The Effect of a Porcine-Derived Small Intestinal Submucosa Product on Wounds With Exposed Bone in Dogs
Supported by the Department of Clinical Sciences, Auburn University.
The abstract of this article was presented at the Small Intestinal Submucosa (SIS) Symposium 2000, Orlando, FL.
Address reprint requests to James T. Winkler, DVM, Hoerlein Hall, Auburn University, Auburn AL, 36849.
Abstract
Objective— To determine the effect of a porcine-derived small intestinal submucosa product (PSIS) on healing time, epithelialization, angiogenesis, contraction, and inflammation of wounds with exposed bone on the distal aspect of the limbs of dogs.
Study Design— Prospective, controlled, experimental study.
Animal Population— 10 young adult, purpose-bred, male Beagles.
Methods— Small wounds with exposed bone were created on the lateral aspect of metatarsal V and the medial aspect of metatarsal II on both hindlimbs. Three sheets of PSIS were sutured into the wounds of the treated limb, and the other limb served as a control. On day 10, punch biopsies of the medial metatarsal wounds were collected and were evaluated microscopically after routine hematoxylin and eosin and phosphotungstic acid hematoxylin (PTAH) staining. The lateral metatarsal wounds were evaluated by planimetry and laser Doppler perfusion imaging on days 7, 14, and 21. Time until complete wound healing was also recorded. The level of significance was set at P≤ .05 for all statistical analyses.
Results— Laser Doppler perfusion measurements were significantly higher in control wounds on day 7, but no differences were noted on days 14 and 21. No significant differences in planimetric values, histopathologic appearance, or time until complete wound healing were noted among treated and control groups.
Conclusions— No objective differences in healing were noted between control wounds and wounds treated with PSIS.
Clinical Relevance— There appears to be no contraindication to the use of PSIS on clean wounds with exposed bone on the distal limbs of dogs. However, our objective data provides no evidence that this product affects epithelialization, contraction, or time to complete healing in wounds with exposed bone.
Wounds of the distal aspect of the limbs are common in small animals. They may be the result of automobile accidents, gunshots, interanimal aggression, oncologic surgery, ischemic episodes caused by snares or tight bandages, limb entrapment, or radiation injuries. With decreased soft tissue coverage compared with the proximal aspect of the limbs and trunk, it is not uncommon for these wounds to have variable-sized areas of exposed bone.1
Areas of exposed bone within a wound require special considerations. Bone without soft tissue coverage is susceptible to desiccation and sequestrum formation. Wound contraction is inhibited because of the lack of fibroblasts or collagen in the center of the wound. Epithelialization is inhibited because of the poor deep blood supply provided by cortical bone,2–4 thus, the process of secondary healing over exposed bone may require more time to achieve complete healing than a wound without exposed bone. Granulation tissue growth has been shown to cover approximately 0.5 mm of bone per day on the human skull. Thus, a 20- mm diameter wound will take approximately 40 days for complete granulation tissue coverage.5 In a retrospective study, it was reported that distal limb wounds with exposed bone or joints had a mean healing time of 6.8 weeks, whereas wounds without exposed bone had a mean healing time of 3.2 weeks.1
Many attempts have been made to develop treatment protocols that decrease healing time of wounds with exposed bone. On the head and proximal limbs, random flaps or axial pattern flaps may be used to cover the defect. When a wound with exposed bone is on a distal limb, however, there is rarely sufficient tissue to close the wound with a local flap. Distant flaps such as pouch flaps on the thorax may be used, but a disadvantage is that the limb must be secured to the trunk until local revascularization has taken place.6 Axial pattern flaps will not consistently reach the distal limbs in dogs.7 The reverse saphenous conduit flap may cover defects of the metatarsus; however, one must ensure that the distal blood supply to the flap is intact before performing this technique.8
To circumvent the problem of having insufficient tissue on the distal limbs for flap transfer, microvascular free tissue transfer may be used. For this technique, a patch of tissue including its direct vessels are isolated and severed. Microvascular techniques are then used to anastomose the flap vasculature to local wound vessels.9 This technique provides immediate tissue coverage with a blood supply; however, it requires microvascular surgical experience.
