Total |
|---|---|---|---|---|---|
| Esocids | |||||
| Mucus | 1/23a | 0/1 | 0/12 | NA | 1/36a |
| Fin | 1/23 | 0/1 | 1/12 | NA | 2/36 |
| Gill | 1/23a | 0/1 | 0/12 | NA | 1/36a |
| Serum | 2/17 | 0/0 | 0/5 | NA | 2/22 |
| Totalb | 4/23 | 0/1 | 1/12 | NA | 5/36 |
| Largemouth Bass | |||||
| K-S-H | 19/63 | 0/58 | 0/71 | 0/60 | 19/252 |
| Serum | 13/50 | 1/27 | 3/69 | 0/60 | 17/206 |
| Totalb | 21/63 | 1/58 | 3/71 | 0/60 | 25/252 |
- a Positive mucus and gill originated from the same individual. bRepresents number of infected individuals (regardless of sample type) to the total number of collected individuals.
In spring 2011, VHSV IVb infection in Largemouth Bass was 33% (21 positive out of a sample size of 63; 95% CI = 22–46%) for all sample types; specifically, infection was 30% (19 positive out of 63; 95% CI = 19–43%) based on visceral tissue samples and 26% (13 positive out of 50; 95% CI = 15–40%) based on serum samples. In summer 2011, VHSV IVb was detected in 4% (1 positive out of 27; 95% CI = 0–19%) of Largemouth Bass serum samples and 0% (0 positive out of 58; 95% CI = 0–6%) of visceral tissue samples. In fall 2011, VHSV IVb was detected in 4% (3 positive out of 69; 95% CI = 0–12%) of Largemouth Bass serum samples and 0% (0 positive out of 71; 95% CI = 0–5%) of visceral tissue samples. The virus was not detected in any of the spring 2012 serum or visceral tissue samples from Largemouth Bass. The TLs of Largemouth Bass that tested positive for VHSV IVb ranged from 15.4 to 46.2 cm.
Based on RT-PCR results, the odds of esocids testing positive for VHSV IVb in spring 2011 were 1.39 times (95% CI = 0.09–21.18) as great in serum samples as in tissue samples. Conversely, the odds of Largemouth Bass testing positive for the virus were 1.23 times (95% CI = 0.50–3.10) as great in visceral tissue samples as in serum samples. In terms of species comparisons, the odds of testing positive for VHSV IVb were 4.47 (95% CI = 0.93–43.13) and 2.60 times (95% CI = 0.49–26.55) as great in Largemouth Bass as in esocids based on tissue samples and serum, respectively. Wide confidence intervals for the odds ratios were a result of small sample sizes, which limited our ability to draw definite conclusions regarding the significance of observed differences in apparent prevalences between sample types of species and between species for both cell culture and RT-PCR results.
Detection of Antibodies against VHSV IVb
Based on cELISA, the apparent prevalence of antibody production in esocids was 74% (14 positive out of a sample size of 19; 95% CI = 49–91%) in spring 2011 and 80% (4 positive out of 5; 95% CI = 28–99%) in fall 2011 (Table 2). The TLs of esocids that were found to produce antibodies ranged from 47.2 to 106.0 cm (Figure 1). For Largemouth Bass, the prevalence of antibody production ranged from 12% in spring and fall 2011 (7 positive out of 59 [95% CI = 5–23%] and 8 positive out of 66 [95% CI = 5–22%], respectively) to 20% in spring 2012 (12 positive out of 59; 95% CI = 11–33%) (Table 2). The TLs of Largemouth Bass that were found to produce antibodies ranged from 10.5 to 42.2 cm (Figure 2).
| Species | Spring 2011 | Summer 2011 | Fall 2011 | Spring 2012 |
|---|---|---|---|---|
| Esocids | 14/19 | 0/0 | 4/5 | NA |
| Largemouth Bass | 7/59 | 4/27 | 8/66 | 12/59 |
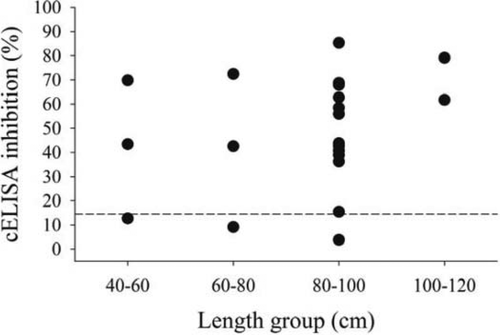
Competitive enzyme-linked immunosorbent assay (cELISA) inhibition levels for esocids sampled in 2011, grouped by total lengths for all sampling seasons. The dashed horizontal line indicates the inhibition level (14.6%) used to distinguish positive binding antibody production.
