Using Larval Fish Community Structure to Guide Long-Term Monitoring of Fish Spawning Activity
Abstract
Larval fishes provide a direct indication of spawning activity and may therefore be useful for long-term monitoring efforts in relation to spawning habitat restoration. However, larval fish sampling can be time intensive and costly. We sought to understand the spatial and temporal structure of larval fish communities in the St. Clair–Detroit River system, Michigan–Ontario, to determine whether targeted larval fish sampling can be made more efficient for long-term monitoring. We found that larval fish communities were highly nested, with lower river segments and late-spring samples containing the highest genus richness of larval fish. We created four sampling scenarios for each river system: (1) using all available data, (2) limiting temporal sampling to late spring, (3) limiting spatial sampling to lower river segments only, and (4) limiting both spatial and temporal sampling. By limiting the spatial extent of sampling to lower river sites and/or limiting the temporal extent to the late-spring period, we found that effort could be reduced by more than 50% while maintaining over 75% of the observed and estimated total genus richness. Similarly, limiting the sampling effort to lower river sites and/or the late-spring period maintained between 65% and 93% of the observed richness of lithophilic-spawning genera and invasive genera. In general, community composition remained consistent among sampling scenarios. Targeted sampling offers a lower-cost alternative to exhaustive spatial and temporal sampling and may be more readily incorporated into long-term monitoring.
Received August 14, 2014; accepted November 28, 2014
In aquatic systems, the richness of families, genera, or species has long been used to determine community integrity in fishes (e.g., Karr 1981) and macroinvertebrates (e.g., Lammert and Allan 1999). Recently, community-level larval fish sampling has been conducted to evaluate riverine spawning and nursery habitats (Niles and Hartman 2011; Nannini et al. 2012) and to assess anthropogenic disturbance in rivers (Humphries and Lake 2000; Ribeiro et al. 2013; Mapes et al. 2015). In freshwater fish communities, degradation of spawning habitat, particularly for lithophilic-spawning species, has been hypothesized to reduce the biotic integrity of the community (e.g., Berkman and Rabeni 1987). Larval fishes can serve as an excellent indicator of fish spawning habitat by providing a direct measure of spawning success, whereas the presence/absence of adult fishes can only be used to indirectly infer spawning habitat quality. Thus, community-level larval fish sampling may provide useful information on spawning habitat quality in light of anthropogenic stressors, invasive species, environmental change, and habitat restoration efforts.
In the Laurentian Great Lakes, connecting channels (rivers that connect one Great Lake to another) once provided spawning habitat to migratory and resident fishes (Goodyear et al. 1982), but habitat degradation, including lock and dam construction, pollution, dredging, and sedimentation, has limited fish production and diversity in these systems (Manny et al. 2010; Hondorp et al. 2014). In the St. Clair–Detroit River system (SCDRS), which acts as the connecting channel between Lake Huron and Lake Erie, spawning habitat restoration has recently been conducted with the goal of increasing the production and biodiversity of native fishes (Manny et al. 2010; Hondorp et al. 2014; SCDRSI 2014). To this end, larval fish sampling has been conducted throughout the system since 2006, and long-term monitoring has been identified as a means to gauge the effectiveness of habitat restoration efforts for meeting fish spawning goals (SCDRSI 2014). Development of long-term monitoring strategies that can inform future habitat restoration through an adaptive management process is paramount to the ongoing success of habitat restoration in the SCDRS (Lindenmayer and Likens 2009; Manny et al., in press).
Although larval fish data can provide useful insights into habitat quality, larval fish sampling is effort intensive and costly (Cyr et al. 1992). However, an understanding of the spatial and temporal structure of larval fish communities may allow for targeted sampling that could reduce sampling effort while achieving monitoring objectives. The goal of this study was to develop an efficient strategy for the long-term monitoring of larval fish diversity in the SCDRS. Our objectives were to (1) characterize the spatial and temporal structure of the larval fish community in the SCDRS, (2) use community structure information to allocate spatial and temporal sampling effort in a way that maximizes taxon accumulation, and (3) evaluate reduced-effort protocols for the long-term monitoring of larval richness, indicator taxa, and community composition. Here, we show that in highly structured communities, spatially and temporally targeted sampling may provide an opportunity to maximize limited sampling resources.
METHODS
Data collection.
