Origin of Atlantic Sturgeon Collected off the Delaware Coast during Spring Months
Abstract
Atlantic Sturgeon Acipenser oxyrinchus oxyrinchus was federally listed under the U.S. Endangered Species Act as five distinct population segments (DPS). Currently, at least 18 estuaries coastwide host spawning populations and the viability of these vary, requiring differing levels of protection. Subadults emigrate from their natal estuaries to marine waters where they are vulnerable to bycatch; one of the major threats to the rebuilding of populations. As a result, identifying the population origin of Atlantic Sturgeon in coastal waters is critical to development of management plans intended to minimize interactions of the most imperiled populations with damaging fisheries. We used mitochondrial DNA control region sequencing and microsatellite DNA analyses to determine the origin of 261 Atlantic Sturgeon collected off the Delaware coast during the spring months. Using individual-based assignment (IBA) testing and mixed stock analysis, we found that specimens originated from all nine of our reference populations and the five DPSs used in the listing determination. Using IBA, we found that the Hudson River population was the largest contributor (38.3%) to our coastal collection. The James (19.9%) and Delaware (13.8%) river populations, at one time thought to be extirpated or nearly so, were the next largest contributors. The three populations combined in the South Atlantic DPS contributed 21% of specimens; the Altamaha River, the largest population in the South Atlantic DPS, only contributed a single specimen to the collection. While the origin of specimens collected on the Delaware coast was most likely within rivers of the New York Bight DPS (52.1%), specimens that originated elsewhere were also well represented. Genetic analyses provide a robust tool to identify the population origin of individual sturgeon outside of their natal estuaries and to determine the quantitative contributions of individual populations to coastal aggregations that are vulnerable to bycatch and other anthropogenic threats.
Received March 17, 2014; accepted September 3, 2014
In the late 19th century, Atlantic Sturgeon Acipenser oxyrinchus oxyrinchus supported large and lucrative in-river fisheries for spawning adults along much of the Atlantic coast of the USA (Atlantic Sturgeon Status Review Team 2007). The U.S. landings peaked at approximately 3,350 metric tons (mt) in 1890 (Waldman and Wirgin 1998). By far, the largest fishery occurred within the Delaware River (Secor and Waldman 1999) with peak landings at nearly 90% of the total U.S. landings (3,000 mt) in 1888 (Smith 1894) and to a lesser extent in tributaries of Chesapeake Bay (Smith 1985). By the early 20th century, these fisheries collapsed, and landings were less than 10% of their former levels. Beginning in the late 1970s the fishery shifted southward, with North Carolina, South Carolina, and Georgia contributing approximately 80% of U.S. landings (Smith and Clugston 1997). The late 1980s to early 1990s witnessed the development of a targeted coastal fishery for subadults and adults in nearshore coastal waters of the New York Bight where annual landings in New Jersey and New York between 1990 and 1994 were up to 115 mt (Waldman et al. 1996). The emergence of this new targeted coastal fishery combined with the observation of continued depletion of spawning stocks led to the imposition of a 40-year coastwide moratorium on the harvest of Atlantic Sturgeon in the U.S. by the Atlantic States Marine Fisheries Commission in 1998. Continued concern over the failure of many spawning populations to rebuild over the next 14 years resulted in consideration of two petitions to federally list Atlantic Sturgeon and as five distinct population segments (DPS) under the U.S. Endangered Species Act (ESA) in 2012 (USOFR 2012a, 2012b). The Gulf of Maine (GOM) DPS was designated as threatened, while the New York Bight (NYB), Chesapeake Bay (CB), Carolinas (CAR), and South Atlantic (SA) DPS were listed as endangered. The final ruling identified several anthropogenic threats to the conservation and recovery of Atlantic Sturgeon populations, including vessel strikes, habitat modification, and compromised water quality, but the most prominent threat among them was the species vulnerability to bycatch in coastal waters and nonnatal estuaries (Stein et al. 2004; USOFR 2012a, 2012b).
Despite their listing, little is known about the abundances of Atlantic Sturgeon within the 18 (3 Canadian and 15 U.S.) riverine populations along the Atlantic coast still believed to support successful natural reproduction (USOFR 2010a, 2010b). Stock abundance estimates for adults in the U.S. exist only for the Hudson River, where the 1985–1986 plus 1995 annual mean was 863 mature individuals (596 males and 267 females; Kahnle et al. 2007), and for the Altamaha River enumerated at 324 in 2004 and 386 in 2005 (Peterson et al. 2008). The Hudson River mean preceded implementation of the 1998 moratorium. Much of this difficulty stems from the unusual life history characteristic of Atlantic Sturgeon that make obtaining accurate population estimates problematic. Even when within natal estuaries to spawn, Atlantic Sturgeon exit within weeks or several months to coastal waters, and in some natal estuaries they are absent for 2–20 years.