Other techniques have been developed to enhance soft tissue coverage over bone. Once a granulation tissue bed is present, epithelialization and contraction will occur at a more rapid pace. Skin grafts may be used once deep vascular tissue is present over bone.10 The vascular supply from the deep surface of the wound may be increased by exposing the medullary cavity and haversian canals of the exposed bone. One disadvantage of this procedure is that it can lead to bone fracture, especially on metatarsal and metacarpal bones.2 Another technique used to provide soft tissue coverage over bone of forelimb defects is a transposition flap of the humeral head of the flexor carpi ulnaris muscle. This muscle has a major distal blood supply from the caudal interosseous artery that allows for rotation of the origin of the muscle.11 We are unaware of any muscle flaps used in the distal hindlimb.
Many biomaterials have been evaluated for their efficacy in stimulating wound healing.12–19 One biomaterial, porcine small intestinal submucosa (PSIS), includes the stratum compactum and submucosa of the swine small intestine as its name implies. It is composed of collagen (types 1, III, IV, V, VI, and VIII), fibronectin, hyaluronic acid, chondroitin sulphate A, heparin, and heparin sulphate.20 Reports have also indicated that swine small intestinal submucosa contains certain growth factors such as fibroblast growth factor-2 (FGF-2), a transforming growth factor beta (TGF-B)–like compound,21 and vascular endothelial growth factor22 that could stimulate healing. It has been used for tissue reconstruction in many organs including the bladder,23,24 urethra,25 vasculature,26–28 fascia,29 dura mater,30 cranial cruciate ligament,31 and meniscus32 and is currently being investigated in various other tissues. In all of these applications, PSIS was gradually replaced by host tissue with characteristics of the normal adjacent tissue. It has also been evaluated for its effect on healing full-thickness cutaneous wounds of rodents.33
A commercially available product containing PSIS is VET BIO SIS T (Cook Veterinary Products, Bloomington, IN). It is supplied in many different forms. The individual sheet configuration of this product is approximately 0.1 mm thick and is available in a lyophilized form. The purpose of our study was to evaluate the effects of PSIS on healing of wounds with exposed bone on the distal aspect of dog limbs. Our hypothesis was that PSIS would decrease healing time of wounds with exposed bone on the distal limbs of dogs by providing a three-dimensional extracellular matrix for the ingrowth of cells and microvasculature. Our objectives were to determine the effect of PSIS on wound perfusion, contraction, epithelialization, and healing time and to also characterize the early morphologic features of the healing tissue.
Materials and methods
This study was approved by the Auburn University Animal Care and Use Committee. Ten 1-year-old healthy, intact male, purpose-bred Beagles were studied. A complete physical examination was performed on each animal before surgery.
Presurgical and surgical procedures
Each dog was premedicated with butorphanol (0.4 mg/kg) and acepromazine (0.05 mg/kg) intramuscularly (IM). A cephalic catheter was placed for induction and fluid administration. Anesthesia was induced with thiopental (10 mg/kg intravenously [IV]) and maintained with isoflurane in oxygen. Both distal hindlimbs were clipped and prepared aseptically from the ends of the digits to the level of mid-tibia. Cefazolin was administered perioperatively (20 mg/kg IV) 30 minutes before surgery and then 2 hours later.
A rectangular skin excision (2 cm long, 1 cm wide) was centered over the lateral surface of metatarsal bone V on both hindlimbs. The subcutaneous tissue and lateral digital extensor tendon in the incisional area was resected to expose the lateral surface of the bone. The periosteum was incised and stripped to the level of the interosseous muscle on the plantar aspect of the bone and the long digital extensor tendon on the dorsal surface of the bone. Simple interrupted sutures of 3–0 polypropylene were used to tack the skin to the underlying tissue at each corner and at the center of the 2 long sides of the rectangle to immobilize the skin edges so the exposed bone remained in the center of the wound (Fig 1). The sutures were removed 7 days postoperatively after the skin had formed a firm adhesion to the underlying tissue. These wounds were used for subjective evaluation, planimetric evaluation, and laser Doppler perfusion imaging.
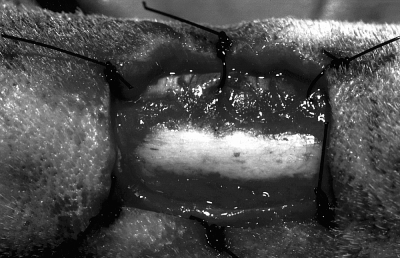
Surgically created wound on the lateral surface of metatarsal bone V on day 0. The periosteum has been stripped, and the skin edges are sutured to the underlying fascia maintaining exposed bone in the center of the wound. These skin sutures were removed on day 7 after an adhesion between skin and fascia was formed. This lesion was used for planimetric and laser Doppler studies.