Our study systems were the St. Clair and Detroit rivers, which are separated by Lake St. Clair and connect Lake Huron to Lake Erie. Both rivers have been heavily modified to accommodate shipping and industrial uses, but unlike other Great Lakes connecting channels, they are free of locks, dams, and other obstructions to fish movement (Hondorp et al. 2014). Larval fishes were sampled in the St. Clair River during 2010–2013 and in the Detroit River during 2006, 2007, and 2010–2013. Sample transects in each river were arranged systematically from upstream to downstream at approximately 7-km intervals, similar to the study of Hatcher et al. (1991). Each transect contained from two to five sampling locations across the river channel. Where multiple channels occurred, at least one sample site was located in each channel. Larval sites were selected to capture cross-channel and longitudinal trends and to represent areas near potential spawning habitat (e.g., near islands; see Pritt et al. 2014 for Detroit River site locations). Sites were consistent across years such that exhaustive sample years (2010 and 2013) included sites of “limited” sample years along with additional sites. Sites were typically sampled weekly, with the exception of five surface sites and one deep site in the lower Detroit River, which were sampled semiweekly through June during 2013. Sampling typically began in early April in the St. Clair River and mid-March in the Detroit River and continued until June, July, or August. Most collections were made with paired bongo nets, each outfitted with 500-µm mesh; however, in 2006 and 2007, a 500-µm-mesh bongo net was paired with a 333-µm- or 1,000-µm-mesh bongo net. Based on preliminary analyses, the composition and abundance of samples collected with 333-µm and 500-µm mesh sizes did not differ, and the 1,000-µm mesh was eliminated to provide consistency in future sampling efforts. Each replicate tow was considered a sample, and differences in mesh sizes were assumed to have no significant effect on the taxa captured in 2006 and 2007. Surface samples (upper 2 m of the water column) were collected at most sites by towing the nets in a circle at a speed of 3.0 km/h for 3–5 min. In addition to surface tows, a subset of deep sites (>5 m) was sampled by anchoring the boat and fishing the bongo nets in the current near the bottom for 15 min. In the Detroit River, 18 deep sites were sampled in 2006, and 7 deep sites were sampled in 2007. One to two deep sites were sampled in both rivers during the remaining sample years.
We preserved larvae in 95% ethanol in the field. In the laboratory, we removed larval fish from samples and identified individuals to the lowest possible taxonomic level following Auer (1982). Since species identification for larval fish is not always achievable, we included only genus-level identifications in this analysis. In addition, because the genera of the family Cyprinidae cannot be distinguished based on morphological characteristics alone, we assumed that all cyprinids belonged to the genus Notropis, which is the most abundant genus in the SCDRS (Hatcher et al. 1991; E. Roseman, unpublished data). For the remaining families, all individuals that could not be identified past the family level were removed from the data set (<3% of collected individuals were removed). Thus, our taxonomic richness estimates reflect not only sampling inefficiencies but also difficulties in identification.
Spatial and temporal structure of larval fish communities.
We first sought to understand the longitudinal and temporal structure of the larval fish communities in the SCDRS. In large rivers, larval fish often hatch in upstream spawning habitats and are transported downriver (e.g., Mion et al. 1998; Roseman et al. 2012); the typical phenology of fishes during the spring hatching season begins with only a few taxa, and more taxa are added as the spring progresses (Auer 1982; Falke et al. 2010). Therefore, lower river reaches during late spring (before early hatching taxa lose their vulnerability to sampling) may integrate taxonomic richness through space and time. Limiting the temporal and spatial sampling to locations and time periods that accumulate taxa may reduce the costs of important long-term monitoring programs while maintaining the value of the programs.
To quantify larval fish community structure and identify opportunities for targeted sampling, we used data collected from the SCDRS during spring and summer of 2011 and 2013, the most intense years of larval fish sampling. We first plotted the observed genus richness by month for all sites sampled in order to determine changes in genus richness through time. Next, we plotted cumulative genus richness by river segment (main channel, delta, and mouth in the St. Clair River; upper, middle, lower, and mouth in the Detroit River) over all sample weeks to determine longitudinal changes in genus richness. Finally, for each river and each sampling year, we created presence/absence matrices of genera by sample week (pooling all locations together) and by river segment (pooling all sample weeks together) to determine nestedness. In nested communities, the species pools at upstream sites are subsets of those at downstream sites, and the species pools at early sampling dates are subsets of those at later sampling dates (Figure 1). We tested for nestedness within each matrix by calculating matrix temperature (see Roberts and Hitt 2010) using the vegan package in R version 3.0 (Oksanen et al. 2011). “Cooler” temperatures (<50°C) indicate nested communities, whereas “warmer” temperatures indicate nonnested communities. From these analyses, we identified the river segment with the highest genus richness and an “optimal window” of sampling for each river system (May–June in the Detroit River; June–July in the St. Clair River) in which most genera were observed as present.