The historical size of the Delaware River population during the late 19th century height of its fishery was estimated to exceed 180,000 adults (Secor and Waldman 1999). Although, quantitative efforts to accurately census its current population size are lacking, it is accepted that fewer than 300 adults persist in its population (ASSRT 2007). Much of this decline, as experienced coastwide, was due to overharvest, but unique to the Delaware River environment was the presence of a severe oxygen block that persisted for decades in the Philadelphia area and probably precluded access of Atlantic Sturgeon and other anadromous fishes to historical spawning locales and may have compromised the successful development of young life stages. Despite improved water quality in recent decades, it is believed by many that dissolved oxygen levels remain insufficient to support successful development of early life stages of Atlantic Sturgeon in the Delaware River (Kahn and Fisher 2012). Furthermore, frequent vessel strikes of adult Atlantic Sturgeon have been invoked as an additional unique threat to the stability and rebuilding of the Delaware River population (Simpson and Fox 2009; Brown and Murphy 2010). Finally, the killing of moderate numbers of subadults and adults in intakes of an upper Delaware Bay power plant was recently discovered.
Atlantic Sturgeon are anadromous, extremely long-lived (up to 60 years), spawn at 1-to-5 year intervals, and emigrate from natal estuaries as subadults at ages 2 to 6 (Smith and Clugston 1997). These general life history characteristics vary among populations and between genders, individuals from southern populations (Smith et al. 1982; Schueller and Peterson 2011) spawning at younger ages than ones from northern populations (Scott and Crossman 1973; Dadswell 2006). Similarly, males are believed to spawn at younger ages than females in most populations (Van Eenennaam et al. 1996). It has long been held that Atlantic Sturgeon coastwide spawn exclusively in the spring months (ASSRT 2007), but recent evidence suggests that late summer to early fall spawning occurs in two tributaries of the Chesapeake Bay (Balazik et al. 2012; Hager et al. 2014) and probably in rivers in the SA DPS (D. Peterson, University of Georgia, personal communication).
As subadults, Atlantic sturgeon migrate seasonally usually (Dunton et al. 2010), although not always (Stein et al. 2004), into shallow coastal waters and to nonnatal estuaries (Waldman et al. 2013) until they return to spawn as adults at 5–25 years of age depending on gender and latitude. The migratory patterns of Atlantic Sturgeon in coastal waters are largely unknown although the duration of these movements is prolonged, geographically extensive (Ludwig et al. 2002; Dunton et al. 2010; Erickson et al. 2011), and may differ among populations. These coastal movements can extend anywhere from central Florida to the entrance of the Gulf of St. Lawrence (D. A. Fox and M. W. Breece, unpublished data), and even be trans-Atlantic to the Baltic Sea (Ludwig et al. 2002). Similarly, subadults from some spawning populations may seasonally enter natal and nonnatal estuaries during the warmer months for presumed foraging opportunities. It is also likely that the duration, extent, and range of movements of Atlantic Sturgeon from different populations in coastal waters and nonnatal estuaries may vary. Thus, efforts to census river-specific and coastwide abundance must account for these complex life history characteristics, which vary among spawning populations. To date, there are no accurate estimates of the number of individuals from individual populations or DPS in coastal waters where the life histories of Atlantic Sturgeon primarily occur.
In recent years, bycatch has elicited concern as a factor responsible for the failure of some Atlantic Sturgeon populations to stabilize and rebuild (Stein et al. 2004; USOFR 2012a, 2012b). Bycatch is known to occur across a variety of target fisheries within coastal waters and most southern estuaries (Shepherd et al. 2007). The greatest concern has been expressed for Atlantic Sturgeon bycatch in coastal waters because of its frequency of occurrence across many commercial fisheries, known mortality in some gear types (up to 20% in large-mesh sink gill nets), and likely impact on many populations, which may differ markedly in abundance and migratory patterns. For example, it has been suggested that a bycatch mortality rate in coastal fisheries of 4% may impede a relatively robust population, such as that in the Hudson River, from rebuilding and may be more devastating for smaller populations (Shepherd et al. 2007). Therefore, to predict the consequences of ocean bycatch to individual populations or DPS, it is imperative to determine the likelihood of their interactions with targeted fisheries. Despite this need, the population or DPS of origin of Atlantic Sturgeon in coastal fisheries bycatch is largely unknown.
