Spatially Dependent Responses of a Large-River Fish Assemblage to Bank Stabilization and Side Channels
Abstract
The alteration of rivers by anthropogenic bank stabilization to prevent the erosion of economically valuable lands and structures has become commonplace. However, such alteration has ambiguous consequences for fish assemblages, especially in large rivers. Because most large, temperate rivers have impoundments, it can be difficult to separate the influences of bank stabilization structures from those of main-stem impoundments, especially because both stabilization structures and impoundments can cause side-channel loss. Few large rivers are free flowing and retain extensive side channels, but the Yellowstone River (our study area) is one such river. We hypothesized that in this river (1) bank stabilization has changed fish assemblage structure by altering habitats, (2) side-channel availability has influenced fish assemblage structure by providing habitat heterogeneity, and (3) the influences of bank stabilization and side channels on fish assemblages were spatially scale dependent. We developed a spatially explicit framework to test these hypotheses. Fish assemblage structure varied with the extent of bank stabilization and the availability of side channels; however, not all assemblage subsets were influenced. Nevertheless, bank stabilization and side channels had different and sometimes opposite influences on the fish assemblage. The effects of side channels on fish were more consistent and widespread than those of bank stabilization; the catches of more fishes were positively correlated with side-channel availability than with the extent of bank stabilization. The influences of bank stabilization and side channels on the relative abundances of fish also varied, depending on species and river bend geomorphology. The variation in river morphology probably contributed to the assemblage differences between stabilized and reference river bends; stabilized alluvial pools were deeper than reference alluvial pools, but the depths of stabilized and reference bluff pools did not differ. The strengths of the relationships among fish assemblages, bank stabilization, and side channels were spatially scale dependent; optimum spatial scales ranged from less than 200 m to 3,200 m up- and downstream, suggesting that bank stabilization and side channels influenced fish assemblages across multiple spatial scales.
Received October 7, 2016; accepted January 23, 2017 Published online July 31, 2017
The alteration of large rivers by anthropogenic bank stabilization structures has uncertain consequences for fish assemblages. Bank stabilization can cause changes in main-channel bathymetry, such as main-channel bed degradation, channel width reduction, and increased stream gradient (Stern et al. 1980; Heede 1986; Shields et al. 1995). Moreover, bank stabilization can reduce floodplain connectivity, lateral channel migration, and the formation of backwaters, braids, and side channels (Leopold et al. 1964; Stern et al. 1980; Florsheim et al. 2008).
Bank stabilization alters fish habitat and probably habitat suitability, albeit in ambiguous ways. Bank stabilization has been associated with decreases in fish abundances in some rivers (e.g., Knudsen and Dilley 1987; Peters et al. 1998; Oscoz et al. 2005) but increases in others (e.g., Knudsen and Dilley 1987; Binns 1994; White et al. 2010) and has had no effect in still others (e.g., McClure 1991; Madejczyk et al. 1998). Similarly, fish species richness has been found to decrease (Oscoz et al. 2005), increase (White et al. 2010), or remain unchanged (Madejczyk et al. 1998) in stabilized reaches. Changes in fish assemblage structure (Madejczyk et al. 1998; Eros et al. 2008) and size-class distributions (Eros et al. 2008) have occurred in bank-stabilized reaches. Thus, bank stabilization has uncertain and possibly multifaceted consequences for fish assemblages.
The discrepancies in the findings of previous studies may result from differences in the rivers studied. In highly altered or naturally homogeneous rivers, bank stabilization may provide habitat diversity that is otherwise lacking (Schmetterling et al. 2001; Zale and Rider 2003) and cause localized increases in fish density and species richness. Conversely, in unaltered or relatively heterogeneous rivers, moderate amounts of bank stabilization may have little or no effect on fish assemblages. Moreover, with the exception of the studies by Zale and Rider (2003) and White et al. (2010), all studies of the effects of bank stabilization in large rivers have been conducted in regulated rivers (e.g., Garland et al. 2002; Eros et al. 2008; Schloesser et al. 2012), where the effects of bank stabilization may be confounded by or interact with the effects of dams.
Differences in study approaches may also underlie the differences in the results of previous research. For example, previous studies differed with regard to the fish taxa studied and the spatial scales at which effects were examined. Many previous studies were limited to a single family of fish (e.g., salmonids) or particular age-classes (e.g., juveniles). The emphasis on a subset of the assemblage may underlie the apparent inconsistencies in the conclusions because effects could remain undetected if some species and age-classes were not sampled (Zale and Rider 2003). Moreover, failing to account for spatial scale–dependence or side-channel availability may lead to differing conclusions.
Ecological theory (e.g., Junk et al. 1989) and empirical field studies (e.g., Copp 1997; Gurtin et al. 2003; Reinhold et al. 2016) suggest that side channels are crucial fish habitats because of the habitat heterogeneity they provide. Fish species richness has been positively associated with increased habitat heterogeneity in the upper Mississippi River (Ellis et al. 1979; Koel 2004). Lateral connectivity is also important; twice as many fish species were found in connected aquatic floodplain habitats than in disconnected habitats in the impounded lower Missouri River (Galat et al. 1998). However, extensive bank stabilization and altered hydrographs in the upper Mississippi and lower Missouri rivers confound the inferences that can be drawn from these studies because both bank stabilization and altered hydrographs reduce side-channel inundation both spatially and temporally. Therefore, the limited amount of remaining side-channel habitats may have concentrated fish.
The Yellowstone River (Figure 1) has co-localized side channels and reaches with and without bank stabilization (Figure 2) and—despite there being impoundments on two major tributaries—it lacks the confounding influence of main-stem impoundments (Koch et al. 1977), making it ideal for study of the effects of bank stabilization and side channels. We examined the influence of bank stabilization and side channels on the structure of the Yellowstone River fish assemblage from Laurel to Sidney, Montana, under late-summer and early-autumn base-flow conditions in 2009–2011. We tested the following hypotheses: (1) bank stabilization changed fish assemblage structure by altering habitats, (2) side-channel availability influenced fish assemblage structure by providing habitat heterogeneity, and (3) the influences of bank stabilization and side channels on fish assemblages were spatially scale dependent.