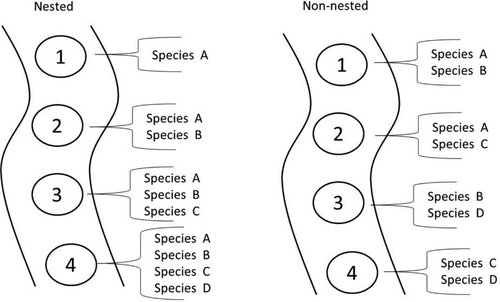
Examples of nested and nonnested communities. In nested communities (left), upstream communities (sites 1 and 2) represent a subset of the downstream communities (sites 3 and 4). In nonnested communities (right), community composition is independent among sites.
Determination of the sampling effort needed for monitoring larval fish communities.
We created four competing sampling scenarios for all study years in the SCDRS to determine the most efficient allocation of sampling effort for long-term monitoring: (1) all available data (all sampling locations and years), (2) limited temporal sampling (using samples from all locations, but only including those samples collected during May–June in the Detroit River and June–July in the St. Clair River), (3) limited spatial sampling (using all available sampling weeks, but only including samples from the river mouth sites for each system), and (4) limited temporal and spatial sampling (including only those samples collected from the river mouth sites during May–June in the Detroit River and June–July in the St. Clair River). A combination of surface and deep larval fish tows was maintained in the reduced-sampling scenarios. For each scenario, we calculated the observed genus richness and the number of samples taken.
Often, taxa are undetected in community sampling, and true richness is greater than observed richness. Two common statistical estimators, Chao 1 and the first-order jackknife (Gotelli and Colwell 2010), were applied to the Detroit River and St. Clair River larval fish data sets to estimate genus richness by accounting for undetected taxa. To extrapolate theoretical richness, the Chao 1 technique uses the number of taxa that occur with an abundance of one or two individuals, and the first-order jackknife uses the number of unique taxa (those found in only one sample; Gotelli and Colwell 2010). All richness estimates were obtained using the vegan package in R. To estimate richness, we built genus accumulation curves through time, treating individual sampling weeks as the unit of observation. We also used Pearson's product-moment correlation to determine (1) the relationship between within-scenario observed and estimated richness values and (2) relationships between observed and estimated richness among sampling scenarios.
Although the overall richness of fishes spawning in the SCDRS is an important metric for evaluating habitat in the system, other metrics may also be important for determining the effectiveness of habitat restoration and biotic integrity. For example, habitat restoration efforts have focused on constructing artificial rock reefs to benefit lithophilic-spawning species (Manny et al., in press). In addition, historic and recent species invasions have been problematic throughout the Great Lakes, and early detection of invasive species has been identified as a priority in community monitoring (Vander-Zanden et al. 2010). To reflect these management aspects, we developed two additional richness metrics: (1) the number of lithophilic-spawning genera and (2) the number of invasive genera. We determined lithophilic spawning behavior based on designations from Balon (1975). Invasive designations were based on the Great Lakes Aquatic Nonindigenous Species Information System (NOAA 2014). We considered Catostomus, Coregonus, Hypentelium, Minytrema, Moxostoma, Percopsis, and Sander as lithophilic genera, and we designated Alosa, Gasterosteus, Neogobius, Osmerus, and Proterorhinus as invasive genera. We compared the magnitude and amount of correlation for each metric among the different sampling scenarios.
We also sought to determine the effects of reduced sampling effort scenarios on observed community composition. To accomplish this, we first calculated pairwise similarities in larval fish communities among the four sampling scenarios for each system in each sampling year by using the Sørensen index of similarity and the Bray–Curtis index of similarity. We chose to use the Sørensen index as a measure of similarity in genus presence/absence, and we used the Bray–Curtis index as a measure of similarity in genus abundances. In addition, we visually analyzed rank abundance curves for each of the four sampling scenarios to determine specific differences in community composition driven by sampling protocol.
RESULTS
Spatial and Temporal Structure of Larval Fish Communities
In general, early spring (March–April in the Detroit River; April–May in the St. Clair River) was relatively genus-poor, with only early hatching genera (e.g., Coregonus) present. In both systems, genus richness increased as the spring progressed, typically peaking during May–June in the Detroit River and during June–July in the St. Clair River. Richness typically declined later in summer (Figure 2). Thus, in subsequent analyses, we defined the optimal sampling window as the 8-week peak period of May–June in the Detroit River and June–July in the St. Clair River. Genus richness in the St. Clair River exhibited no apparent changes with river segment, whereas genus richness in the Detroit River was typically greater in the lower segments than in the upper segments (Figure 2). The larval fish communities in the St. Clair and Detroit rivers both exhibited a high amount of temporal and spatial nestedness (Table 1). Temporal nestedness in both systems was higher in 2013 than in 2011; spatial nestedness was higher in the Detroit River than in the St. Clair River. Thus, samples from upper-river sites were a nested subset of samples from lower-river sites, and early spring samples were a nested subset of late-spring samples.