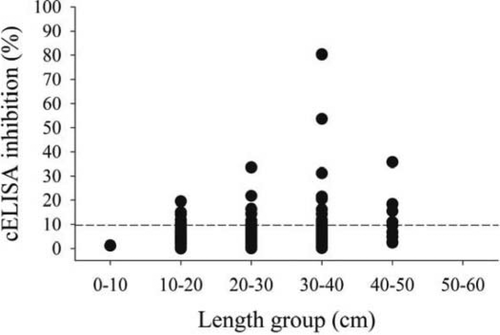
Competitive enzyme-linked immunosorbent assay (cELISA) inhibition levels for Largemouth Bass sampled in 2011 and 2012, grouped by total lengths for all sampling seasons. The dashed horizontal line indicates the inhibition level (9.6%) used to distinguish positive binding antibody production.
Based on the calculated odds ratios, the odds of producing VHSV IVb antibodies in spring 2011 were nearly 20 times greater for esocids than for Largemouth Bass (Table 3). For Largemouth Bass, the odds of individuals producing antibodies in spring 2012 were around 1.5–1.9 times as great as for any of the other sampling periods (Table 3). It is again important to note that small sample sizes resulted in wide confidence intervals for the odds ratios, which limits our ability to draw definite conclusions regarding the significance of observed differences in antibody production between species and sampling periods.
| Scenario | Odds ratio | 95% confidence interval |
|---|---|---|
| Esocid Spring 2011 versus LMB Spring 2011 | 19.53 | 4.92–93.69 |
| LMB Spring 2012 versus LMB Spring 2011 | 1.88 | 0.62–6.16 |
| LMB Spring 2012 versus LMB Fall 2011 | 1.84 | 0.63–5.66 |
| LMB Spring 2012 versus LMB Summer 2011 | 1.46 | 0.39–6.91 |
| LMB Summer 2011 versus LMB Spring 2011 | 1.29 | 0.25–5.68 |
| LMB Summer 2011 versus LMB Fall 2011 | 1.25 | 0.25–0.74 |
| LMB Fall 2011 versus LMB Spring 2011 | 1.02 | 0.30–3.57 |
Correspondence between VHSV IVb Infection and Antibody Production
Of the esocids that tested positive for either VHSV IVb infection or antibody production in spring 2011, 69% (9 of 13; 95% CI = 39–91%) produced only antibodies, while 15% were positive for the virus only (2 of 13; 95% CI = 2–45%), and 15% were positive for the virus and antibodies (2 of 13; 95% CI = 2–45%). In fall 2011, 100% (4 of 4; 95% CI = 40–100%) produced antibodies only.
Of the Largemouth Bass that tested positive for either VHSV IVb infection or antibody production in spring 2011, 76% (16 of 21; 95% CI = 53–92%) were found to be positive for the virus only, 14% (3 of 21; 95% CI = 3–36%) were found to produce only antibodies, and 10% (2 of 21; 95% CI = 1–30%) were positive for both the virus and antibodies. In summer 2011, 80% (4 of 5; 95% CI = 28–99%) were positive for antibodies only and 20% (1 of 5; 95% CI = 0–72%) were positive for the virus only. In fall 2011, 70% (7 of 10; 95% CI = 35–93%) were positive for antibodies only, while 20% (2 of 10; 95% CI = 3–56%) were positive for the virus only and 10% (1 of 10; 95% CI = 0–45%) were positive for both the virus and antibodies. In spring 2012, 100% (12 of 12; 95% CI = 74–100%) of these individuals were positive for antibodies only.
DISCUSSION
The presence of VHSV IVb in both the Muskellunge and Largemouth Bass collected from Budd Lake in 2011 was somewhat unanticipated given that sampling conducted between 2007 and 2010 failed to detect the virus. These past surveillance findings, in conjunction with the results of this research, raise the question as to whether the virus persisted in Budd Lake and simply went undetected by the conventional diagnostic assay recommended by both the OIE and American Fisheries Society or was perhaps reintroduced by recreational fishing activities or other biological means. After numerous mass mortality events that were attributed to VHSV IVb outbreaks throughout 2005 and 2006 in the Great Lakes region, federal, state, and provincial regulations were implemented to restrict intra- and interstate (provincial) movement of fish and other measures were taken to prevent the transfer of the virus, such as requiring anglers to empty live wells (Faisal et al. 2012). While these regulations undoubtedly lowered the risk of spread, the possibility of the virus being transferred among waterbodies cannot be entirely excluded.