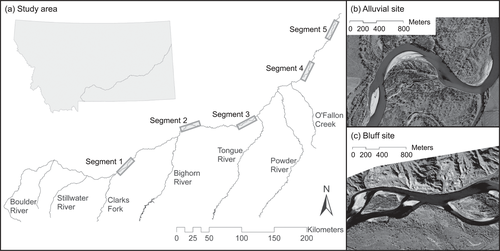
(a) Locations of the five study segments in the Yellowstone River, Montana, and (b)–(c) examples of alluvial and bluff sites.
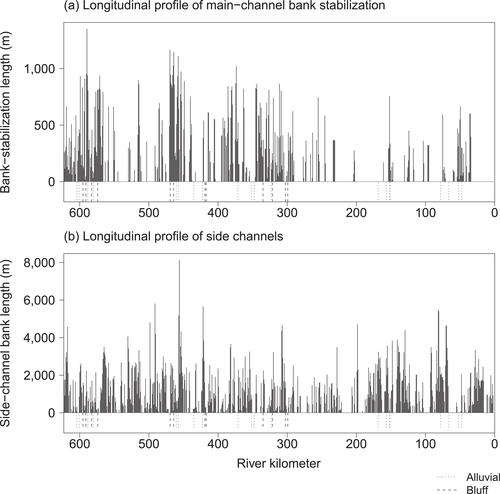
Longitudinal profiles of (a) main-channel bank-stabilization lengths and (b) side-channel bank lengths in the Yellowstone River study area. Alluvial and bluff sampling sites are indicated by dashed lines along the x-axes. Segment 1 (Billings) included the sites near rkm 600; segment 2 (Hysham) included the sites near rkm 440; segment 3 (Miles City) included the sites near rkm 340; segment 4 (Glendive) included the sites near rkm 160; and segment 5 (Sidney) included the sites near rkm 75.
Study Area
Our study area was the main-stem Yellowstone River between the confluence of Clarks Fork with the Yellowstone River and that of the Yellowstone River with the Missouri River. The Yellowstone River is an eighth-order stream in a 182,336-km2 basin (White and Bramblett 1993). It originates in the Rocky Mountains in northwestern Wyoming and flows generally northeast to its confluence with the Missouri River in western North Dakota (Figure 1). The spring freshet occurs in June as a result of snowmelt runoff; however, impoundments on the Bighorn and Tongue rivers and water withdrawals have reduced mean monthly flows by approximately 28% during June near the confluence of the Yellowstone River with the Missouri River (estimated from Chase 2013). Anthropogenic alterations to Yellowstone River fluvial geomorphology include six low-head irrigation dams, in-stream linear bank armoring, floodplain levees, dikes (Silverman and Tomlinsen 1984; Boyd and Thatcher 2004), and removal of riparian vegetation (Boyd and Thatcher 2004). In this article, the term “linear bank stabilization” refers to in-stream bank armoring structures consisting of rock and concrete riprap constructed longitudinally along river banks to prevent bank erosion.
Channel slope, substrate (Koch et al. 1977; Bramblett and White 2001), water temperature (White and Bramblett 1993), and water clarity (A. M. Reinhold, unpublished data) shift longitudinally in the study area. Concomitant with these abiotic trends, the fish assemblage structure (White and Bramblett 1993) exhibits strong longitudinal trends (Reinhold 2014; Duncan et al. 2016). Throughout the study area, cyprinids and catostomids are common. Common cyprinids shift from Longnose Dace in the upstream reaches to Western Silvery Minnows, Flathead Chub, Emerald Shiners, and Sand Shiners in the downstream reaches (see Table 1 for scientific names); Sturgeon Chub occur downstream of the confluence of the Powder River (Duncan et al. 2012; Reinhold 2014). Common catostomids include Mountain and Longnose suckers in the upstream study reaches and White Suckers and Shorthead Redhorses throughout the study area. Shovelnose Sturgeons are common downstream of the confluence with the Tongue River, and although rare, Pallid Sturgeons are present below the confluence with O'Fallon Creek. Native game fish (in order of capture frequency) include Channel Catfish, Saugers, and Burbot. With the exception of Smallmouth Bass, introduced game fish are generally rare in the study area; these include Walleyes, Black Crappies, White Crappies, White Bass, Green Sunfish, Pumpkinseeds, and Bluegills (Reinhold 2014).
| Family | Code | Species name | Total catch |
|---|---|---|---|
| Acipenseridae | past | Pallid Sturgeon Scaphirhynchus albus | 9 |
| shst | Shovelnose Sturgeon Scaphirhynchus platorynchus | 291 | |
| Catostomidae | bibu | Bigmouth Buffalo Ictiobus cyprinellus | 7 |
| blsu | Blue Sucker Cycleptus elongatus | 19 | |
| cato | Juvenile suckers | 2,304 | |
| losu | Longnose Sucker Catostomus catostomus | 1,500 | |
| mosu | Mountain Sucker Catostomus platyrhynchus | 385 | |
| rica | River Carpsucker Carpiodes carpio | 773 | |
| shre | Shorthead Redhorse Moxostoma macrolepidotum | 3,427 | |
| smbu | Smallmouth Buffalo Ictiobus bubalus | 39 | |
| whsu | White Sucker Catostomus commersonii | 767 | |
| Centrarchidae | blcr | Black Crappie Pomoxis nigromaculatus | 7 |
| blue | Bluegill Lepomis macrochirus | 20 | |
| centr | Juvenile sunfishes | 10 | |
| grsu | Green Sunfish Lepomis cyanellus | 44 | |
| laba | Largemouth Bass Micropterus salmoides | 5 | |
| pump | Pumpkinseed Lepomis gibbosus | 3 | |
| smba | Smallmouth Bass Micropterus dolomieu | 241 | |
| whcr | White Crappie Pomoxis annularis | 7 | |
| Cyprinidae | coca | Common Carp Cyprinus carpio | 475 |
| crch | Creek Chub Semotilus atromaculatus | 7 | |
| cypr | Juvenile minnows | 2,473 | |
| emsh | Emerald Shiner Notropis atherinoides | 5,618 | |
| fami | Fathead Minnow Pimephales promelas | 1,906 | |
| flch | Flathead Chub Platygobio gracilis | 18,301 | |
| lach | Lake Chub Couesius plumbeus | 13 | |
| loda | Longnose Dace Rhinichthys cataractae | 16,791 | |
| sash | Sand Shiner Notropis stramineus | 5,035 | |
| sich | Sicklefin Chub Macrhybopsis meeki | 3 | |
| stch | Sturgeon Chub Macrhybopsis gelida | 652 | |
| wesi | Western Silvery Minnow Hybognathus argyritis | 31,023 | |
| Esocidae | nopi | Northern Pike Esox lucius | 5 |
| Fundulidae | noki | Northern Plains Killifish Fundulus kansae | 12 |
| Gasterosteidae | brst | Brook Stickleback Culaea inconstans | 42 |