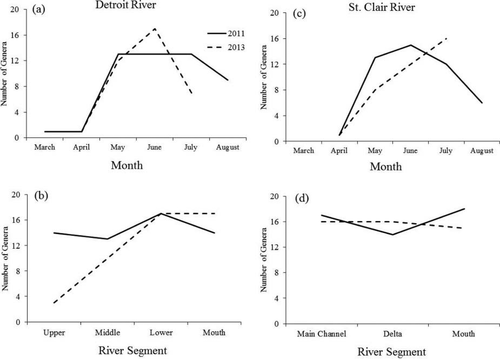
Genus richness during 2011 and 2013 by (a) sample month in the Detroit River; (b) river segment in the Detroit River; (c) sample month in the St. Clair River; and (d) river segment in the St. Clair River.
| Type | River | Year | Matrix temperature (°C) |
|---|---|---|---|
| Temporal | Detroit | 2011 | 17.2 |
| 2013 | 7.9 | ||
| St. Clair | 2011 | 11.2 | |
| 2013 | 9.0 | ||
| Spatial | Detroit | 2011 | 15.2 |
| 2013 | 7.9 | ||
| St. Clair | 2011 | 17.7 | |
| 2013 | 27.0 |
Determination of the Sampling Effort Needed for Monitoring Larval Fish Communities
The amount of annual sampling effort in the full data sets for the St. Clair and Detroit rivers ranged from 374 to 1,000 samples and covered from 13 to 22 sampling weeks (Table 2). Reducing the full data set to a limited spatial sampling (river mouth sites only) produced an approximate 65% decline in the number of samples taken (the number of weeks sampled remained the same as that of the full data set). Limited temporal sampling led to a 55% decline in the total number of samples taken and the number of weeks sampled (Table 2). Use of both limited spatial sampling and limited temporal sampling produced an approximate 85% decline in the number of samples taken and an approximate 55% decline in the number of weeks sampled (Table 2).
| Measure of effort | River | Year | Full | Limited spatial | Limited temporal | Limited spatial and temporal |
|---|---|---|---|---|---|---|
| Weeks sampled | Detroit | 2006 | 13 | 12 | 6 | 6 |
| 2007 | 15 | 13 | 6 | 6 | ||
| 2011 | 21 | 20 | 8 | 8 | ||
| 2012 | 14 | 13 | 7 | 7 | ||
| 2013 | 15 | 16 | 8 | 8 | ||
| St. Clair | 2010 | 20 | 20 | 8 | 8 | |
| 2011 | 20 | 20 | 8 | 8 | ||
| 2012 | 22 | 22 | 8 | 8 | ||
| 2013 | 18 | 18 | 8 | 8 | ||
| Samples taken | Detroit | 2006 | 880 | 255 | 394 | 135 |
| 2007 | 671 | 193 | 306 | 100 | ||
| 2011 | 1,383 | 472 | 575 | 192 | ||
| 2012 | 743 | 410 | 392 | 192 | ||
| 2013 | 940 | 424 | 458 | 228 | ||
| St. Clair | 2010 | 993 | 238 | 401 | 81 | |
| 2011 | 1,032 | 280 | 416 | 80 | ||
| 2012 | 374 | 132 | 132 | 72 | ||
| 2013 | 578 | 218 | 288 | 72 |
Observed genus richness in the full data set for the Detroit River varied from 16 genera in 2012 to 21 genera in 2007; observed richness in the full data set for the St. Clair River ranged from 16 genera in 2012 to 20 genera in 2011 (Figure 3). Reducing effort by limiting spatial sampling produced a decline of 2–5 observed genera in the Detroit River (average decline = 3 genera; 83% of full data set) and a decline of 2–6 observed genera in the St. Clair River (average decline = 4 genera; 78% of full data set). Reducing effort by limiting temporal sampling produced a decline of 0–5 observed genera in the Detroit River (average decline = 2 genera; 90% of full data set) and a decline of 2–6 observed genera in the St. Clair River (average decline = 3 genera; 82% of full data set). Reducing effort by limiting both spatial and temporal sampling produced a decline of 3–7 observed genera in the Detroit River (average decline = 5 genera; 76% of full data set) and a decline of 6–10 observed genera in the St. Clair River (average decline = 8 genera; 55% of full data set). For the St. Clair and Detroit rivers, observed genus richness from the full data set was highly correlated with observed richness from the reduced-effort data sets. Based on Pearson's product-moment correlation coefficients across systems and years, the observed genus richness in the full data set was positively correlated with the richness from limited spatial sampling (r = 0.63), limited temporal sampling (r = 0.72), and limited spatial and temporal sampling (r = 0.74), indicating that limited-sampling protocols may be useful for assessing annual trends in richness.