A smaller wound was created with an 8-mm biopsy punch centered on the medial surface of metatarsal bone II on both hindlimbs (Fig 2). The periosteum was resected, and the skin was tacked to the underlying tissue with 4 evenly spaced simple interrupted sutures of 3–0 polypropylene to keep the exposed bone centered in the wound. Tissue from this wound was used for histopathologic evaluation on day 10.
Surgically created (8-mm biopsy punch) wound on the medial surface of metatarsal bone II. The periosteum has been stripped, and the skin has been sutured to the fascia to ensure that the exposed bone is in the center of the wound. This small wound was created for histopathologic examination on day 10.
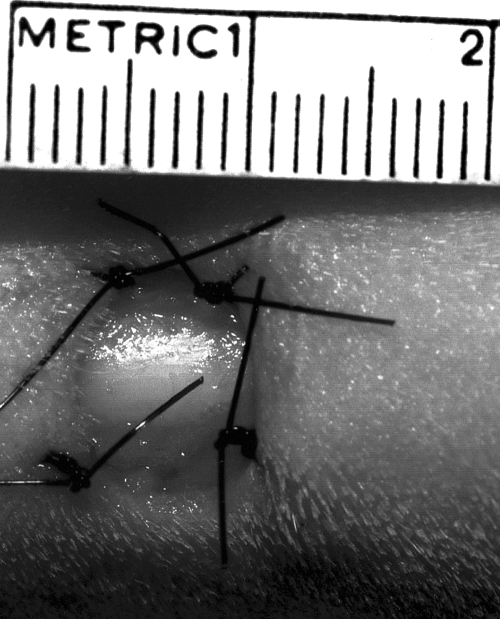
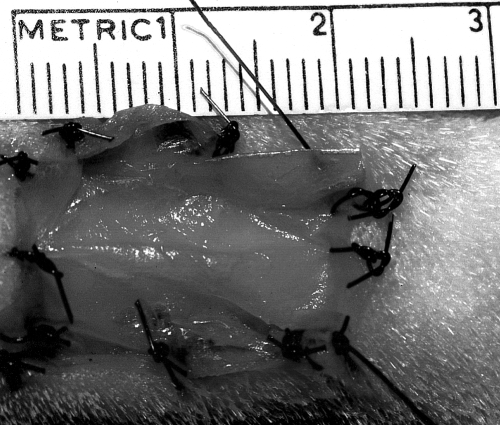
One hindlimb on each dog was treated with PSIS, and the other served as a control (no treatment other than routine bandage changes), thus each dog served as its own control. PSIS was placed on the wounds on the day they were created (day 0); 5 dogs had PSIS on the right hindlimb and 5 dogs had PSIS on the left hindlimb. Immediately after wound creation, 3 sheets of PSIS (Fig 3) were cut slightly larger than the wound so that there was a 1- to 3-mm overlap of PSIS around the wound edges. One sheet was fenestrated using a 1.5-mm diameter biopsy punch placing holes approximately 5 mm apart. The sheets were stacked on top of one another with the fenestrated sheet placed in the most superficial position to allow for wound drainage. The 3 stacked sheets were then laid over the bone.

Three sheets of PSIS cut slightly larger than the wound in which it is to be placed. The top sheet is fenestrated to allow drainage.
On the first dog, only the top sheet was sutured to the skin edges; however, the deeper sheets on the large wound migrated out of the wound within 3 days. Thus, on the other dogs, all 3 sheets were sutured to the skin. We chose to use 3 sheets instead of 1 because of the depth of the defect. On the large wounds, PSIS was secured by simple interrupted 3–0 polypropylene sutures placed at each corner and at the center of each edge (Fig 4). On the small wounds, 4 to 6 simple interrupted sutures were evenly spaced around the wound to anchor the PSIS to the skin edge (Fig 5). After all sutures were in place, a saline-moistened gauze was used to rehydrate the PSIS.
Treated wound on the lateral aspect of metatarsal V on day 0 with 3 sheets of PSIS sutured into the wound.