Despite the wealth of information on VHSV physical and chemical characteristics and genetics, extremely little is known about its ecology—in particular, its latency. There are several ancillaries suggesting that VHSV IVb has persisted in Budd Lake and simply gone undetected. First, the laboratory methods approved by the OIE and American Fisheries Society for monitoring VHSV have detection limits so it is conceivable that fish in Budd Lake could have been infected, just at undetectable VHSV concentrations. Alternatively, VHSV IVb could have been actively circulating in fish populations at very low prevalences, which, given the sizes of samples collected during previous surveillance efforts, may have resulted in a very low probability of actually detecting the virus. Third, fish might have become more resistant to infection, which might explain why other large-scale mortality events clearly attributed to VHSV IVb have not occurred. According to Kim and Faisal (2012), infected Muskellunge can shed the virus for up to 15 weeks after infection and can resume shedding after exposure to a stressor event. Such shedding could have contributed to the development of this resistance by exposing naive fish to very low virus doses. This assumption is supported by our observation that Muskellunge and Largemouth Bass were infected in 2011 but exhibited no or minor external clinical signs characteristic of VHSV IVb infection. We cannot rule out the possibility that VHSV IVb has caused smaller-scale fish mortality events, relative to the 2007 event, that have gone unnoticed. This could easily be the case for particularly susceptible fish, such as juveniles or stocked individuals that might be naive to the virus. If the virus caused acute infections in these fish, they may have died and these mortality events went unnoticed.
The presence of antibodies in fish also suggests that the virus has been present in Budd Lake for a relatively long period. According to the results of Millard et al. (2014), in the case of Muskellunge, antibodies against VHSV IVb are detectable at 7–15 weeks postinfection. If this holds for a natural system, this would suggest that fish would have needed to have been exposed as early as January–March 2011, when water temperatures would have been below that considered optimal for a viral outbreak. Additionally, Millard et al. (2014) found that some infected Muskellunge can continue producing antibodies detectable by cELISA up to 1 year postinfection, meaning that fish in Budd Lake may have been exposed to the virus even longer ago. If the virus has been persisting in the environment since the 2007 outbreak, fish may have been continuously exposed for several years through either small-scale outbreaks or virus shedding, which would have contributed to sustained antibody production over the years.
Interpreting the antibody results for Largemouth Bass as to what they suggest concerning VHSV IVb persistence in Budd Lake is complicated by the fact that less is known about the relationship between the virus and this species, relative to that of a species such as Muskellunge. In particular, the timing and duration of antibody production in Largemouth Bass have not yet been studied as far as we are aware. Considering interspecific variations in factors such as spawning temperature, diet, habitat preference, and innate susceptibility to viruses, it is not surprising that esocids and Largemouth Bass would have differing levels of infection and antibody production. The fact that Largemouth Bass were found to be actively infected with the virus during some of the sampling events and in some cases individuals were producing antibodies at similar levels to Muskellunge, yet no mortality events were detected, suggests that Largemouth Bass have acquired at least some protection against the virus.
Lastly, in terms of previous surveillance efforts that turned up negative results, including the 2012 sampling that was conducted as part of this study, there was wide variability in sampling dates and presumably water temperatures. Immune system performance would have varied across these sampling dates depending on stress level (e.g., proximity to spawning). It is entirely possible that these previous surveillance efforts simply did not coincide with when individuals were most vulnerable to a viral outbreak. With respect to the negative surveillance results in 2012 as part of this research, because Largemouth Bass were processed on site rather than being transported to and contained at the Michigan State University Research Containment Facility, it is conceivable that differences in methods between the 2 years contributed to the observed differences in prevalences. First, it is possible that the Largemouth Bass collected in 2011 that were transported and contained had higher levels of stress, which could have led to increased viral concentration levels and resulted in the virus being more easily detected in infected individuals. It is also possible that as a result of elevated stress infected individuals began shedding the virus during transport and containment, which resulted in elevated infection prevalences as a consequence of disease spread. Kim and Faisal (2010b) found that mortality can occur in as little as 3 d postinfection, suggesting rapid uptake and proliferation of the virus, at least in some species. However, even if this was the case, it still would have been necessary for at least some individuals to have been infected with the virus initially, meaning our overall finding of positive infections in 2011 continues to be supported.