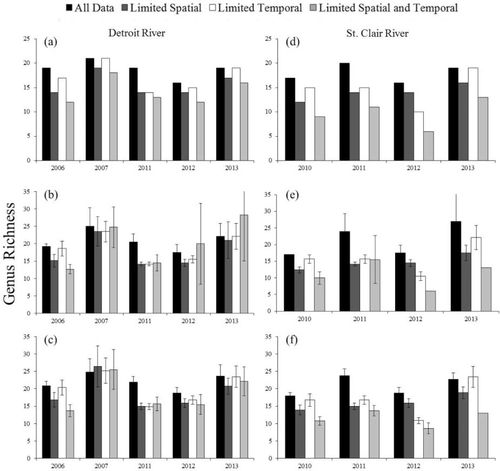
(a) Observed genus richness of larval fish in the Detroit River; (b) estimated richness in the Detroit River based on the Chao 1 estimator (±SE); (c) estimated richness in the Detroit River based on the jackknife estimator (±SE); (d) observed genus richness of larval fish in the St. Clair River; (e) estimated richness in the St. Clair River based on the Chao 1 estimator; and (f) estimated richness in the St. Clair River based on the jackknife estimator. Four sampling scenarios were examined.
In general, richness estimators predicted 1–4 genera greater than observed richness for all sampling scenarios (Figure 3). Genus richness estimates in the limited spatial sampling, limited temporal sampling, and limited spatial and temporal sampling scenarios were typically less than estimates derived from the full data set. However, estimates from the limited temporal sampling scenario were most similar to those derived from the full data sets. The first-order jackknife estimator typically produced the highest richness estimate, but both the Chao 1 and jackknife estimators were typically within 1 SE of each other (Figure 3). The jackknife estimator produced more precise estimates of richness than did the Chao 1 estimator (Figure 3). For the Detroit River, observed and estimated richness values were higher for 2007 and 2013 than for the other study years. Similarly, for the St. Clair River, 2013 had higher observed and estimated richness than the other sampling years, indicating that similar processes control richness in both systems (the St. Clair River was not sampled in 2007). Using the first-order jackknife, estimated genus richness in the full data set was positively correlated with estimated richness from the limited spatial sampling (r = 0.68), limited temporal sampling (r = 0.68), and limited spatial and temporal sampling (r = 0.72) scenarios. Using the Chao 1 estimator, positive correlations also existed between estimated genus richness in the full data set and estimated richness from the limited spatial sampling (r = 0.62), limited temporal sampling (r = 0.41), and limited spatial and temporal sampling (r = 0.39) scenarios; however, these correlations were weaker than those associated with observed richness and estimated richness from the first-order jackknife.
Lithophilic-spawning genus richness and invasive genus richness performed similar to total genus richness among the four sampling scenarios. Lithophilic richness in the limited spatial sampling scenario was typically 0–1 genus less than that in the full data set, with the exception of the Detroit River in 2006 (difference = 3 genera; Figure 4). Similarly, observed lithophilic richness in the limited temporal sampling scenario was 0–1 genus less than that in the full data set except for the St. Clair River in 2012 (difference = 2 genera; Figure 4). However, observed lithophilic richness in the limited spatial and temporal scenario was less than lithophilic richness from the full data set in all but one instance (St. Clair River in 2013), and the difference was as great as 4 genera (Figure 4). Invasive genus richness in all of the limited sampling scenarios was 0–2 genera less than invasive richness in the full data set, with all three limited sampling scenarios performing similarly (Figure 4). Based on Pearson's product-moment correlation coefficients across systems and years, the observed lithophilic genus richness in the full data set was positively correlated with lithophilic richness in the limited spatial sampling (r = 0.65), limited temporal sampling (r = 0.89), and limited spatial and temporal sampling (r = 0.36) scenarios. Observed invasive genus richness in the full data set was also positively correlated with invasive richness in the limited spatial sampling (r = 0.49), limited temporal sampling (r = 0.49), and limited spatial and temporal sampling (r = 0.64) scenarios.