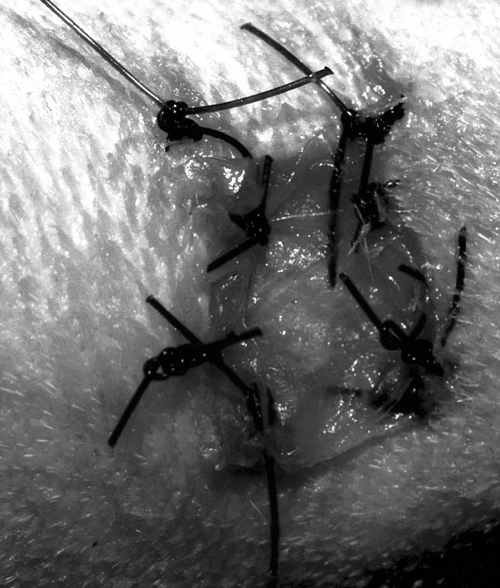
Treated wound on day 0 on the medial surface of metatarsal bone II.
Postoperative care
Each dog was administered butorphanol (0.4 mg/kg IM, every 4 hours) after surgery for 24 hours. Ketoprofen was administered on day 1 (2 mg/kg subcutaneously [SC]) after surgery and 1 mg/kg SC on day 2.
Each hindlimb was covered with a bandage until the wounds were healed. A non-adherent, semi-occlusive bandage (Telfa pad; Kendall, Mansfield, MA) with a thin layer of 0.1% garramycin ointment (Schering Corporation, Kenilworth, NJ) was applied directly to the wound and secured with an absorbent secondary wrap (Sof-Band; Johnson and Johnson Medical, Arlington, TX) that covered the paws and extended proximal to the hocks. Two- inch porous adhesive tape (Zonas; Johnson and Johnson Medical) was used for the outer layer. Bandages were changed daily for 7 days, then every other day unless bandage loosening required more frequent changes.
Because of the gradual degradation or incorporation of PSIS into the wound bed, an additional sheet of PSIS was added when the original PSIS did not cover at least 75% of the wound. The method for securing the new PSIS depended on when it was added. On days 7 and 10 when dogs were anesthetized for other diagnostic purposes, PSIS was sutured to the wound. On other days, PSIS was either laid or taped in place with one thin strip of adhesive tape applied circumferentially above and below the wound. Unincorporated PSIS was removed from wounds on day 21.
Evaluation procedures
Subjective evaluation
At each bandage change, the condition of the PSIS was checked and wounds were evaluated for exudate, swelling, and odor. In most instances, the PSIS wound bed could not be evaluated completely because of the PSIS. Each dog was evaluated for any evidence of lameness or pain associated with the wound. Number of days until healing (complete epithelial coverage) of each wound was recorded.
Laser doppler perfusion imaging
Blood flow in the area of the wound on the abaxial surface of metatarsal V of both hindlimbs was evaluated on days 7, 14, and 21 with the dog anesthetized, as described above, to prevent movement. The scanner (PIM II Laser Doppler Perfusion Imager; Lisca, Linkoping, Sweden) was positioned 7.5 cm above the wound. An area of 10 × 10 pixels in the center of the wound was measured with high resolution. This area was approximately 1 cm2 and corresponded to the region of exposed bone on day 7. Over the course of 3 weeks, granulation tissue and skin began to cover the exposed bone and was included in the scan area. The laser Doppler generated a mean perfusion (recorded in units of perfusion) that was a product of cell velocity and cell density within that region. This value was compared between control and treated wounds.
Planimetry
Hand tracings of the wounds on the abaxial surface of metatarsal V were made on day 7, 14, and 21. The wound edge traced on day 7 was considered the original wound area because this was the day that skin stay sutures were removed. The area of granulation tissue could not be traced because of visual interference by PSIS. The edge of the wound was traced on day 14, whereas the outer and inner edges of new epithelial tissue were traced on day 21. Wound tracings were retraced into a computer program (Sigma Scan Pro 5.0; SPSS, Chicago, IL) for area calculations. Percent total wound healing was a combination of epithelialization and contraction. The percent of total wound healing at each measurement time compared with the original wound size was calculated by subtracting the area of the wound with no epithelial tissue from the original wound area, dividing this number by the original wound area, and multiplying by 100. This was calculated for days 14 and 21. Percent of wound epithelialization was calculated by dividing the area of new epithelial tissue by the original wound area and multiplying by 100. The area of new epithelial tissue was calculated by subtracting the area of the wound with no epithelialization from the area of the wound within the outer edge of new epithelial tissue. This was calculated on day 21. Percent wound contraction compared with the original wound size was calculated by subtracting the area of the wound at the outer edge of new epithelial tissue from the original wound area, dividing this value by the original area of the wound, and multiplying by 100. Percent of wound contraction was evaluated on day 21.
