Capture and Brief Invasive Procedures Using Electronarcosis Does Not Appear to Affect Postrelease Habits in Male Atlantic Sturgeon During the Spawning Season
Abstract
With advances in technology and demand for life history information, researchers are increasingly conducting invasive procedures on fish that require an anesthetic. This study examined the effectiveness of electronarcosis as a field anesthetic on Atlantic Sturgeon Acipenser oxyrinchus oxyrinchus during the spawning season in the James River, Virginia. Concerns about sampling Atlantic Sturgeon during spawning runs prompted our examining whether movements after capture, narcosis, and tagging were noticeably modified. An electronarcosis system, which consisted of a power supply, fiberglass tank, and hardware cloth, were set up both on land and on a sampling boat. During the spawning season Atlantic Sturgeon were caught and implanted with Vemco V16 telemetry tags using electronarcosis as an anesthetic. Anesthesia induction, surgery, and recovery averaged 5 min. Telemetry data from the tagging year was compared with returning fish tagged in previous years. This showed movements during the spawning season and spawning exit dates were similar between the two groups, suggesting that electronarcosis was effective and time-efficient for conducting invasive procedures. Capturing and implanting transmitters in Atlantic Sturgeon during spawning runs does not appear to modify spawning movements. Managers may benefit from targeting adult Atlantic Sturgeon and other iteroparous anadromous fishes in rivers during spawning periods, which is typically very efficient due to high densities of fish during that period.
Received February 21, 2014; accepted December 31, 2014
Sturgeons (family Acipenseridae) are threatened worldwide and have been extirpated from various parts of their historical ranges (Smith 1985; Boreman 1997). With increased effort in restoration of sturgeon species, invasive procedures to track their movements and observe internal biological characteristics are being conducted around the world. The large size of many sturgeon species makes invasive procedures difficult without anesthetics. Atlantic Sturgeon Acipenser oxyrinchus oxyrinchus can grow to 4.4 m total length and 364 kg (Scott and Crossman 1973), but fish of this size have not been documented in decades; currently, Atlantic Sturgeon, up to 2.5 m FL and 135 kg have been collected over the past few years in the James River (M.T.B., unpublished data). Atlantic Sturgeon was federally listed as an endangered or threatened species in 2012 and invasive procedures are required to help restoration efforts.
Anesthetics that have immediate induction and recovery are vital to fisheries research (Trushenski et al. 2013). Electronarcosis, a physical anesthetic frequently used in salmonid field studies (Roth et. al 2003; Hudson et al. 2011), has been used on captive Lake Sturgeon A. fulvescens, Shortnose Sturgeon A. brevirostrum (Henyey et al. 2002), and Atlantic Sturgeon (Balazik et al. 2013). Balazik et al. (2013) determined laboratory Atlantic Sturgeon had higher cortisol (stress hormone) plasma levels during invasive procedures without anesthesia than those anesthetized. Henyey et al. (2002) and Balazik et al. (2013) concluded electronarcosis was easier, faster, better for the environment, and safer than tricaine methanesulfonate (MS-222), a common fish anesthetic. Electronarcosis is effective in a laboratory setting, but its effect on wild adult Atlantic Sturgeon in the field is unknown. Our two study goals were to examine (1) examine electronarcosis effectiveness and efficiency for anesthetizing adult Atlantic sturgeon in a field setting, and (2) the effects of sampling and handling on adult male Atlantic Sturgeon movement patterns.
METHODS
To determine whether electronarcosis was effective and practical on adult Atlantic Sturgeon in the field, an experimental setup was built in 2011 at Presquile National Wildlife Refuge located at river kilometer (rkm) 123 of the James River, Virginia. To increase our ability to sample and conduct surgeries in areas away from the refuge an electronarcosis system was positioned on the collection boat in 2013 (Figure 1). Both the refuge and boat electronarcosis apparatus consisted of a fiberglass tank (244 × 61 × 36 cm) with electrodes on each end. The electrodes were 0.6 cm square-mesh galvanized hardware cloth supported by polyvinyl chloride frames (55 × 40 cm) designed to stand upright and be placed various distances from each other in the tank. A 0–60-V DC, 1.5-A (BK Precision: Model 1623A) power supply was connected to the hardware cloth with 10-gauge wire attached to alligator clips (Henyey et al. 2002; Hudson et al. 2011; Balazik et al. 2013). At the refuge the power supply was plugged directly into a 120-V AC plug. Boat electronarcosis was powered by the boat's 12-V battery via an inverter. The tanks were filled with James River freshwater for both setups, and fish were placed with their head toward the positive electrode (Henyey et al. 2002; Balazik et al. 2013). A stretcher was positioned underneath the fish so that only the incision area was elevated above the water (Figure 1). The power supply was set to 0 V, and the voltage was increased until stage-4 anesthesia was reached (Summerfelt and Smith 1990; Hudson et al. 2011; Balazik et al. 2013). Stage-4 anesthesia is characterized by complete loss of spinal reflexes and slowed opercular movement (Summerfelt and Smith 1990). The difference with electronarcosis is there is no slowed opercular movement (Balazik et al. 2013). Voltage was reduced if opercula movement was erratic or the mouth protruded (Balazik et al. 2013). Amperage, voltage, and distance between the electrodes were recorded. Vemco V16-4x or V16-6x tracking tags were surgically implanted following Mohler (2004). Fish fork length (cm), weight (kg), water salinity, (‰) and temperature (°C) were measured during tag implantation. After tag implantation fish were monitored for positive upright taxis and returned to the James River at rkm 123, which was within 1 km of capture.