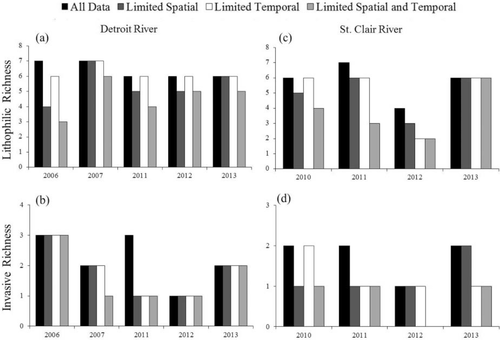
Observed (a) lithophilic genus richness of larval fish in the Detroit River; (b) invasive genus richness of larval fish in the Detroit River; (c) lithophilic genus richness in the St. Clair River; and (d) invasive genus richness in the St. Clair River. Four sampling scenarios were examined.
Pairwise values of the Sørensen index of similarity between sampling scenarios were typically greater than 0.80, indicating that community composition based on genus presence/absence was highly similar among sampling scenarios (Table 3). The Bray–Curtis index of similarity, which accounts for differences in abundance, took on pairwise values that were lower than Sørensen index values, with the greatest disparities occurring between the full data set and the scenario of limited spatial and temporal sampling (Table 3). Thus, community composition remains highly similar among sampling scenarios, but limiting the sampling effort may change the observed relative abundance of genera within the community.
| River | Year | F vs. S | F vs. T | F vs. ST | S vs. T | S vs. ST | T vs. ST | Mean |
|---|---|---|---|---|---|---|---|---|
| Sørensen index of similarity | ||||||||
| Detroit | 2006 | 0.86 | 0.95 | 0.79 | 0.79 | 0.93 | 0.84 | 0.86 |
| 2007 | 0.90 | 1.00 | 0.88 | 0.90 | 0.97 | 0.88 | 0.92 | |
| 2011 | 0.86 | 0.89 | 0.82 | 0.97 | 0.97 | 0.93 | 0.91 | |
| 2012 | 0.90 | 0.90 | 0.83 | 0.86 | 0.92 | 0.92 | 0.89 | |
| 2013 | 0.92 | 0.95 | 0.92 | 0.97 | 1.00 | 0.97 | 0.96 | |
| St. Clair | 2010 | 0.91 | 0.91 | 0.76 | 0.87 | 0.85 | 0.85 | 0.86 |
| 2011 | 0.95 | 0.86 | 0.76 | 0.80 | 0.81 | 0.90 | 0.85 | |
| 2012 | 0.92 | 0.87 | 0.87 | 0.95 | 0.95 | 1.00 | 0.93 | |
| 2013 | 0.86 | 0.97 | 0.82 | 0.82 | 0.97 | 0.85 | 0.88 | |
| Mean | 0.90 | 0.92 | 0.83 | 0.88 | 0.93 | 0.90 | 0.89 | |
| Bray–Curtis index of similarity | ||||||||
| Detroit | 2006 | 0.65 | 0.70 | 0.39 | 0.82 | 0.66 | 0.62 | 0.64 |
| 2007 | 0.67 | 0.75 | 0.38 | 0.81 | 0.55 | 0.55 | 0.62 | |
| 2011 | 0.87 | 0.65 | 0.49 | 0.76 | 0.62 | 0.78 | 0.69 | |
| 2012 | 0.84 | 0.54 | 0.51 | 0.60 | 0.56 | 0.84 | 0.65 | |
| 2013 | 0.80 | 0.67 | 0.84 | 0.53 | 0.70 | 0.77 | 0.72 | |
| St. Clair | 2010 | 0.58 | 0.77 | 0.46 | 0.59 | 0.70 | 0.64 | 0.62 |
| 2011 | 0.70 | 0.75 | 0.53 | 0.60 | 0.75 | 0.48 | 0.63 | |
| 2012 | 0.91 | 0.67 | 0.60 | 0.70 | 0.63 | 0.92 | 0.74 | |
| 2013 | 0.57 | 0.70 | 0.44 | 0.51 | 0.74 | 0.43 | 0.56 | |
| Mean | 0.73 | 0.69 | 0.52 | 0.66 | 0.66 | 0.67 | 0.65 | |
Rank abundance curves showed that community composition was highly similar among sampling scenarios for the Detroit River, as little deviation in relative abundance or rank was observed among the scenarios (Figure 5). However, for the St. Clair River, community composition based on the limited spatial sampling scenario and the limited spatial and temporal sampling scenario differed from the community composition based on the full data set or the limited temporal sampling scenario (Figure 5). When spatial sampling was limited, some genera (e.g., Catostomus, Moxostoma, and Perca) were underrepresented in relative abundance, whereas others (e.g., Labidesthes, Notropis, and Percopsis) were overrepresented (Figure 5).