Histopathology
On day 10, each dog was administered 0.4 mg/kg butorphanol IM and 0.01 mg/kg medetomidine IV. All sutures in the small wounds on the medial surfaces of metatarsal bones II were removed. An 8-mm diameter biopsy punch was used to remove all tissue down to bone in the wound center. This tissue specimen was fixed in 10% buffered formalin, and sections were stained with hematoxylin and eosin (H & E). Various histologic variables were scored (0 to 3) at 400× magnification.
The inflammatory stage of healing was evaluated by scoring neutrophils, eosinophils, macrophages, lymphocytes, plasma cells, and mast cells: 0 = <3 cells/field, 1 = 3 to 10 cells/field, 2 = 11 to 30 cells/field, and 3 = >31 cells/field. For necrosis and fibrin deposition: 0 = none identified, 1 = few scattered areas, 2 = multiple focal dense areas, and 3 = necrosis or fibrin present throughout the slide. The stage of repair was evaluated by scoring collagen, fibroblasts, and angiogenesis. Scoring of fibroblasts and angiogenesis was 0 = <3 fibroblasts or capillary buds/field, 1 = 3 to 10/field, 2 = 11 to 30/field, and 3 = >30/field. Collagen was scored as 0 = no collagen, 1 = scant collagen bundles slightly separating fibroblasts, 2 = dense accumulations of collagen between fibroblasts, and 3 = extensive separation of fibroblasts by collagen.
Because PSIS could not be definitively identified on H & E stains, we used phosphotungstic acid hematoxylin (PTAH) staining on 9 samples in an attempt to distinguish partially degraded PSIS from host fibrin strands. PTAH stains fibrin, nuceli, muscle fibers, keratin, and terminal bars blue, and collagen, reticular fibers, basement membranes, amyloid, cartilage, and thrombocytes are stained various shades of red.34 PTAH staining was not performed for 1 dog because insufficient tissue remained after the initial specimen was prepared for H & E staining.
Data analysis
The mean wound size was calculated at day 7. Percent total wound healing on days 14 and 21, percent epithelialization and contraction on day 21, laser Doppler perfusion values on days 7, 14, and 21, and days until complete wound healing were recorded. Variables from treated and control groups were compared for significant differences by use of a paired t test. Mean histopathologic scores for each category, for treated and control groups, were compared for significant differences by use of the Wilcoxon Signed Rank Test. Significance was set at P < .05.
Results
Subjective evaluation
All dogs were fully weight bearing on both hindlimbs within 12 hours of surgery. Few complications were noted. One dog was carrying the treated limb on day 23, but no cause for the lameness was identified, and lameness was no longer evident after the limb was re-bandaged. Fluid that accumulated under the PSIS of dog 8 on day 7 was expressed by gentle pressure; no further fluid accumulated. A purulent discharge occurred from the control wound of 1 dog on day 14. The wound was cleaned with 0.5% chlorhexidine solution, and the discharge resolved 2 days later. Because this infection appeared superficial and resolved within 2 days, the data from this dog were not discarded.
Five to 14 days after initial application, PSIS appeared to become incorporated into the wound bed of 8 dogs; an additional sheet of PSIS was added when >25% of the wound bed was not covered by PSIS. Only 1 dog required replacement of the original PSIS before incorporation into the wound. On day 2, the dog's bandages came off, and no PSIS remained, so it was replaced. The second application of PSIS remained in place for 7 days when at least 25% appeared to become incorporated into the wound; another sheet of PSIS was added at that time. Two had no PSIS added throughout the first 3 weeks. In both dogs, a thin, firm membrane formed on the wound surface. This membrane was removed on day 21. Although not evaluated histologically, this membrane grossly appeared to be a combination of dried wound exudate and dehydrated PSIS. Normal healing had occurred beneath the membrane, leaving a central area of granulation tissue without epithelial covering. In many instances where an additional sheet was laid or taped in place, migration occurred prior to PSIS incorporation into the wound resulting in frequent replacement of PSIS during the last 10 days of the study. All PSIS not incorporated into the wound was removed on day 21. PSIS was not removed at other times to avoid disturbing healing; however, this interfered with objective evaluation of granulation tissue production over bone.
Complete healing for both wound groups occurred within 25 to 48 days. Control and treated wounds healed within 2 days of each other in 8 dogs. There was no significant difference (P= .87) between days until complete healing for treated (mean, 33.8 ± 6.78 days) and control wounds (mean, 34.3 ± 7 days).