| Hiodontidae | goey | Goldeye Hiodon alosoides | 726 |
| Ictaluridae | blbu | Black Bullhead Ameiurus melas | 7 |
| chca | Channel Catfish Ictalurus punctatus | 837 | |
| icta | Juvenile catfishes | 1 | |
| stca | Stonecat Noturus flavus | 248 | |
| Lepisosteidae | shga | Shortnose Gar Lepisosteus platostomus | 4 |
| Lotidae | burb | Burbot Lota lota | 18 |
| Moronidae | whba | White Bass Morone chrysops | 5 |
| Percidae | saug | Sauger Sander canadensis | 213 |
| wall | Walleye Sander vitreus | 13 | |
| yepe | Yellow Perch Perca flavescens | 2 | |
| Salmonidae | brtr | Brown Trout Salmo trutta | 69 |
| mowh | Mountain Whitefish Prosopium williamsoni | 45 | |
| ratr | Rainbow Trout Oncorhynchus mykiss | 29 | |
| Sciaenidae | frdr | Freshwater Drum Aplodinotus grunniens | 59 |
| Unknown | unkn | 14 |
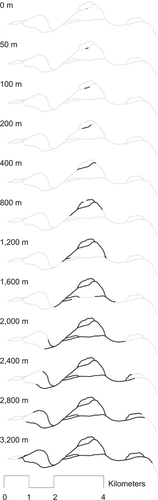
Depiction of the 12 spatial scales around one subsample, wherein the channel network is shown in gray and the spatial scale indicated is shown in black. Bank-stabilization, side-channel bank, and main-channel border lengths were extracted at each spatial scale for each subsample.

Species-specific estimated changes in gear-group relative abundance as a function of a 10% increase in (a)–(c) bank-stabilization and (d)–(f) side-channel proportions at alluvial and bluff sites. Error bars represent the 95% bootstrap confidence intervals. Estimates with confidence intervals not spanning zero were interpreted as satistically singnificant. The bank-stabilization proportion was the linear length of bank stabilization per main-channel bank length at the optimal spatial scale for each species and gear combination. The side-channel proportion was the linear length of side-channel banks divided by the total length of the side- and main-channel banks at the optimal spatial scale for each species and gear combination.
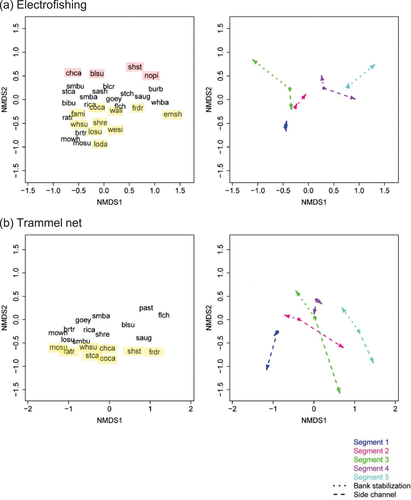
Two-dimensional NMDS ordinations of fish assemblage structure at alluvial sites for (a) electrofishing and (b) trammel net assemblage subsets. Species (Table 1) were arrayed in mesohabitat ordination space (left panels). The bank-stabilization and side-channel eigenvector lengths (right panels) correspond to the magnitudes of the correlation coefficients; eigenvector directions correspond to the directions of the shifts in assemblage structure related to bank stabilization and side channels. In the left panels, pink denotes a positive correlation to bank stabilization, a negative correlation to side channels, or both; yellow denotes a positive correlation to side channels, a negative correlation to bank stabilization, or both.
Methods
Sampling Design
The sampling design was a nested hierarchy. We divided the study area into five longitudinal segments (Figure 1). These segments were selected to include proximate locations of bank stabilization and unaltered banks as well as similar local geomorphology, braiding, and slope. Major tributary confluences and diversion dams were excluded from segments wherever possible. However, segment 2 included the Myers Diversion Dam to circumvent dividing this segment by both the Cartersville Diversion Dam and the confluence with the Bighorn River. Additionally, the Cartersville Diversion Dam impedes fish passage more than the Myers Diversion Dam (Helfrich et al. 1999).
Each site consisted of an upstream and downstream channel crossover and a main pool. We used stratified random sampling to select stabilized and reference alluvial and bluff sites (Figure 1) from braided and anabranching reaches (Boyd and Thatcher 2004) within segments. Alluvial sites were unconstrained laterally by bedrock bluffs, whereas bluff sites were constrained laterally by bedrock bluffs on one bank. We omitted all potential sites within 1.5 km of either a major tributary confluence or a diversion dam. Other exclusion criteria were related to having a unique feature; for example, one potential site was excluded because it was about twice as long as all the other potential sites.
Reference sites had no armoring on main pools or crossovers. Stabilized sites had at least 35% stabilization of outside-bend bank length in main pools. However, we made some exceptions to the reference site criteria where no other potential reference sites existed. In segment 1, sections of bank stabilization were present on the upstream crossover (34 m of stabilization; 7% of the upstream crossover banks) of one reference bluff site, the downstream crossover (188 m; 34%) of one reference bluff site, and the upstream crossover (220 m; 37%) and pool (30 m; 1%) of one reference alluvial site. Bank stabilization was present on one bank of the upstream crossover (79 m; 19%) of one reference bluff site in segment 3. We accounted for these exceptions to our reference site criteria by quantitatively assessing the lengths of bank stabilization and treating bank stabilization as a continuous rather than a categorical variable in our analyses of the potential effects of bank stabilization on the fish assemblage.
Fish Sampling
Fish sampling was conducted under late-summer and autumn base-flow conditions: September through early November 2009 and mid-August through mid-October in 2010 and 2011. Fish sampling occurred in mesohabitats: the inside bends of pools, outside bends of pools, and channel crossovers.