Genetic analyses provide the opportunity to determine the population or DPS of origin of single specimens or aggregations of Atlantic Sturgeon in coastal bycatch within acceptable limits of accuracy. Previous genetic studies using microsatellite and mitochondrial DNA (mtDNA) markers and their combination have demonstrated that individuals and aggregations of Atlantic Sturgeon from most spawning populations and DPSs are distinguishable (Wirgin et al. 2000; King et al. 2001; Grunwald et al. 2008). Specifically, a combination of microsatellite and mtDNA genetic markers have been used to determine the origin of subadult and adult Atlantic Sturgeon from mixed aggregations in the inner Bay of Fundy (Wirgin et al. 2012), Long Island Sound, and its tributary Connecticut River (Waldman et al. 2013). Furthermore, using less sensitive mtDNA restriction fragment-length polymorphism (RFLP) markers and having fewer reference spawning populations, Waldman et al. (1996) determined that Atlantic Sturgeon collected in the early 1990s from the targeted nearshore fishery in the New York Bight were almost all of Hudson River origin. Finally, Dunton et al. (2012), using the mtDNA control-region sequencing haplotypes reported in Grunwald et al. (2008), reported that 70% of sturgeon that they collected in fishery-independent trawl surveys during the spring and autumn in the New York Bight were still of Hudson River origin but contributions from other populations were greater than reported in Waldman et al. (1996). Whether the decreased Hudson River contribution reported in Dunton et al. (2012) reflected rebuilding of other populations or differences in the mtDNA markers between the two studies used was not determined.
Our fishery-independent sampling targeted for migratory Atlantic Sturgeon indicated a seasonal presence of moderate numbers of adult and subadult Atlantic Sturgeon off the Delaware coast during the spring months (M. W. Breece and D. A. Fox, unpublished results). We collected in total 528 specimens during springs of 2009–2012. The population origin of these specimens had not been empirically determined, although preliminary acoustic tagging results suggested that this is a mixed aggregation of fish from several different spawning areas, spanning much of the U.S. Atlantic coast (Fox and Breece, unpublished data). Given the position of this mixed collection near the center of the species’ coastal distribution and our springtime sampling, we hypothesized that its composition should be diverse and reflective of many target fisheries in the New York Bight and beyond. Furthermore, it should provide a measure of the status of populations in the NYB, CB, and SA DPSs that may be partially rebuilt as a result of the imposition of the federal coastwide moratorium on Atlantic Sturgeon harvest.
METHODS
Sample collections.
Sampling for Atlantic Sturgeon took place between late March and mid May from 3 to 12 km from the coast in the vicinity of Bethany Beach, Delaware (Figure 1). Our sampling effort was focused on the Delaware coast to optimize the opportunity of encountering and acoustically tagging specimens of Delaware River origin, but it also offered the opportunity to interact with spawning adults migrating to estuaries in the NYB DPS and beyond. Gill nets and techniques utilized in sampling were similar to those developed in the Atlantic Sturgeon coastal intercept fishery prior to the 1998 moratorium. To target adult Atlantic Sturgeon, monofilament anchor gill nets consisted of 10 panels (each 91.0 × 3.65 m) strung together and fished as one long string of nets. Mesh sizes were 30-cm and 33-cm stretch mesh, twine size was 0.90–1.20 mm, and the hanging ratio was 0.5. Up to four strings of gill nets were fished daily during suitable conditions for 7–10 h/d and were tended every 2–3 h to reduce bycatch and minimize stress and potential mortality of Atlantic Sturgeon.
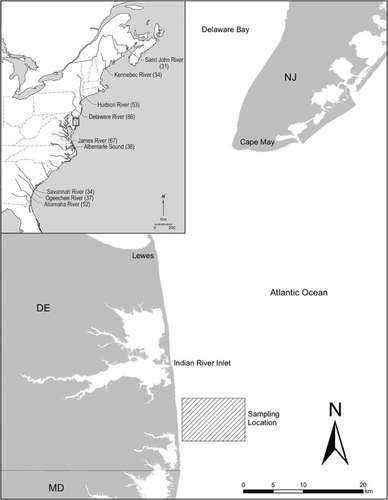
The Delaware coast depicting where 261 adult and subadult Atlantic Sturgeon specimens were collected during the spring months of 2009–2012. The inset includes the locales of the spawning locations from which our nine reference collections were made and sample sizes for those collections. For details on reference collections see Waldman et al. (2013).
In total, we determined the population and DPS origin of 261 Atlantic Sturgeon, the majority of which (95%) were > 130 cm TL and that were collected off the Delaware Coast between 2009 and 2012. Of these, 50 were collected in 2009 (April 5–26), 55 in 2010 (April 1–23), 81 in 2011 (April 2 to May 12), and 75 in 2012 (March 29 to April 30). Mean total length of the entire collection was 178.4 cm (SE = 1.8) and mean weight was 46.2 kg (SE = 1.3).