Planimetry
Wounds expanded immediately after creation. There was no significant difference in mean wound size between the control and treated wounds at day 7. Wound size ranged from 2.9 cm2 to 4.8 cm2 (mean, 3.7 cm2 for both groups). There was no significant difference (P= .17) in percent total wound healing at day 14 (Table 1; Fig 6). The amount of epithelialization was not measurable at day 14 in most dogs, therefore total wound healing was almost completely because of wound contraction. On day 21, there was no significant differences in percent total wound healing, contraction, or epithelialization between treated and control wounds (Table 1).
| Variable | Treated | Control | P Value |
|---|---|---|---|
| % Contraction (day 21) | 35.3 ± 11 | 35.2 ± 15 | .99 |
| % Epithelialization (day 21) | 50.1 ± 4.7 | 48.6 ± 12.2 | .7 |
| % Total wound healing (day 21) | 85.4 ± 6.6 | 83.8 ± 4.9 | .44 |
| % Total wound healing (day 14) | 24.8 ± 19.8 | 34.2 ± 23.2 | .17 |
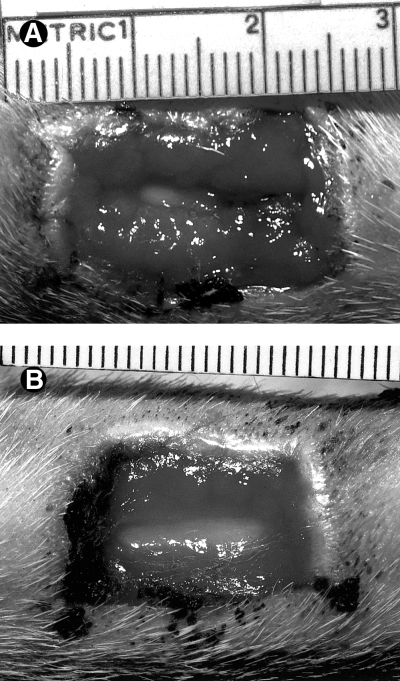
Treated wound (A) and control wound (B), day 10. PSIS no longer inhibits observation of the treated wound bed. There is no observable difference in wound size.
Laser doppler perfusion imaging
At day 7, laser Doppler perfusion imaging showed a significantly higher mean perfusion value (P < .001) of the control wounds compared with treated wounds; however, there were no significant differences between wound groups on days 14 and 21 (Table 2).
| Control | Treated | P Value | |
|---|---|---|---|
| Day 7 | 2.26 ± 0.60 | 0.855 ± 0.54 | <.001 |
| Day 14 | 1.67 ± 1.27 | 2.09 ± 0.98 | .29 |
| Day 21 | 2.85 ± 1.32 | 2.65 ± 1.2 | .47 |
Histopathologic evaluation
Insufficient tissue was collected from 2 dogs for inclusion in histopathologic scoring. One dog had insufficient tissue from both the control and treated wounds, and 1 dog had insufficient tissue from the control wound. For statistical analysis, the treated biopsy sample from this dog was also not included in the histopathologic scores.
On day 10, neutrophilic inflammation was observed in all samples; mean neutrophil score for both groups was 2.3. There were a few scattered plasma cells, lymphocytes, and macrophages, but no differences were noted between groups. There was a variable amount of fibrin and necrotic debris present in all specimens. The mean necrosis score for the treated (1.4) and control (1.7) groups was not significantly different. Similarly, the mean fibrin score for control (1.8) and treated (1.4) wounds were not significantly different.
Fibroblast proliferation appeared to be increased in all samples evaluated, with a mean score of 3 in both treated and control groups. Angiogenesis (control 1.3, treated 1.6; P= .25) and collagen production (control 1.5, treated 1.6) were mildly increased but not significantly different between groups. Most of the collagen appeared randomly oriented, but there were also areas of more mature collagen organized in parallel bundles (Fig 7).
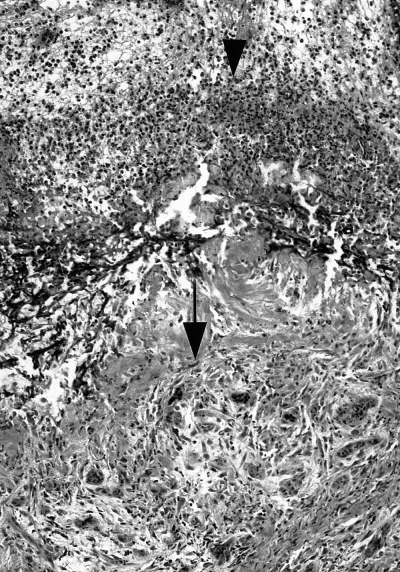
Control wound, day 10 (100× magnification, phosphotungstic acid hematoxylin [PTAH] stain). Neutrophils are present at the superficial wound edge (arrowhead). Granulation tissue (fibroblasts, collagen, and newly formed capillaries) are noted in the deep surface of the specimen (arrow).