Electronarcosis apparatus on the boat used to collect Atlantic Sturgeon in the James River, Virginia, showing the power supply (usually placed in a ventilated plastic container for protection from splashed water), which was hooked to an inverter powered by the boat's 12-V battery. The adjustable electrodes are hooked to the power supply by leads about 5 m long. The webbed stretcher within the nonmetallic, fiberglass tank was used to elevate the fish to the proper level so the incision area was out of the water but the fish remained in the electrical current.
As part of another study five Vemco V13-1x tags were tethered externally in 2009 and another 25 were tethered externally in 2010 at rkm 122 during the fall spawning season (Balazik et al. 2012a). No anesthesia was used for the external tagging and all 2009 and 2010 fish were males (Balazik et al. 2012a). Sturgeon were captured via anchored gill nets (<4 m high, 25–36 cm stretch mesh) set in water depths of 4–12 m. All sampling followed National Marine Fisheries Service protocols. Nets were set parallel to river flow blocking less than 10% of navigable waters, to allow only a small percentage of fish to be intercepted and others to move by unhindered. Nets were set about 9 rkm downstream of the closest hypothesized spawning grounds. All Atlantic Sturgeon were captured at rkm 122 and released at rkm 123 between August 5 and October 15 of 2011–2013; we captured 305 individual adult male Atlantic Sturgeon and tagged 136 (10 in 2011, 72 in 2012, and 54 in 2013). Tagged fish averaged 154 cm FL, (range, 127–207 cm) and 41 kg (range, 21–74 kg); they were implanted with Vemco telemetry tags using electronarcosis as an anesthetic (Table 1). During the tagging period water temperature varied from 18°C to 27°C and salinity was ≤0.1‰. In 2011, the first tags placed were later in the year than in subsequent years because the electronarcosis apparatus was not built until later in the sampling season (Table 1). All but eight surgeries were conducted on the national wildlife refuge. The eight that were conducted on the boat were done adjacent to the refuge.
| New tags | Returning tags | Effect size | ||||||
|---|---|---|---|---|---|---|---|---|
| Year | No. tagged | Average tagging date (range) | Average exit date (range) | No. (year tagged) | Average exit date (range) | D-value (P) | Stand. | 95% CI |
| 2011 | 10 | Sep 24 (Sep 19–Oct 04) | Oct 10 (Oct 03–19) | 2 (2009) 10 (2010) | Oct 12 (Sep 22–Nov 02) | 0.17 (0.44) | 0.21 | −0.59 to 1.01 |
| 2012 | 72 | Sep 17 (Aug 22–Oct 14) | Oct 12 (Sep 18–Nov 07) | 1 (2010)9 (2011) | Oct 13 (Oct 07–Oct 18) | 0.31 (0.32) | 0.08 | −0.58 to 0.74 |
| 2013 | 54 | Sep 09 (Aug 16–Oct 16) | Oct 10 (Sep 15–Nov 04) | 7 (2011) 52 (2012) | Oct 11 (Sep 21–Oct 27) | 0.37 (0.37) | 0.08 | −0.29 to 0.45 |
Postsurgery spawning migration patterns were monitored throughout the fall using a Vemco VR2W array with about 42 receivers in the river per year. Telemetry data from returning adult Atlantic Sturgeon tagged (internally or externally) in previous years were compared with newly tagged fish movements. I assumed that returning fish displayed normal spawning movements and behavior and, as such, served as a control group. Because of poor receiver coverage in the lower part of the James River, the furthest downstream receiver gate at rkm 53 was considered as the exit point. When a fish moves downstream of this exit point, it was never observed any to move back upstream to the spawning area again that year. Kolmogorov–Smirnov two-sample tests using relative cumulative-frequency plots were run comparing exit date for returning fish and fish that were tagged that same year. Because of low sample sizes, Hedges’ bias-corrected standardized-effect size with 95% confidence intervals (CIs) were calculated to determine the effect of the tagging procedure on leave date (Hedges 1981; Kelly 2007). Statistical tests were considered significant at α = 0.05. Movements were plotted to determine differences between the two groups.