DNA isolations.
Total DNAs were isolated from fin clips that were stored at ambient temperature in 95% EtOH. Fin clips were initially washed with PBS buffer, incubated in CTAB buffer (Saghai-Maroof et al. 1984), digested with proteinase K at 65°C, and purified by standard phenol-chloroform extractions and alcohol precipitations. Concentrations and purities of DNA isolates were evaluated using a Nanodrop ND-1000 Spectophotometer (NanoDrop Technologies, Wilmington, Delaware). Sampled DNA concentrations were adjusted to 50 ng/μL for standardization of subsequent procedures.
Mitochondrial DNA control region sequence analysis.—Atlantic Sturgeon-specific primers S1 (5′-ACATTAAACTATTCTCTGGC-3′) and G1 (5′-GAATGATATACTGTTCTACC-3′; Ong et al. 1996) amplified an approximate 560-bp portion of the mitochondrial DNA (mtDNA) control region and were used to sequence a portion of it. We reported only 205-bp of the amplicon to allow for comparison of Delaware coast specimens of unknown origin to previously characterized reference populations (Wirgin et al. 2000; Grunwald et al. 2008).
Polymerase chain reactions (PCRs) were in 50-μL volumes containing 5 μL of 10× reaction buffer (Roche Applied Science, Indianapolis, Indiana), 0.25 μL of each dNTP (25 mM stocks; GE Healthcare, Piscataway, New Jersey), 0.07 μL of S1 primer, 0.05 μL of G1 primer (Integrated DNA Technologies, Coralville, Iowa), 50 ng of template DNA, 1 unit of Taq DNA Polymerase (Roche Applied Science), and 43.9 μL of H2O. Amplification conditions were 94°C for 5 min followed by 40 cycles at 94°C for 45 s, 56°C for 45 s, and 72°C for 60 s, followed by a final extension at 72°C for 10 min in MJ Research PTC-100 thermal cyclers (Waltham, Massachusetts). The PCR amplicons were purified with QIAquick PCR Purification kits (Qiagen, Valencia, California).
Purified PCR products were Dye-Terminator Cycle Sequenced as recommended by the manufacturer (Beckman Coulter, Inc., Fullerton, California). Sequencing conditions were 30 cycles at 96°C for 20 s, 50°C for 20 s, and 60°C for 4 min. Sequencing products were EtOH precipitated as recommended by Beckman Coulter (except no EDTA was added), resuspended in 40 μL of Beckman Coulter CEQ Sample Loading Buffer, loaded into a Beckman Coulter CEQ 8000 automated capillary-based DNA sequencer, run using the standard long fast-read method (LFR-1), and analyzed with the Sequence Analysis Module of the CEQ 8000 Genetic Analysis System.
Microsatellite analysis.
Eleven informative microsatellite loci were scored in specimens collected off the Delaware coast. These diploid markers included LS19, LS39, LS54, and LS68 (May et al. 1997); Aox23 and AoxD45 (King et al. 2001); and Aox44, AoxD165, AoxD170, AoxD188, and AoxD24 (Henderson-Arzapalo and King 2002). These loci were selected because they could be reliably scored, are unlinked, and in previous studies were effective in distinguishing reference specimens from spawning populations (King et al. 2001; Wirgin et al. 2012; Waldman et al. 2013).
Characterization of microsatellite genotypes was performed using the CEQ 8000 sequencer. The PCR reactions were multi-pooled and were diluted up to 1:3 with Sample Loading Solution (Beckman Coulter). Diluted PCR reactions (0.5–2 μL) were loaded onto 96-well plates along with 0.5 μL of CEQ DNA Size Standard-400 and 40 μL of Sample Loading Solution (Beckman Coulter) and run with the FRAG 1 program (Beckman Coulter). Micro-Checker software (Oosterhout et al. 2004) was used to test for the presence of null alleles, errors due to microsatellite stuttering and large-allele dropout.
Statistical analysis.
Reference population data used in this study were from Waldman et al. (2013; Figure 2). Reference data were obtained from spawning adults (≥130 cm TL) or river-resident juveniles (≤50 cm TL) collected from nine spawning rivers out of a hypothesized 18 such rivers and all five DPSs. Other populations were deemed too small to collect a sufficient number of specimens of the requisite length. Only specimens of these lengths were included in reference collections to help ensure that reference specimens were natal to the rivers in which they were collected. The baseline dataset consisted of multilocus genotypes for 427 specimens genotyped at 11 microsatellites and mtDNA control region sequence haplotypes coded as a homozygous diploid locus (e.g., haplotype A, 001001). Empirical analyses performed to estimate the accuracy of identification of individuals to natal origin indicated mean accuracy to individual population was 85% and to DPS was 97% (Waldman et al. 2013; Figure 2).