Samples of rehydrated PSIS appeared as an acellular dense lamellar collagenous structure on both PTAH and H & E stains (Fig 8). When examined in biopsy samples, PSIS could not be definitively distinguished from host tissue in H & E or PTAH stains. However, there were 2 sections from treated wounds where linear acellular collagen structures could be identified. In 1 specimen, granulation tissue appeared to be growing between these structures (Fig 9); however, it is unknown whether this represented granulation tissue proliferation between strands of PSIS.
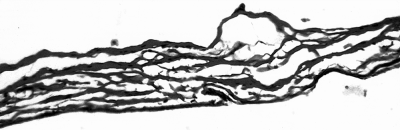
Rehydrated PSIS (100× magnification, PTAH stain) showing acellular linear strands of collagen.
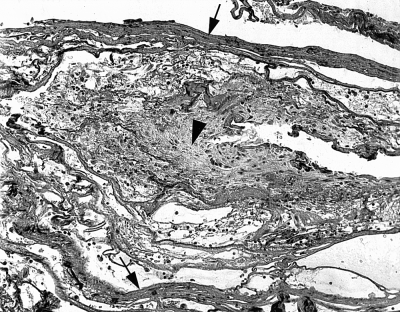
Treated wound at day 10 (100× magnification, PTAH stain). Acellular linear strands of collagen (arrows)are noted at the top and bottom of the field. Granulation tissue is present between these 2 areas of linear acellular collagen (arrowhead).
Discussion
All wounds healed completely within 48 days without the need for surgical interference or wound healing stimulants. PSIS appeared grossly to become gradually incorporated and degraded in the granulation tissue of the wound bed, but sheets of PSIS had a high tendency for migration when not sutured into the wound bed. No differences in total wound healing, contraction, or epithelialization between control wounds or wounds treated with PSIS were noted. Histopathologic examination showed a neutrophilic inflammatory response, fibroblast proliferation, and collagen production in all wounds typical of second intention wound healing, but there were no significant differences between treated and control wounds. Laser Doppler perfusion imaging showed higher values on day 7 for control wounds, but no differences were noted on days 14 and 21 between control and treated wounds.
Many nonautogenous materials placed into host tissue have the potential to increase the risk of infection by providing a surface for bacterial growth. In many studies in which PSIS has been used in various organs such as bladder, dura mater, stifle, vasculature, and skin, no increased incidence of infection has been noted.23–33 This may be partially because of its degradation by host tissue, so it likely does not provide immunologically privileged sites for bacterial growth. In fact, PSIS used as a biological dressing may help to decrease the risk of infection. In vitro research indicates that certain peptides contained within PSIS have bacteriostatic properties against Staphylococcus aureus and Escherichia coli.35 In our study, no PSIS-treated wounds developed clinically apparent infection, supporting the idea that PSIS does not lead to an increased risk for wound infection. Only 1 wound developed a superficial infection, and this was a control wound not treated with PSIS. Further in vivo studies would be needed to provide evidence that PSIS decreases the risk for wound infection.
As mentioned previously, PSIS has been used for bladder wall and vascular grafts23–28 because of its relative impermeability. This impermeability could be disadvantageous when treating wounds if drainage is inhibited and fluid accumulates between the wound bed and the PSIS. To facilitate drainage, we fenestrated the top sheet, and the layered sheets were secured to the wound with an interrupted pattern. On wounds where marked drainage is anticipated or if simple continuous sutures are used for stabilization, fenestration of all sheets may help to prevent discharge accumulation.
On gross inspection, the PSIS appeared to gradually be replaced by granulation tissue over 5 to 10 days in most dogs. Studies using PSIS in other organs have also shown that it is gradually replaced by host tissue.23–33 Previous work has demonstrated the ability of exogenous collagen matrices to serve as 3-dimensional templates for the ingrowth of fibroblasts and the production of new collagen.36 In in vitro studies, PSIS has supported the growth of various cells within and along the surface of the PSIS.37,38 As host tissue is produced, PSIS is gradually degraded. In 1 study, 14carbon-labeled PSIS placed in porcine bladder wall was 90% degraded over 2 months.39
The manufacturer recommends that PSIS be replaced if damaged. There are no specific recommendations for adding additional sheets as healing progresses and PSIS becomes incorporated or degenerates before complete healing. Suturing additional sheets of PSIS in place once the original sheets no longer cover the wound bed requires general anesthesia, which is time-consuming and not without risk to the patient. Therefore, we placed additional sheets without sutures hoping that PSIS would adhere to the underlying healing tissue; however, it was very difficult to keep PSIS in place without sutures.