We sampled fish with five gears. Fyke nets, bag seines, and otter trawls were deployed to target small-bodied fishes, whereas electrofishing and trammel nets were employed to target large-bodied fishes. Nevertheless, large-bodied fishes were occasionally captured with fyke nets, bag seines, or otter trawls, and small-bodied fishes were occasionally captured with electrofishing or trammel nets. Otter trawls were only deployed in segments 4 and 5 because they effectively capture Sicklefin and Sturgeon chubs (Duncan et al. 2012) and the ranges of these species did not include segments 1–3 (Duncan et al. 2016). Fyke nets, bag seines, and boat electrofishing were used to sample fish along shorelines. Trammel nets and otter trawls were used to sample the fish in the deep portions of the channel in each mesohabitat.
Each gear deployment was a subsample, and the GPS coordinates of each subsample were recorded. We randomly selected the locations of fyke, seine, trammel net, and otter trawl subsamples by dividing each mesohabitat into equal longitudinal sections, then dividing each section into tenths and randomly selecting a starting location therein using the second digit in the seconds field of a digital watch. When sampling crossovers, we flipped a coin to decide which bank to sample. Fyke nets had two 1.2-m-wide and 0.6-m-high rectangular steel frames and two 0.6-m-diameter circular steel frames covered with 3-mm nylon mesh. Each fyke net had a 4.5-m-long lead and a 3-m-long cab and extended 7.5 m from the shoreline when deployed. Our fyke nets were identical to the “mini-fyke nets” described in detail in the upper Mississippi River system Long Term Resource Monitoring Program Procedures (Gutreuter et al. 1995). Three fyke nets were set at the shoreline in channel crossovers, inside bends, and outside bends. Seines were constructed of 6.4-mm mesh and were 9.1 m long and 1.8 m high with a centrally located cubic bag measuring 0.9 m on each side. Two 100-m-downstream seine hauls were made in wadable (less than 1.5 m deep) portions of channel crossovers and inside bends. Boat electrofishing was conducted with a Smith-Root Model 5.0 Generator Powered Pulsator using pulsed direct current at 60 pulses per second. One-pass upstream-to-downstream electrofishing was conducted along the entire shoreline of each mesohabitat where depths were 0.8 m or greater. Two 200-m trammel net and trawl subsamples were collected in each mesohabitat. Trammel nets were 38.1 m long with a 9.5-mm float line at the top and a 13.6-kg lead line at the bottom. Trammel nets had two panels; the inner panel was 2.4 m in height with 2.5-cm-bar mesh, and the outer panel was 1.8 m in height with 20.3-cm-bar mesh. Otter trawls were 4.9 m wide, 0.91 m tall, and 7.6 m long. The inner mesh size was 6.4 mm and the outer mesh size was 38.1 mm. Trawl doors were 76.2 cm × 38.1 cm and weighed 13.6 kg each. The trawl was towed downstream with two 30-m ropes. Otter trawls were identical to the “Model OT16” used for the Missouri River Pallid Sturgeon Population Assessment Program (Hamel et al. 2009).
We used MS-222 to anesthetize fish prior to handling. All fish greater than 30 mm total length (TL) were identified to species and counted. Catostomids, cyprinids, and centrarchids less than 30 mm TL and ictalurids less than 20 mm TL were identified to family, counted, and treated as separate taxa in subsequent analyses. Fish that we were unable to identify to family (0.01% of our total catch) were omitted from subsequent analyses. We captured 45 species of fish (Table 1) and 94,504 fish in total. We captured 72,917 fish with fyke nets, 11,667 with seines, 7,212 by electrofishing, 2,121 with trammel nets, and 587 with otter trawls (see Supplementary Figure S.A.1 available in the the online version of this article).
Spatial Analyses
We used ESRI ArcGIS 10.0 and existing digitizations of bank stabilization, flow lines (Yellowstone River Corridor Resource Clearinghouse 2013), and channel margins (T. Thatcher, DTM Consulting, unpublished data) for all spatial analyses. We examined the longitudinal variation in bank stabilization and side channels in the Yellowstone River to ensure that the selected sites reflected a diverse sample (Figure 2).
We calculated the spatial center of each subsample (“sampling point”) from its GPS coordinates and established its position in the channel network. The spatial scales at which bank stabilization and side channels influenced fish assemblages were unknown. Specifically, we were unsure how far up- and downstream from a sampling point to summarize bank stabilization and side-channel length to capture any potential effect on the fish assemblage. Therefore, we incorporated 12 different channel network distances (“spatial scales”: 0, 50, 100, 200, 400, 800, 1,200, 1,600, 2,000, 2,400, 2,800, and 3,200 m) into our analyses. We used ArcGIS Network Analyst to extract the network of main and side channels for each distance upstream and downstream of each sampling point (Figure 3). We then applied a 200-m lateral buffer to each extracted network and summed the bank stabilization and side-channel bank lengths within each buffer. We chose the range from 0 to 3,200 m based on the assumption that side channels and bank stabilization more than 3,200 m from the sampling point would not have an effect on the fish assemblage at the sampling point.
We standardized the lengths of bank stabilization and side-channel banks for each subsample for each spatial scale by calculating bank-stabilization and side-channel proportions. Each bank-stabilization proportion was calculated by dividing the length of bank stabilization by the main-channel bank length. Each side-channel proportion was calculated by dividing the length of side-channel banks by the length of all banks. The bank-stabilization and side-channel proportions for all subsamples of each gear type within each mesohabitat were averaged and resulted in one bank-stabilization proportion and one side-channel proportion for each combination of mesohabitat, gear type, and spatial scale.
Statistical Analyses
We examined the relationships of individual fish species and fish assemblage structure to the bank-stabilization and side-channel proportions. Catch per unit effort (CPUE) was calculated for each species captured by each gear type. Trammel net, otter trawl, and seine haul CPUEs were calculated by dividing catch by the distance sampled. Electrofishing CPUE was calculated by dividing catch by the time electrofished. Fyke net CPUE was calculated by dividing catch by the set duration. The CPUE for each mesohabitat × gear combination was averaged for each site because our sampling design was balanced at the mesohabitat level. All statistical analyses were conducted in R 3.0.1 (R Development Core Team 2013).