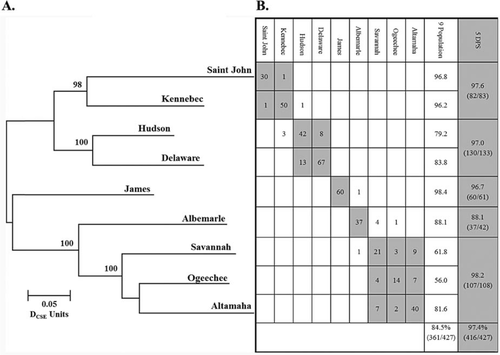
(A) Neighbor-joining tree depicting evolutionary relationships (as the Cavalli–Sforza and Edwards chord distances) among nine Atlantic Sturgeon reference spawning populations that were surveyed at 11 microsatellite loci. Numbers represent bootstrap support at each branch with 5,000 replicates of resampling across loci. (B) Results of assignment testing of the baseline individuals from the nine reference spawning populations using 11 microsatellite loci and mitochondrial DNA control region haplotypes (scored as a homozygous locus) to determine the likelihood of each individual's multilocus genotype–haplotype being found in the reference collection and distinct population segment (DPS) from which it was collected (without replacement) using the program GeneClass2. The numbers in the shaded cells represent the total number of individual specimens from the populations in that DPS correctly assigned to DPS.
Individual-based assignment (IBA) tests and mixed stock analysis were used to estimate the stock origin of Atlantic Sturgeon in our Delaware coast collection. Individual-based assignment tests, using multilocus likelihood functions (Paetkau et al. 1995) were used to determine the likelihood of each individual's genotype being found in the collection from which it was sampled (without replacement) using the program GeneClass2 (Piry et al. 2004). The analysis determines the likelihood ratio assuming the gene pool is in Hardy–Weinberg equilibrium with frequencies as specified in the collection. Individual assignments were based on the most likely population (highest relative likelihood score). Note that our analysis of a combination of diploid and haploid mtDNA data violates an assumption of this Monte Carlo resampling method.
Mixed stock analysis (MSA) was also used to estimate the proportion of each baseline population in the mixed samples of unknown origin off the Delaware coast and to determine the probability of an individual belonging to each of the baseline DPS and spawning rivers. In contrast to IBA, MSA applies mixture modeling, which takes into account the genotypes of individual fish across multiple loci, the multilocus genotype distributions of the baseline samples, and the multilocus genotype distribution in the mixture sample. We used ONCOR (Kalinowski 2008) and the same reference data set used for the IBA to perform the MSA. We estimated mixture proportions with 95% confidence limits based on 10,000 bootstraps. Results were reported for each population in the baseline as well as for each DPS. Simulation analysis (100%) was used in ONCOR with reference sample sizes to evaluate the probability of correct assignments in MSA with 95% confidence intervals. The data were subjected to 1,000 simulations, and 400 fish were selected for assignment.
Interannual temporal stability of genotypes across 4 years of collections off the Delaware coast (2009–2012) were evaluated using pairwise allelic differentiation tests in GENEPOP version 4.0.10 using default Markov chain parameters (dememorization number = 1,000; number of iterations per batch = 1,000; Rousset 2007). Allelic differentiation tests were also used to evaluate genetic similarity between specimens larger and smaller than the median total length of 188 cm. Only microsatellite data were used for these comparisons.
We used entropy analysis to compare the diversity of reference population origin of our Delaware coast collection to that we previously reported for collections from the Bay of Fundy (Wirgin et al. 2012) and Long Island Sound (Waldman et al. 2013). We hypothesized that population origin diversity would be greater for the Delaware coast collection than to collections previously analyzed. To do this, we measured variability of the categorical variable (i.e., identity of the reference river population origin) by the entropy of the observed relative frequency distribution of the variable in an SP-PLUS program that was written for us (A. Nadas, New York University School of Medicine). Entropy is the expected value of the base 2 logarithm of the reciprocal of the relative frequencies, and the resulting units of entropy are in bits. For this study, the variable can take on nine values identifying the population of origin and their relative frequencies based on the counts and on the sample sizes at their respective collection sites (Delaware coast = 261; Long Island Sound = 275; and Bay of Fundy = 181). Here the expected value at each site is the negative sum of the relative frequencies multiplied by their corresponding base 2 logarithms. Chi-square tests were then used to determine the significance (α = 0.05) of differences in the three distributions.