If the virus was instead reintroduced to Budd Lake at some point after the initial outbreak, there are several explanations for how this could have occurred. First, other animals could have transferred the virus back to the lake; these would most likely be piscivorous poikilotherms, such as turtles, which have been shown to be possible vectors, and snakes (Goodwin and Merry 2011b). As well, invertebrates have been shown to carry VHSV IVb in their tissues (Faisal and Schulz 2009; Faisal and Winters 2011). Second, although regulations were implemented that required boaters to drain water from the bilge and live well(s) upon leaving a body of water and required anglers to comply with specific bait restrictions, it is possible that in some cases these restrictions were not properly followed. An extension of this is the possible undocumented stocking of fish into Budd Lake without a permit or without ensuring that fish were disease-free prior to stocking. Any one or several of these events could have occurred in Budd Lake, leaving the discussion open as to whether the virus had persisted in the lake since 2007 or was reintroduced at a later time. Regardless, VHSV IVb has been confirmed in the lake 4 years later, and management actions should take these possibilities into consideration.
The isolation of VHSV IVb in Budd Lake has been an issue of concern for fishery managers not simply from the standpoint of the effect the virus could have on the lake's fish community and fishery but also the potential risk of the virus spreading to other inland lakes in the region. Given that there have been no additional VHSV IVb outbreaks in systems close to Budd Lake, the strict regulations adopted by the Michigan Department of Natural Resources have contributed to the overall noticeable restriction of virus spread throughout the state (Faisal et al. 2012). Before this study was conducted, it was uncertain whether VHSV IVb was still present in Budd Lake and thus whether managers still needed to be concerned about the possible spread of the virus or whether management actions (e.g., stocking) might result in another disease outbreak. Based on the results of this study, VHSV IVb still very much appears to be circulating in the system; therefore, managers should continue to be vigilant about the potential for the spread of the virus and perhaps revisit their prevention and monitoring plans with this fact in mind. A simple prevention tool for minimizing pathogen transfer is the cleaning and disinfection of boats entering and leaving infected bodies of water. It would be beneficial to have a clear guide placed at boat launches outlining the best method for this, and boat wash facilities should be installed near the lake. Managers may want to consider the use of molecular assays for the surveillance of VHSV IVb as opposed to the current “gold standard” method of virus isolation on cell culture, which may not be sufficiently sensitive. In addition, the detection of antibodies may be just as important for information on virus status as detection of the virus itself, so we encourage the implementation of serological techniques for monitoring purposes, although it is important that these methods are optimized for each species on which they are conducted.
While there have been no mortality events caused by VHSV IVb that have been detected in Budd Lake since the initial outbreak in 2007, there remains the potential for future mortality events to occur given that we now know there is still virus in this system. Both natural and anthropogenic stressors could trigger another viral outbreak and additional fish kills, similar to what has been observed in other VHSV IVb–enzootic systems. Clearly, gaining a better understanding of the complete host range of VHSV IVb and its ability to persist in environments would be immensely helpful in the development of efforts to limit spread or future outbreaks of the virus.
There are numerous possibilities regarding reservoirs for VHSV IVb, and more effort is needed to identify both incidental and natural reservoirs and to include them in general surveillance plans. As previously mentioned, shedding of the virus may be one source of infection. As well, invertebrate species, such as piscicolid leeches and amphipods, have been identified as potential VHSV IVb reservoirs for the Great Lakes (Faisal and Schulz 2009; Faisal and Winters 2011). Thus, it may be beneficial for surveillance efforts to be expanded to include organisms other than just fish. Continued study of VHSV IVb in Budd Lake could provide beneficial information with regards to reservoirs and vectors of the virus in systems where outbreaks have occurred, as well as provide a better understanding of transmission dynamics, latency, and outbreak triggers.
ACKNOWLEDGMENTS
Funding for this research was provided by the U.S. Fish and Wildlife Service Great Lakes Fish and Wildlife Restoration Program. The authors thank Chris Schelb, Donald Barnard, Vince Balcer, Tammy Newcomb, and Gary Whelan from the Michigan Department of Natural Resources for their field assistance, guidance, and helpful discussions regarding this project. The authors also thank Kyle Molton, Heidi Jerrils, Carson Prichard, Chad Burton, Jared Ross, Kelly Donohue, and Christine Rabaut for assistance in the field and laboratory.