Single-species analyses
We calculated subsample relative abundances by dividing the catch of each species in the subsample by the total catch in the subsample. Subsample relative abundances were averaged within gear types and mesohabitats to generate a gear × mesohabitat relative abundance (GMRA) for each species (Figure S.A.1). We then generated three gear-group relative abundances (GGRAs) from the GMRAs. First, we averaged the electrofishing GMRAs and the trammel net GMRAs within each mesohabitat type because these gears targeted large-bodied fishes (Figure S.A.1, panels a and d) and were deployed in all mesohabitat types. Second, we averaged the seine GMRAs and the fyke GMRAs within channel crossover and inside bend mesohabitat types because these gears targeted small-bodied fishes (Figure S.A.1, panels b and c); we omitted outside bends from this analysis because we were unable to seine outside bends. Third, we considered otter trawls separately because we deployed them solely in segments 4 and 5; thus, the otter trawl GMRAs and GGRAs were equivalent.
We used ordinary least-squares regression to determine the estimated changes in GGRAs as a function of bank-stabilization and side-channel proportions at both bluff and alluvial sites; the GGRAs from alluvial and bluff sites were analyzed separately because we expected that the effects of bank stabilization on fish would be different at bluff and alluvial sites. We restricted the regressions to the primary longitudinal range for each species (i.e., segments where a species had 25% or fewer absences) to avoid zero inflation. To limit overparameterization, we restricted our regression analyses to species GGRAs for which we had a minimum of 1.5 degrees of freedom for each regression term. This constraint limited our regression analysis to 12 species × GGRA combinations. We nested sites within segments to account for longitudinal differences in relative abundance and modeled changes to relative abundances using terms for bank-stabilization proportion, side-channel proportion, mesohabitat, year, and site. The GGRA of each species was modeled at all combinations of the 12 bank-stabilization and side-channel spatial scales. The combination of bank-stabilization and side-channel spatial scales that maximized the adjusted R2 for each species GGRA was selected as the best-fitting model and was the only model for that species GGRA for which coefficients were interpreted. We resampled our data 5,000 times with replacement to generate 95% nonparametric, bootstrapped confidence intervals for the regression coefficients because the data were not normally distributed. Although we applied the same statistical test to the 12 GGRAs, we did not narrow the range of our confidence intervals to account for potential multiple-testing error. We decided that an increased type I error rate was preferable to failing to identify true but weakly significant results because of a multiple-test correction (sensu Brosi and Biber 2009). However, true departures from the null hypotheses (no effect of bank stabilization or side channels) cannot be discerned with certainty from departures caused by random chance alone. We estimated changes in GGRA for a 10% increase in the bank-stabilization and side-channel proportions because a 10% change was within the range of the bank-stabilization and side-channel proportions in our data. Interpretation of these estimated changes indicated general trends in relative abundances. However, this analysis was simplified because an assumption of our statistical model structure was that relative abundance changed linearly with changes in the bank-stabilization and side-channel proportions. Consequently, this analysis was unable to detect possible threshold, interaction, or density-dependent effects; nevertheless, it improves our understanding of which species were influenced by bank stabilization.
Assemblage structure analyses
The fish assemblage subset captured by each gear at each geomorphic site type was analyzed separately. A Bray–Curtis dissimilarity matrix (Bray and Curtis 1957) was generated from the CPUEs of species from each assemblage subset (function vegdist in R's vegan package; Oksanen et al. 2013) and analyzed with permutational multivariate analysis of variance (perMANOVA; function adonis in R's vegan package), an analog to multivariate analysis of variance (Anderson 2001). Because sites were nested within segments, each perMANOVA was stratified by river segment to restrict the permutations therein and included terms for bank-stabilization proportion, side-channel proportion, mesohabitat, year, and site. Each assemblage subset was modeled at the 12 bank-stabilization and side-channel spatial scales and all combinations thereof. The model that maximized the R2 for each assemblage subset was selected as the best-fitting model. Bank-stabilization and side-channel terms were only interpreted for the best-fitting model for each assemblage subset.
Nonmetric multidimensional scaling (NMDS; function metaMDS in R's vegan package; Oksanen et al. 2013) of each Bray–Curtis dissimilarity matrix was used to visualize similarities among the fish assemblage subsets. Correlation coefficients were calculated for bank-stabilization and side-channel proportions (function envfit; vegan; Oksanen et al. 2013) and the corresponding eigenvectors were plotted from the centroids of each river segment (function ordispider; vegan; Oksanen et al. 2013) to visualize how assemblage structure varied with the bank-stabilization and side-channel proportions. We examined longitudinal consistency in eigenvector directions across segments for each NMDS. Where eigenvector directions were not longitudinally consistent, inferring fish responses to bank-stabilization and side-channel proportions was problematic because responses varied according to longitudinal river position. However, where eigenvector directions were longitudinally consistent, we qualitatively inferred which fish species had longitudinally consistent responses to the bank-stabilization and side-channel proportions. We highlighted these species in the NMDS figure (Figures 5, S.B.1) and reported them in the NMDS table (Table S.B.1). However, we could not discern between positive correlation to bank stabilization and negative correlation to side channels (or vice versa), because either could result in the same species position in ordination space.
Model fitting and interpretation
We were primarily interested in determining whether main-channel fish assemblages differed as a function of bank-stabilization proportion. We were secondarily interested in determining whether main-channel fish assemblages differed as a function of side-channel proportion. Therefore, we fitted all regression and perMANOVA models using sequential sums of squares, such that the effects of bank stabilization were accounted for prior to estimating the effects of side channels on the fish assemblage. Accordingly, the lack of a statistically significant effect of side channels cannot be interpreted as a lack of a side-channel effect but only as a lack of a side-channel effect after accounting for a potential bank-stabilization effect.
Depth and Velocity Profiling
Each site was surveyed with an Acoustic Doppler Current Profiler (ADCP) during base flow in 2011 to determine whether depths and velocities differed significantly between stabilized and reference pools. Profiles were conducted along evenly spaced transects that varied in number as a function of pool length (<550 m, two transects; 550–850 m, three transects; 850–1,150 m, four transects; 1,150–1,450 m, five transects; and >1,450 m, six transects).