RESULTS
We used mtDNA control region sequencing and analyses at 11 informative microsatellite loci to quantify the population and DPS origin of 261 subadult and adult Atlantic Sturgeon collected off the Delaware coast during the spring months of 2009–2012. Using IBA testing, we found that the Hudson River was the predominant contributor to this coastal collection with 100 specimens (38.3%) assigned to the Hudson River (Figure 3). The James River was the second largest contributor with 52 specimens (19.9%) assigned ancestry there. Consistent with its severely depleted status, the Delaware River population contributed only a moderate number of specimens, 36 (13.8%), to this mixed coastal aggregation, despite the geographic proximity of our coastal collection site to the entrance of Delaware Bay and the fact that our sampling was initially designed to target adults from this system. Despite being roughly 1,000 km from our sampling site, two populations in the SA DPS also contributed moderate numbers of specimens to our coastal collection, the Savannah River at 17 (6.5%) and Ogeechee River at 24 (9.2%). Surprisingly, the Altamaha River population, believed to be the largest in the SA DPS and one of the largest coastwide, contributed only a single specimen to our collection. The Albemarle Sound-Roanoke River population, the only one genetically characterized from the CAR DPS contributed 14 individuals (5.3%) in our coastal collection. Contributions of populations within the GOM DPS made much smaller contributions to our Delaware coast collection, 4 (1.5%) specimens originating from the Saint John River and 14 (5.3%) from the Kennebec River. In total, all nine populations and five DPSs were represented in our mixed coastal collection, but populations in closest proximity to the Delaware coast were the greatest contributors (Hudson, James, Delaware (in rank order)).
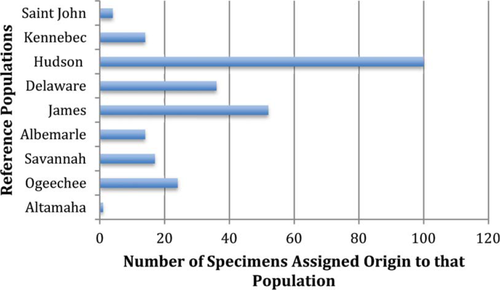
population assignment to nine reference populations determined using individual-based assignment (IBA) testing implemented in GeneClass2, as applied to 261 adult and subadult Atlantic Sturgeon collected off the Delaware coast during the spring months of 2009–2012.
Results from MSA were accurate (Table 1) and very similar to those for IBA testing, the Hudson River population being the largest contributor (44%) followed by the James River (20.6%), and Delaware River (10.6%; Table 2). On a DPS basis, the NY Bight contributed 54.6% of the samples collected along the Delaware coast followed by the CB DPS at 20.6%, SA DPS at 16.3%, GOM DPS at 4.6%, and CAR DPS at 4.1%.
| Population | Average | SD | 95% confidence limits |
|---|---|---|---|
| Saint John | 0.9873 | 0.0024 | 0.9822, 0.9915 |
| Kennebec | 0.9953 | 0.0015 | 0.9923, 0.9982 |
| Hudson | 0.9899 | 0.0027 | 0.9845, 0.9948 |
| Delaware | 0.9859 | 0.0027 | 0.9806, 0.9913 |
| James | 0.9999 | 0.0001 | 0.9996, 1.0000 |
| Albemarle | 0.9990 | 0.0007 | 0.9975, 1.0000 |
| Savannah | 0.9058 | 0.0056 | 0.8944, 0.9163 |
| Ogeechee | 0.8176 | 0.0072 | 0.8036, 0.8320 |
| Altamaha | 0.9766 | 0.0034 | 0.9698, 0.9829 |
| Origin | Estimate (%) and (95% confidence limits) | Correctly assigned (%) | Largest misassigned DPS or population (%) |
|---|---|---|---|
| DPS | |||
| Gulf of Maine (GOM) DPS | 4.6 (2, 9.4) | 94.8 | NYB (3.9) |
| New York Bight (NYB) DPS | 54.6 (46.9, 60.7) | 94.6 | GOM (4.6) |
| Chesapeake Bay (CB) DPS | 20.6 (15.8, 26.4) | 93.8 | GOM (2.1) |
| Carolina (CA) DPS | 4.1 (1.5, 7.8) | 87.2 | SA (12.8) |
| South Atlantic (SA) DPS | 16.3 (10.4, 20.6) | 98.0 | CA (2.0) |
| Population (DPS) | |||
| Saint John River (GOM) | 0.4 (0, 1.9) | 96.3 | Kennebec (3.7) |
| Kennebec River (GOM) | 4.1 (1.5, 8.9) | 94.0 | Hudson (6.0) |
| Hudson River (NYB) | 44 (33.1, 49.9) | 84.6 | Delaware (11.5) |
| Delaware River (NYB) | 10.6 (6.4, 18.7) | 76.9 | Hudson (23.1) |
| James River (CB) | 20.6 (15.8, 26.4) | 91.7 | Kennebec (4.2) |
| Albemarle Sound (CA) | 4.1 (1.5, 7.8) | 89.7 | Savannah (7.7) |
| Savannah River (SA) | 6.9 (2.4, 11.4) | 60.6 | Altamaha (27.3) |
| Ogeechee River (SA) | 8.8 (4.2, 13.3) | 52.6 | Altamaha (36.8) |
| Altamaha River (SA) | 0.4 (0, 1.7) | 89.6 | Savannah (8.3) |
We quantitatively compared the diversity of source reference populations to the Delaware coast collection to that in collections that we previously made from the Bay of Fundy (Wirgin et al. 2012) and Long Island Sound (Waldman et al. 2013). As expected, we found that variation of the number of reference populations contributing to the Delaware coast collection expressed as bits was significantly greater (P < 0.001) than for the Bay of Fundy or Long Island Sound collections (Delaware coast = 2.53 bits, Long Island Sound = 1.83 bits, and Bay of Fundy = 1.14 bits).