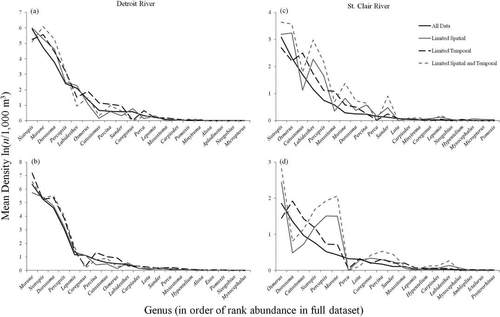
Rank abundance curves for (a) the Detroit River in 2011; (b) the Detroit River in 2013; (c) the St. Clair River in 2011; and (d) the St. Clair River in 2013. Four sampling scenarios were examined. Genera are ranked in order of abundance in the full data set.
DISCUSSION
Aquatic biodiversity is important for sustaining ecosystem function and economically important fisheries (Dudgeon et al. 2006; Worm et al. 2006). Habitat restoration in the SCDRS has been aimed at increasing the production and diversity of native fishes (Hondorp et al. 2014; SCDRSI 2014). Long-term monitoring of fish spawning activity in the SCDRS is crucial to evaluating the success of management practices and informing future restoration efforts through an adaptive management process (SCDRSI 2014). However, larval fishes are transient, are difficult to detect, and have abundances that are dynamic through space and time; sampling must be adequate to capture spatial and temporal variability (Cyr et al. 1992; DuFour et al. 2014; Pritt et al. 2014). We sought to use an existing larval fish data set to aid in the development of an efficient long-term monitoring strategy given limiting sampling resources.
The larval fish communities in the Detroit and St. Clair rivers were highly structured in space and time, and we used this information to develop sampling effort scenarios. In general, the Detroit River larval fish community had higher amounts of spatial nestedness than the St. Clair River community (i.e., Detroit River genera were aggregated at the river mouth). As a result, sampling only the lower river and mouth segments in the Detroit River produced richness estimates that were similar to those of the full data set, whereas the limited spatial sampling scenarios for the St. Clair River produced genus estimates that were considerably (2–6 genera) lower than that of the full data set. Similarly, rank abundance curves of genera were more similar among the four sampling scenarios for the Detroit River than for the St. Clair River. Therefore, the degree of nestedness appears to be positively related to the ability to reduce sampling effort while still observing a high proportion of the total richness and obtaining an accurate representation of community composition. Researchers could consider exhaustive preliminary sampling to guide long-term monitoring designs. In this case, we used two exhaustive sampling years to create sampling scenarios for the remaining years. This approach could be easily applied to set up efficient long-term monitoring efforts.
In both the St. Clair River and the Detroit River, reducing the sampling effort by sampling only in the late spring produced genus richness estimates that were nearest those from the full data sets; limiting the spatial sampling decreased richness estimates more substantially. However, estimates derived from all of the sampling scenarios were highly correlated, indicating that reduced sampling effort protocols may be useful to track changes in larval fish richness through time. In general, larval genera remained present in each system for many weeks. Thus, in the SCDRS, spatial variability in genus occurrences appears to have a greater influence on richness estimates than temporal variability.
Rank abundance curves and similarity indices also showed that community composition was generally similar among sampling scenarios, but the amount of spatial and temporal nestedness may influence the effect of reducing sampling effort on relative abundances. For example, rank abundance curves were highly similar among sampling scenarios for the Detroit River; thus, reducing sampling effort did not have a strong influence on relative abundance of genera in the Detroit River. However, similar to genus richness, limiting the spatial sampling in the St. Clair River produced rank abundance curves that deviated from those generated by the full data set. As a result, characterization of relative abundance in the St. Clair River may be biased when based on a limited sampling effort scenario. Researchers must carefully evaluate the tradeoffs between sampling effort and the information needed to achieve monitoring objectives (Lindenmayer and Likens 2009).
Richness estimators such as Chao 1 and the first-order jackknife may be useful for determining richness when sampling effort is not exhaustive (Gotelli and Colwell 2010). Chao 1 and the first-order jackknife are nonparametric estimators that have been shown to perform well for estimating richness in a variety of communities (Walther and Moore 2005). In this study, the richness estimates produced by the Chao 1 and jackknife techniques were nearer to estimates produced from the full data sets than the differences in observed richness between the full data sets and the reduced-effort scenarios. In general, the Chao 1 and jackknife estimators were within 1 SE of each other, and they were within 2 SEs (approximate 95% confidence intervals) in every instance. The first-order jackknife gave more precise estimates of richness and therefore may be preferable to the Chao 1 technique for estimating genus richness in larval fish data sets.