RESULTS
Anesthesia induction and recovery were instantaneous similar to laboratory experiments observed in Balazik et al. (2013). Once the current was increased to the appropriate level, prone fish rolled to the supine position, if already supine that fish stayed in that position. It usually took about 2 s to adjust the voltage. Gill movements remained constant throughout all procedures. Induction metrics averaged 0.17 V/cm (range, 0.14–0.19) and 0.08 A (range, 0.07–0.09) and procedures (induction, surgery, and recovery) averaged 5 min (range 4–6 min). One outlier surgery took 10 min because old gill net material was cut from fish's head and gills and was not included in the surgery time calculation. The current was maintained until the surgery was over. All fish were released and swam away in apparent good health.
All but one fish left the James River the year they were tagged (Table 1). The one fish that did not leave was active for 11 d after implantation before movement stopped on September 7, 2013, and did not move through December 3, 2013. The tag may have been shed or the fish may have perished; this fish was removed from data analysis. The tag is located in an area of high boat traffic where boat strikes occur (Balazik et al. 2012b). All of the returning fish in 2011 and one in 2012 had external tags placed during the spawning season of 2010 (Balazik et al. 2012a) (Table 1). Nine of the 10 fish tagged in 2011 returned in 2012 and seven returned in 2013. Of the 72 fish tagged in 2012, 52 returned in 2013. There were no significant differences (Kolmogorov–Smirnov test) between exit dates of returning fish and newly tagged fish (Table 1). The relative cumulative-frequency plots (Figure 2) used for the Kolmogorov–Smirnov test show similar patterns in 2011 and 2013. The 2012 plot (Figure 2) shows returning fish leaving in a much narrower timeframe than fish tagged that year. The difference is probably due to low number of returning fish compared with fish tagged that year (Table 1). According to Cohen (1988) a standardized effect size of 0.2–0.3 signifies there might be a small effect due to the variable. So in 2011 (effect size 0.21), there is a small chance of an effect, while in 2012 and 2013 (effect size 0.08) there is very little chance of an effect (Table 1). The 95% CIs of the standardized effect size includes 0 for all 3 years, suggesting if there is an effect it is small.
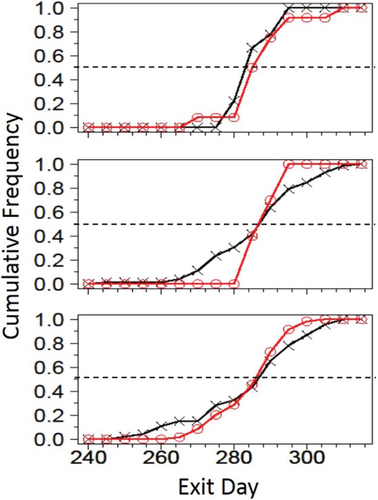
Relative cumulative-frequency plots of exit date (day of the year) for returning male Atlantic Sturgeon in the James River, Virginia, tagged previously (O) via electronarcosis and ones tagged that year (X).
Analysis of recaptures and telemetry data showed that fish moved passed the sampling area at least 91% of the time without being captured. Plots of telemetry data of returning and fish tagged that year show similar patterns (Figure 3). Approximately half of the male fish tagged during the early part of the spawning season (September 7) moved 10–30 rkm downstream within a few days of tagging (Figure 3B), but the remainder stayed within a few river kilometers or moved upstream. Early returning males also exhibit a bimodal response, some moving upstream and staying while others move back and forth (Figure 3A). Males caught and tagged after September 7 remained in the area or moved upstream from the capture location (Figure 3C, D).
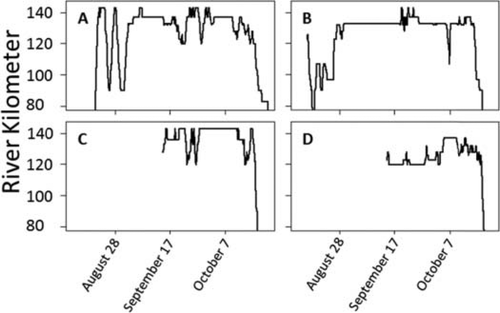
Plots of four different telemetry day-of-year movements among male Atlantic Sturgeon during the spawning season in the James River, Virginia. Telemetry data are available downstream of river kilometer (rkm 80); however, spawning occurs above rkm 120 based on the general location of the salt wedge. (A) The earliest male to return to the freshwater portion of the river in 2013. (B) The first fish tagged in 2013 showing the general pattern of a fish that drops back after tagging (dropped down to rkm 79 before moving back upstream). Drop-back is often seen in fish tagged before September 7 and is observed in many returning fish, as shown in panel A. (C) The observed pattern of a fish tagged later in the year after September 7 that immediately moves upstream. (D) The pattern of a fish that stays close to the tagging area.