We determined whether main-channel bank stabilization was present within 50 m upstream or downstream of each ADCP transect to locate partially stabilized pools because some “reference” pools had small amounts of bank stabilization. Partially stabilized pools were classified as stabilized because even short lengths of bank stabilization can cause local changes in pool depth or current velocity. We calculated the means and 95th percentiles of depth and velocity of each pool and regressed these against bank stabilization and river segment using ordinary least-squares regression. We used the 95th percentiles of depth and velocity instead of the true maxima to avoid reporting overestimations resulting from potentially spurious ADCP readings. Nevertheless, we refer to the 95th percentile as the “maximum” in this text.
Results
Individual Species Responses
Bank stabilization and side channels influenced the relative abundances of many fish species. Individual species responses to bank stabilization and side channels generally varied according to site geomorphology and sampling method (Figure 4). The responses of Flathead Chub to bank stabilization varied according to sampling method in alluvial sites; the relative abundances of Flathead Chub captured with fyke nets and seines increased with the bank-stabilization proportion, but the relative abundances of those caught by electrofishing and trammel nets did not differ. Only White Suckers captured with electrofishing and trammel nets responded to bank stabilization consistently across site geomorphologies, their relative abundances decreasing with bank stabilization at both alluvial and bluff sites. Goldeye responses to side channels and Goldeye, Shorthead Redhorse, and Longnose Sucker responses to bank stabilization varied according to site geomorphology when captured with electrofishing and trammel nets. Moreover, Goldeye responses to bank stabilization and side channels were opposite at alluvial sites; relative abundances increased with the bank-stabilization proportion and decreased with the side-channel proportion.
Assemblage Subset Responses
Bank stabilization and side channels influenced the fish assemblage structure of many assemblage subsets (Table 2), although the R2 values for these model terms indicate that their explanatory power was modest. For example, the terms for site and year generally explained a greater portion of the assemblage structure than the terms for either bank stabilization or side channels (compare the R2 values in Table 2). Nevertheless, the eigenanalysis of the NMDSs generally confirmed the perMANOVA results for the bank-stabilization and side-channel terms. The influences of bank stabilization and side channels on the fish assemblage subsets differed, as indicated by the differing and often opposing eigenvector directions for the bank-stabilization and side-channel proportions (Figures 5, S.B.1). Where eigenvector directionalities were generally longitudinally consistent, species CPUE correlations with side channels were more frequent and consistent than those with bank stabilization (Table S.B.1).
| Degrees of freedom | Bank stabilization | Side channels | Mesohabitat | Year | Site | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Capture method | Residual | Total | Model R2 | P | R2 | P | R2 | P | R2 | P | R2 | P | R2 |
| Alluvial sites | |||||||||||||
| Electrofishing | 36 | 59 | 0.61 | 0.081 | 0.02 | 0.013 | 0.03 | 0.041 | 0.04 | <0.001 | 0.08 | <0.001 | 0.44 |
| Fyke net | 35 | 58 | 0.60 | <0.001 | 0.06 | 0.019 | 0.03 | 0.001 | 0.07 | 0.008 | 0.05 | <0.001 | 0.39 |
| Seine | 14 | 36 | 0.81 | 0.001 | 0.05 | 0.049 | 0.03 | 0.475 | 0.01 | <0.001 | 0.13 | 0.002 | 0.60 |
| Trammel net | 35 | 58 | 0.68 | 0.021 | 0.02 | 0.010 | 0.02 | 0.007 | 0.04 | <0.001 | 0.12 | <0.001 | 0.46 |
| Otter trawl | 12 | 23 | 0.52 | 0.612 | 0.03 | 0.542 | 0.03 | 0.747 | 0.05 | 0.050 | 0.09 | 0.167 | 0.32 |
| Bluff sites | |||||||||||||
| Electrofishing | 20 | 35 | 0.65 | <0.001 | 0.10 | 0.026 | 0.04 | 0.011 | 0.07 | <0.001 | 0.14 | <0.001 | 0.31 |
| Fyke net | 19 | 34 | 0.69 | 0.082 | 0.03 | 0.001 | 0.08 | 0.039 | 0.06 | <0.001 | 0.25 | 0.002 | 0.28 |
| Seine | 8 | 22 | 0.72 | 0.353 | 0.04 | 0.024 | 0.09 | 0.429 | 0.03 | 0.004 | 0.18 | 0.225 | 0.38 |
| Trammel net | 19 | 34 | 0.62 | 0.117 | 0.03 | 0.145 | 0.03 | 0.613 | 0.03 | <0.001 | 0.16 | <0.001 | 0.37 |
Spatial Scale Dependence
Clear patterns in scale dependence emerged from the relative abundance models of single species (Figure 6). Modeling each species at the optimum spatial scale resulted in substantial improvements in adjusted R2 values; for example, the adjusted R2 for modeling White Sucker relative abundances in bluff habitats (Figure 6, bottom right) improved fourfold (from 0.15 to 0.57) simply as a result of measuring the bank-stabilization and side-channel proportions at appropriate spatial scales. In contrast with the models of assemblage structure, the scale-dependent distributions in explanatory power attributable to bank stabilization and side channels were generally unimodal for the single-species models.
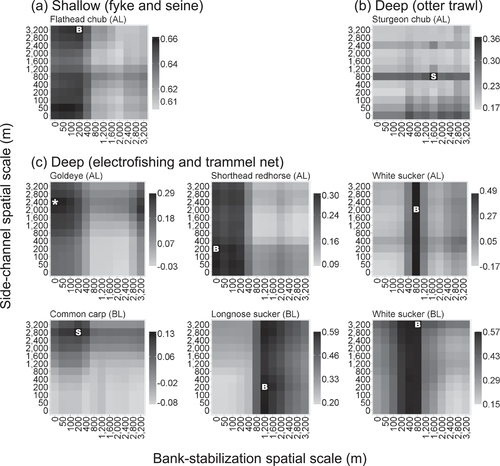
Spatial scale–dependence patterns in adjusted R2 values for relative abundance regressions for species captured in (a) shallow and (b)–(c) deep habitats at alluvial (AL) and bluff (BL) sites. Better-fitting models are indicated by black and poorer-fitting models by light gray. The maximum adjusted R2 values are shown for species–habitat combinations with significant terms for bank stabilization (B), side channels (S), or both (*) (Figure 4).