Lithophilic-spawning genus richness and invasive genus richness from the reduced-sampling scenarios were positively correlated with observed values from the full data set. Similar to total observed genus richness, lithophilic richness from the limited temporal sampling scenario was nearest that of the full data set. Thus, limiting temporal sampling only may provide the most information on sensitive lithophilic-spawning taxa. By contrast, the limited temporal sampling and limited spatial sampling scenarios performed similarly to the full data sets in terms of observed invasive genus richness. It is important to note that out of nine total instances (five study years in the Detroit River; four study years in the St. Clair River), at least one invasive genus was missed by reducing the sampling effort: three times by reducing spatial sampling, three times by reducing temporal sampling, and six times by reducing both spatial and temporal sampling. Introduced species are often rare during the early stages of invasion (Jerde et al. 2011), which may increase the sampling effort required for detection (Pritt et al. 2014). If early detection of invasive taxa is a goal for long-term monitoring (e.g., Vander-Zanden et al. 2010), exhaustive sampling effort may be necessary. Conversely, a reduced-effort larval fish sampling protocol could be conducted in concert with other invasive detection methods (e.g., sampling environmental DNA; Jerde et al. 2011) to improve detectability, validate environmental DNA samples, and provide physical information (i.e., density, size, life stage, etc.) on organisms present that cannot be obtained through environmental DNA sampling alone.
This study is limited by the lack of species-level identifications. Larval fish are notoriously difficult to identify based on morphological characters alone, and the identification of large numbers of individuals (>100,000) through genetic techniques is cost prohibitive. As a result, valuable ecological information may be lost due to coarse taxonomic resolution. Specifically, in this study, genera in the family Cyprinidae could not be distinguished and were analyzed as only one genus. Cyprinidae is a diverse family in the Great Lakes (Scott and Crossman 1973), and the loss of genus-level identification may limit the usefulness of our methods for assessing trends in biodiversity through time. In addition, coarse taxonomic resolution may have limited our ability to detect invasive species. However, in organisms such as macroinvertebrates, genus-, family-, or order-level identifications are routinely used to assess habitat quality (e.g., Lammert and Allan 1999; Mapes et al. 2015). The benefit of species-level identification for habitat assessment must therefore be weighed against the cost of obtaining such information.
Even though larval fish sampling was intense on a riverwide scale, the river segments were not all sampled equally, which may have contributed to inconsistencies between rivers. Additionally, the St. Clair River forms the largest Great Lakes delta that may be concentrating and retaining larval fish instead of allowing the fish to continue drifting downstream to the river mouth area and Lake St. Clair (a natural lake between the delta of the St. Clair River and the head of the Detroit River). Consideration of physical habitat and hydrology specific to the study system may be necessary when designing a larval fish sampling program.
Long-term monitoring is important for determining temporal trends in relation to environmental changes (Lindenmayer and Likens 2009; Magurran et al. 2010) and management activities (Whiteway et al. 2010). Improving spawning habitat is often the goal of fish restoration efforts (e.g., Manny et al. 2010), and larval fishes provide a useful indicator of the success and extent of fish spawning (Roseman et al. 2012; Mapes et al. 2015). However, intensive sampling of larval fish is costly and may therefore be difficult to sustain over long time periods. We have demonstrated that understanding the spatial and temporal structure of larval fish communities can be used to reduce sampling effort and overcome this problem. Similar methodologies could be used by researchers designing long-term monitoring programs for highly structured communities to reduce the high cost inherent in long-term monitoring.
ACKNOWLEDGMENTS
This work would not have been possible without the support of Nick Arend, David Bennion, Emily Bouckaert, Dustin Bowser, Nelson Cordero, Jaquie Craig, Ellen George, Darryl Hondorp, Robert Hunter, Stacey Ireland, Christina Jovanovic, Greg Kennedy, Kelsey Lincoln, Maureen Lynch, Bruce Manny, Darrin McCullough, Erik McDonald, Matthew McLean, Tim O'Brien, Stacy Provo, Lismar Rodriguez, Jenny Sutherland, and Patricia Thompson. This work was supported by the Great Lakes Restoration Initiative Project Number 70 (Fish Habitat Enhancement Strategies for the Huron-Erie Corridor). This is Contribution Number 1895 of the U.S. Geological Survey, Great Lakes Science Center. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.