We also compared microsatellite allelic frequencies among the four collection years using Fisher's exact test and found no evidence of genetic heterogeneity (χ2 = 16.5, P = 0.792). Because of the possibility that contingents of fish of different sizes may be from different populations, we also compared microsatellite frequencies between specimens >188 cm TL (median) and those <188 cm and found no difference between the two sized samples (χ2 = 25.1, P = 0.293).
DISCUSSION
Although federally listed under the U.S. Endangered Species Act as endangered and threatened, depending on the DPS, little is known of the migratory behavior of Atlantic Sturgeon outside of their natal estuaries. Knowledge of the population and age-specific migratory patterns of subadult and adult Atlantic Sturgeon is critical because most of their life history occurs outside of natal estuaries, the vast majority of time being spent in coastal waters where their vulnerability to bycatch is great. We found that the Hudson River was the major contributor to our fishery-independent sampling of Atlantic Sturgeon off the Delaware coast with a 4-year mean of 38.3% using IBA testing, and 44% using MSA. The James River at 19.9% and Delaware River at 13.8% were the second largest contributors in IBA testing and likewise with MSA at 20.6% and 10.6%, respectively. Also, we found no statistical difference in allelic frequencies among the four collection years, suggesting that contingents of specimens with similar population origins migrate annually off the Delaware coast. In total, we determined that all nine of our reference populations contributed to our Delaware coast spring collections, including those at the extremes of our analyses in the Gulf of Maine and the South Atlantic.
We proceeded to compare contribution determinations for our Delaware coast collection to those reported previously using the same genetic markers on collections from the Bay of Fundy (Wirgin et al. 2012), Long Island Sound, and its tributary, the Connecticut River (Waldman et al. 2013). Not unexpectedly, the predominant contributors to the Bay of Fundy collection were the proximal Saint John River (>60%) and the more distant Kennebec River (34–36%) in the GOM DPS; there were only possible minor (1–2%) contributions from the James and Hudson rivers. In contrast, the Saint John and Kennebec rivers made significantly smaller contributions to our Delaware coast collection. We found that our Long Island Sound–Connecticut collections were predominated by migrants from the proximal Hudson River (64–76%) but also included moderate numbers of specimens from the Delaware (7.6–12%) and James rivers (6–7.3%). There is a growing body of evidence that migratory Atlantic Sturgeon tend to remain within the geographic province of the river in which they were spawned, as we reported for the Bay of Fundy and Long Island Sound collections; however, this boundary is variable and penetrable by representatives from more distant populations (Wirgin 2012; Wirgin et al. 2012; Waldman et al. 2013). The population origin of specimens in our Delaware coast collection was significantly more diverse than those from other locales, as revealed by our entropy analysis. This probably results from the Delaware coast being more central to the overall coastal distribution of Atlantic sturgeon, our collection occurring during the spring months when adults are migrating to their spawning rivers, and the larger mean size of these specimens. Our results support the hypothesis that sturgeon from many populations mix in coastal aggregations and that the migratory patterns of populations differ spatially along the coast.
The selection and timing of our sampling program was predicated on our desire to intercept adult Atlantic Sturgeon of Delaware River origin. Although our target population collections were comparatively low, the central position of our sampling within the species’ coastwide distribution, and the likelihood that adults from many populations pass during their seasonal coastal movements made it ideally situated to collect from a wide range of populations. Our genetic results indicated that we sampled specimens from a variety of reference populations, and given their large size (median TL = 188 cm), it is likely that some were destined to enter spawning estuaries shortly after collection or in subsequent years. Furthermore, we found that the diversity of source reference populations contributing to the Delaware coast collection was significantly greater than that determined for collections previously made in the Bay of Fundy (Wirgin et al. 2012) and Long Island Sound (Waldman et al. 2013). These results demonstrate that the Delaware coast seasonally hosts aggregations of adult and subadult Atlantic Sturgeon of diverse origins.