The spatial scales of measurement for bank stabilization and side channels influenced the explanatory power of the models of assemblage structure (Figure 7), but to a lesser degree than in the single-species models. The patterns of scale dependence of explanatory power were bimodal for side channels in the electrofishing subset at alluvial and bluff sites and for bank stabilization in the trammel net subset at alluvial sites. The results for the remaining assemblage subsets were unimodal or lacked a clear pattern. Additionally, although changes in overall R2 values across spatial scales were small (Figure 7), these changes are attributable solely to the scale dependence of bank stabilization and side channels; other model variables are not scale dependent. Changes in overall R2 can be considered relative to the R2 of model terms for bank stabilization and side channels (0.02 to 0.10; Table 2). Thus, a 0.01 increase in overall R2 equates to a 10–50% improvement in the R2 of bank stabilization and side channels (i.e., 0.01 is 50% of 0.02 and 10% of 0.10).
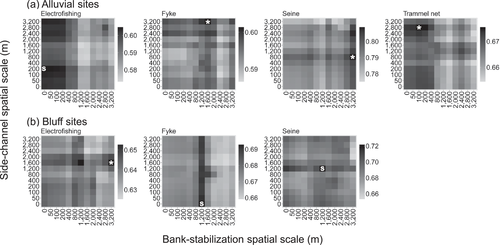
Spatial scale–dependence patterns in R2 values for Bray–Curtis perMANOVA results for (a) alluvial and (b) bluff sites. Better-fitting models are indicated by black and poorer-fitting models by light gray. The maximum R2 values (Table 2) are shown for assemblage subsets with significant terms for bank stabilization (B), side channels (S), or both (*).
Depths and Velocities in Stabilized and Reference Pools
Depths differed between reference and stabilized alluvial pools (Figure S.C.1a), but velocities did not (Figure S.C.2a). Stabilized alluvial pools were deeper (mean depths: t5, 14 = 2.18, P = 0.047; maximum depths: t5, 14 = 3.12, P = 0.008) than reference alluvial pools. The mean depths of stabilized alluvial pools were 0.41 m greater than those of reference pools (95% confidence interval [CI] = 0.01–0.81 m), and the maximum depths were 1.26 m greater (95% CI = 0.39–2.13 m). The mean velocities (linear regression: t5, 14 = −0.12, P = 0.907) and maximum velocities (t5, 14 = −0.46, P = 0.654) were not different between stabilized and reference alluvial pools.
Neither depths nor velocities differed between reference and stabilized bluff pools (Figures S.C.1b, S.C.2b). The mean depths (t3, 8 = 1.93, P = 0.089) and maximum depths (t3, 8 = 1.58, P = 0.153) were not different between stabilized and reference bluff pools. The mean velocities (t3, 8 = 0.58, P = 0.577) and maximum velocities (t3, 8 = 0.20, P = 0.851) were not different between reference and stabilized bluff pools.
Discussion
Consistent with our hypotheses, the lower Yellowstone River fish assemblage varied with bank-stabilization extent and side-channel availability in a scale-dependent manner. However, the responses of fish to bank stabilization and side channels often varied with longitudinal location, sampling year and site, and site geomorphology. Moreover, these responses were dependent on species and life history stage.
Bank Stabilization
Bank stabilization influenced fish habitat (i.e., pool depth) and fish habitat use differently at alluvial and bluff sites. Bank stabilization probably caused the deepening of alluvial pools by attenuating lateral erosion and accelerating bed degradation, whereas bedrock bluffs constrained lateral erosion regardless of bank stabilization. The trammel net subset may have responded directly to such changes in depth, because the assemblage structure of this subset differed according to the bank-stabilization proportion at alluvial but not bluff sites (Table 2). At alluvial sites, the catches of Shovelnose Sturgeons and Channel Catfish were positively correlated with bank stabilization in the electrofishing subset but negatively correlated in the trammel net subset (Figure 5; Supplement B). This apparent contradiction in responses to stabilization may have been caused by fish shifting their habitat use from deepened portions of the channel near the thalweg (which were sampled with trammel nets) to shorelines (which were sampled with electrofishing). For instance, Shovelnose Sturgeons most often use depths less than 2.5 m in the Yellowstone River (Bramblett and White 2001), and Channel Catfish may have exploited riprap for cover or spawning habitat (McMahon and Terrell 1982).
The effects of bank stabilization can be indirect and vary according to pool geomorphology, fish species, and life history stage. Stabilized alluvial pools were deeper than reference pools, which probably resulted in smaller shallow, slow-current velocity (SSCV; sensu Bowen et al. 2003) patches along inside bend margins. These decreases in SSCV patch size reduced suitable habitat for small fish that use shallow habitats for refuge from aquatic predators and slow current velocities to avoid downstream displacement. Thus, the reduction in SSCV patch size may have increased the densities of some small fish, contributing to the differences in the fyke net and seine assemblage subsets between stabilized and reference alluvial pools. For example, the fyke net and seine relative abundances of small Flathead Chub were greater in inside bend and channel crossover shorelines of stabilized alluvial pools than in reference alluvial pools. However, the relative abundances of large Flathead Chub captured using electrofishing and trammel nets did not differ according to stabilization extent (Figure 4a).
Bank stabilization may be detrimental to the persistence of fish, including fishes that were positively associated with bank stabilization during base flow, because bank stabilization can reduce floodplain connectivity (Florsheim et al. 2008), which is critical during high-water periods (Sheaffer and Nickum 1986b; Galat et al. 1998). Although the relative abundances of small Flathead Chub were positively correlated with bank stabilization during base flow, the abundances of Flathead Chub may be limited by seasonally inundated floodplains (Reinhold et al. 2016). For example, Flathead Chub were extirpated from the unimpounded upper Mississippi River concomitant with the anthropogenic disconnection of its floodplain (Barko et al. 2004b). Therefore, if the extent of bank stabilization and floodplain disconnection exceeds threshold levels, fishes that depend on floodplain habitats will probably experience population declines.