DISCUSSION
Construction of an electronarcosis setup is relatively inexpensive, and I recommend this technique for future field work on Atlantic Sturgeon and other sturgeon species in freshwater. Unlike various chemicals analgesics, electronarcosis equipment does not expire or degrade and require the handling and disposal of toxic chemicals. Reduced handling time probably benefits the fish because they fish resume natural behavior sooner. Both the refuge and boat electronarcosis setups were efficient for sex determination and tag implantation. Induction and recovery from anesthesia using electronarcosis were instantaneous allowing more rapid processing than other typically used methods (i.e., clove oil, MS-222) (Munday and Wilson 1997; Henyey et al. 2002; Matsche 2011; Balazik et al. 2013). Because the amperage is relatively low (about 0.08 A), no sensation was felt by the surgeon during surgeries. However, a slight electric sensation was felt if the surgeon's hands were spread apart close to each electrode, which is much farther apart than needed to perform surgery. The sensation was felt with and without latex gloves but was not considered painful by any of the researchers.
The telemetry data show fish moved passed the sampling area most of the time without being intercepted, suggesting nets settings were successful in allowing most fish to pass by the sampling area and to spawning grounds unhindered. Telemetry data also show no noticeable difference between spawning of fish tagged in prior years and movements of fish recently tagged (2, 3). About half of the males tagged prior to September 7, swam about 30 rkm downstream within a few days of capture and tagging; however, returning fish have similar upstream–downstream movements (Figure 3). Males tagged after September 7, tended to stay at the capture location or move upstream similarly to returning males.
Spawning runs are an effective and efficient time to sample adult Atlantic Sturgeon, and because their capture appears to not modify male behavior, more managers may want to sample sturgeon during spawning migrations. River sampling typically requires smaller boats, is less costly, generally safer, and more efficient than ocean sampling because fish density is much higher in the river. Another positive aspect is that the fish being sampled in the river are likely to be part of the targeted local population. Given this increased efficiency and knowledge of population membership, river sampling probably provides better opportunities for managers studying or rehabilitating sturgeon populations.
The ineffectiveness of electronarcosis in brackish or marine waters continues to hinder its use for various marine species (Balazik et al. 2013). However, on the St. Lawrence River, Quebec, adult Atlantic Sturgeon were caught in water with a salinity of 10‰ and placed in freshwater briefly for surgery. Telemetry data shows all fish survived (G. Verreault, Quebec Ministry of Natural Resources, unpublished data). More research is needed describing the physiological effects of briefly moving fish from saltwater to freshwater for electronarcosis. More information is also needed on the effects of handling and electronarcosis on spawning females. The speed of anesthesia induction and recovery and non-use toxic chemical analgesics makes electronarcosis a better choice for freshwater. Considering that sturgeon spawn in freshwater and male spawning behavior does not appear to be modified due to capture and tag implantation, using electronarocsis on adult-male sturgeon during spawning periods seems to be an effective research tool.
ACKNOWLEDGMENTS
I thank Briana Langford, Greg Garman, Stephen McIninch, Thomas Huff, Anne Wright, Leonard Smock, Michael Fine, Casey Seelig, and Peter Sturke (Virginia Commonwealth University), Albert Spells, Cyrus Brame, Michael Odom, and Ed Darlington (U.S. Fish and Wildlife Service), Chuck Frederickson and Jameson Brunkow (James River Association), Kevin Reine and Douglas Clarke (U.S. Army Corps of Engineers), Boyd Kynard (University of Massachusetts, Amherst), Jason Kahn and Malcolm Mohead (National Oceanic and Atmospheric Association), Bob Greenlee (Virginia Department of Game and Inland Fisheries) and Carter Watterson (U.S. Department of the Navy) for assistance with this project. Martin Balazik and Thiwaporn Balazik spent countless hours assisting with gill-net sampling and surgical procedures. The work was greatly improved by four anonymous reviewers. This work was completed following VCU IACUC AD20127 protocols and Nation Marine Fisheries Service Endanger Species Permit 16547-1. This research was partially funded by the PADI Foundation (7831) and NOAA (NA13NMF4720037). This is VCU Rice Rivers Center contribution 50.