The actual accuracy of our individual-based assignments will be empirically addressed by results of a companion acoustic telemetry study on a subset of these specimens and additional ones not characterized here. By combining acoustic telemetry with receivers monitored within spawning rivers at spawning time and genetic analysis on the same specimens we will be able to empirically evaluate the accuracy of our results in assigning ancestry to the individual adult specimens characterized in this study.
There are several possible deficiencies in our reference collection data set that should be considered. First, we only used data from nine reference collections, rather than the 18 spawning populations believed to persist coastwide. Therefore, the contributions of other uncharacterized populations that may contribute to our coastal collection were ignored and their omission may have inflated the contribution estimates of the reference populations that were included. However, most of the other extant populations are small, and their contributions are likely minor. Also, the strong pattern of isolation by distance in Atlantic Sturgeon that we report in our neighbor joining tree (Figure 2) suggests that most, if not all, putative spawning populations not represented in our baseline will be genetically similar to the adjacent populations included and would fall within the same DPS. Second, we have not empirically evaluated the interannual temporal stability of diagnostic genotypes and haplotypes in our reference collections. This may be particularly pronounced for reference collections from the CAR DPS and SA DPS, which were predominated by age-0–2 specimens. Temporal instability of genotypes and haplotypes among year-classes may be a particular problem for very small populations of Atlantic Sturgeon in which year-class production may be dependent on a limited number of parents. Clearly, more exhaustive spatial and temporal characterization of reference populations is needed. Finally, our assignment results, while accurate for large adult and subadult specimens collected off the Delaware coast during the spring months, may not be accurate for smaller specimens collected during the same period or even adult specimens at this locale during other times of the year. It is likely that assignments results will differ seasonally and among different size-classes of specimens.
Our results support the likelihood of persistent successful natural reproduction and recruitment at very low levels over recent decades in the Delaware River population. However, they also indicate that its abundance remains extremely low compared with historical levels and that it has not rebounded to the extent of other populations. For example, we identified 38 (10.6–13.8%) specimens of Delaware River origin in our coastal sample, despite the location of our collection site just south of the mouth of Delaware Bay. The continued depressed size of the Delaware River population may be due to the chronic presence of compromised water quality (Kahn and Fisher 2012) and chemical stressors at the Delaware River nursery grounds and a disproportionate impact from vessel strikes compared with other systems (Simpson and Fox 2009; Fisher 2011). The low adult abundance, very sporadic successful natural reproduction, and presence of damaging environmental stressors may suggest that the Delaware River population deserves protection above that of neighboring populations.
In summary, genetic analysis provides a cost-effective and accurate method to determine the population origin of Atlantic Sturgeon collected in coastal waters. We found that the population-origin profile of specimens collected off the Delaware coast was temporally stable over 4 years of collections and, not unexpectedly, differed significantly from those that we previously reported for the Bay of Fundy and Long Island Sound–Connecticut River using the same genetic markers. However, unlike the Bay of Fundy and Long Island Sound–Connecticut River studies, the majority of the specimens collected off the Delaware coast were not from the closest known spawning river, the Delaware River. This is probably due to the larger median size of the specimens in the current study (188 cm TL), collection during the spring months when fish from many populations are on migratory runs, and the central position of our Delaware coast collection site along the coastwide migratory route of Atlantic sturgeon. Finally, we suggest that quantitative analysis of the population origin of specimens collected in coastal aggregations, such as the Delaware coast, may provide a means to determine trends in the relative abundances of individual populations.
ACKNOWLEDGMENTS
We thank New York Sea Grant (Grant R/SG-20) and the Molecular Facility Core of the NYU National Institute of Environmental Health Sciences Center ES00260 for their support. Funding for our coastal sampling program was provided by the National Marine Fisheries Service's Anadromous Fish Conservation Act (NOAA Award NA08NMF4050611, NOAA-NMFS-NERO contract EA133F-08-CN-0151) and Species Recovery Grants to States (NOAA Award NA10NMF4720030) project. We acknowledge the statistical assistance of Arthur Nadas. We thank Lori Brown for her assistance with database management and sampling handling, as well as Michael Lohr for his assistance during field collections. Atlantic Sturgeon sampling following their ESA listing was carried out under the auspices of National Marine Fisheries Service research permit 16507. Prior to listing Atlantic Sturgeon were sampled under annual state of Delaware collection permits. The use of trade names does not imply endorsement by the U.S. Government.