There was much overlap between the species compositions of stabilized and reference sites, which was concordant with the results of similar investigations from other large rivers (Barko et al. 2004a; White et al. 2010; Schloesser et al. 2012). Additionally, the relative abundances of some species were higher at stabilized sites than at reference sites, whereas other species exhibited the opposite trend. This variability in individual species responses was consistent with the findings of similar investigations in the Kansas River (White et al. 2010) and the upper Mississippi River (Madejczyk et al. 1998). However, unlike investigations in other large rivers, our study was not confounded by the influences of main-stem impoundment, dredging, channelization, locks and dams, or a largely disconnected floodplain. Such differences complicate direct comparison of our study with many other studies. For example, in our study Shorthead Redhorse used stabilized alluvial sites less than reference alluvial sites, whereas in the Mississippi River Moxostoma species preferentially occupied wing dikes (Madejczyk et al. 1998), which are not present in our study area. However, in the heavily stabilized upper Mississippi River, Moxostoma species may have been concentrated at wing dikes because wing dikes offered some of the only slow-velocity habitat (Barko et al. 2004a, 2004b). In our study area, fish could access slow-velocity habitats in both the main stem and side channels (Bowen et al. 2003; Reinhold et al. 2016).
Side Channels
Consistent with our hypothesis, side channels influenced fish assemblages (Table 2). The catches of more species were positively correlated with side-channel availability than were negatively correlated with it (Figures 5, S.B.1; Table S.B.1). We speculate that one cause of positive correlations is proximal food sources in side channels; side channels had higher densities of zooplankton (Bothar 1981), macroinvertebrates (Eckblad et al. 1984; Sheaffer and Nickum 1986a), and larval fish (Eckblad et al. 1984) than main channels in other large rivers. Moreover, main-channel habitats with connected side channels had higher densities of zooplankton (Bothar 1981) and larval fish (Eckblad et al. 1984; Sheaffer and Nickum 1986b) than main-channel habitats without connected side channels. Positive correlations may also be caused by proximal habitat heterogeneity for spawning and rearing habitat, as the densities of larval (Eckblad et al. 1984) and juvenile fish (Eckblad et al. 1984; Sheaffer and Nickum 1986b) were greater in side channels than in main channels. Thus, proximal side channels may increase local recruitment; in our study area, the total catches of small-bodied fish were higher in river bends with side channels than in river bends without side channels (Reinhold et al. 2016). Proximal food sources may also cause negative correlations if fish concentrate in side channels, where foraging efficiency is maximized. Also potentially contributing to negative correlations is the use of side channels by small fish as refuges from predation by larger fish (sensu Jackson et al. 2001). Although our speculations on the effects of side-channel availability assume movement between side and main channels, the spatial and temporal details of lateral fish movement in the Yellowstone River are not known and merit future study.
Spatial Scale Dependence
The influences of bank stabilization and side channels on fish were spatially scale dependent, but the patterns in this scale dependence were clearer and more robust for the relative abundance models than for the assemblage subset models (Figure 6 versus Figure 7). Perhaps the scale dependence was clearer in the relative abundance models because they are inherently simpler—focusing only on one species—than the assemblage subset models, which incorporate data from several species. Thus, the variability and weak patterns in the assemblage subset models suggest that the species within each subset are responding to bank stabilization and side channels at different scales.
Bank stabilization may increase fish species richness and diversity at finer spatial scales by creating novel bank habitat, but it may also influence fish at coarser spatial scales by altering normal riverine function (Schmetterling et al. 2001; White et al. 2010). Our results broadly support this idea; the bimodal distribution in the scale dependence of some of the assemblage subsets indicated that at least two processes structured those subsets—one at finer scales and another at coarser scales. We speculate that introducing novel substrates (riprap) and increasing pool depth contributed to assemblage structure at finer scales and that channel simplification or land use practices associated with bank stabilization contributed to assemblage structure at coarser scales (Figures 6, 7) .
Concluding Remarks
The influence of bank stabilization on the fish assemblages in our study area depended on the natural riverine template and how bank stabilization altered the physical habitat therein. Fish assemblage subsets responded differently based on longitudinal river position, local geomorphology, and access to side channels. Additionally, the influences of bank stabilization generally differed from, and often opposed, the influences of side channels on the fish assemblage subsets. Therefore, conservation or restoration of side channels may provide an appropriate mitigation strategy for bank stabilization. Although the shifts in the fish assemblage associated with bank stabilization did not result in completely different assemblage structures at stabilized and reference sites, shifts in the fish assemblage were widespread, as bank stabilization was present in many lower Yellowstone River reaches (Figure 2; Boyd and Thatcher 2004). Moreover, we suspect that the present fish assemblage reflects the incremental changes in fish habitat that have resulted from the installation of bank-stabilization structures and the loss of side channels concomitant with increased land use and the development of the Yellowstone River basin.
ACKNOWLEDGMENTS
We thank the Yellowstone River Technical Advisory Committee and the Yellowstone River Conservation District Council for making much of this work possible. We thank Karin Boyd, Tony Thatcher, and Matt Jaeger for their seminal work on the Yellowstone River and their continual collaboration. We thank the staffs of Regions 5 and 7 of Montana Fish, Wildlife and Parks for their guidance and assistance. We thank Rosa McIver, Chris Naus, Drew Pearson, Dave Ritter, Elliot Johnson, Caleb Mitchell, Nate Laulainen, Nick Rubino, and Michael Moore for many long days of fieldwork. We thank Sean Lawlor and his USGS crew for collecting the ADCP data. We thank George Jordan, John Powell, Mike Duncan, Warren Kellogg, Stan Proboszcz, Chris Guy, and Bret Olson for their thoughtful feedback as this project developed. We thank Lynn DiGennaro for her tremendous administrative and logistical support. We thank Adam Sepulveda for his thoughtful comments on an earlier draft of this manuscript.
This article is based on work supported in part by U.S. Army Corps of Engineers (USACE) military interdepartmental purchase requisitions (MIPR-59XQG72647975 and MIPR-W59XQG6913) and in part by National Science Foundation Experimental Program to Stimulate Competitive Research cooperative agreement EPS-1101342. Any opinions, findings, conclusions, and recommendations expressed herein are those of the authors and do not necessarily reflect the views of the USACE or the National Science Foundation. All work was conducted under applicable state permits and under the auspices of the Montana State University Institutional Animal Care and Use Committee (protocol 88-03). The Montana Cooperative Fishery Research Unit is jointly sponsored by the U.S. Geological Survey, Montana Fish, Wildlife and Parks, Montana State University, and the U.S. Fish and Wildlife Service. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.