Laser Doppler perfusion imaging (LDPI) was used to assess wound microcirculation on days 7, 14, and 21. We speculated that PSIS would stimulate increased neovascularization on wounds with exposed bone and would therefore lead to increased laser Doppler perfusion values.
The only significant difference in LDPI values occurred at day 7, when the LDPI value was significantly lower for the treated group. Whereas it is possible that the treated wounds had less blood flow at day 7, it is more likely that the PSIS inhibited penetration of the laser beam. This laser beam can penetrate a maximum depth of 1.5 mm but in most instances penetrates a depth of less than 1 mm.40–42 On days 7 and 14, most wounds were covered with PSIS that had not been sufficiently ingrown with microvasculature to result in markedly elevated LDPI readings. Therefore, we speculate that superficial unvascularized PSIS inhibited penetration of the laser beam into underlying granulation tissue, leading to falsely lowered LDPI values on days 7 and 14. At the onset of the study, we did not anticipate that intact superficial PSIS would cover granulation tissue. Instead we speculated that invasion would occur at all levels of the PSIS simultaneously, resulting in increased LDPI values compared with control wounds. However, it appeared that such ingrowth began from the depths of the wound and progressed to the superficial levels. On day 21, any unincorporated PSIS on the wound was removed before LDPI imaging and no significant differences in wound perfusion were detected between treated and control wounds.
Planimetry results indicated that both groups had almost identical mean wound size at day 0, so wound size should not have affected days to healing for either group. Percent wound contraction was not significantly different between treated and control wounds. These results are in contrast to those of Prevel et al where PSIS inhibited contraction, by approximately 23%, in full-thickness wounds in rodents.33 The reason for decreased wound contraction was not determined. Thus, the effect of PSIS on contraction is not completely understood at this time.
We found no significant difference in percent epithelialization on day 21, which is in agreement with Prevel et al.33 The manufacturer recommends that PSIS be placed under the skin edges when possible to allow for faster epithelialization over the membrane. We did not do this because the skin edges were sutured to the underlying tissue to maintain exposed bone in the center of the wound. PSIS did overlap the skin edges in some areas, which could have made it difficult for epithelialization to occur over the most superficial sheet.
There were no significant differences in total wound healing on days 14 or 21 between treated and control groups. We do not know whether PSIS migration that occurred when additional sheets were added later in the study had any detrimental effect on wound healing. We are unaware of any information concerning the effect that additional sheets might have once the original PSIS is incorporated into the wound bed. Studies evaluating other wound- healing stimulants have shown that the greatest effect on wound healing was noted early in the wound healing process (first 10 to 15 days).43 Thus, the benefit of keeping PSIS on the wound bed after the early stages of healing is unknown.
Average wound size in our study was 3.7 cm2. We limited wound size for humane reasons because we created 4 wounds on each dog. It is possible that larger wounds with a greater area of exposed bone may have shown a greater difference between healing in treated and control wounds. However, contraction, epithelialization, and time until complete wound healing were essentially the same for treated and control wounds, with no trend to indicate that PSIS would show a greater effect on larger wounds.
Our microscopic observations were similar to those reported for full-thickness wounds in rodents.33 On day 10, a moderate inflammatory response consisting of mainly neutrophils was evident. No specific or marked inflammatory response was observed in wounds treated with PSIS. The lack of a foreign body reaction to PSIS has been consistent in many studies involving multiple species23–33; however, the reason for this is not completely understood. It has been shown that PSIS induces a TH2 immune response without a TH1 immune response. TH2 cells are T-helper cells that secrete cytokines that stimulate B cell proliferation and immuoglobulin secretion but are not cytotoxic and do not result in a delayed hypersensitivity reaction. TH1 cells are T-helper cells that may be cytotoxic and also secrete cytokines responsible for delayed hypersensitivity reactions. However, recent research has shown that even in an experimental environment of cytokines favoring a TH1 response, no rejection was noted.44 There is also evidence that certain peptides in PSIS induce activation-induced cell death in certain human helper T cells.45 It is unknown why this occurs, and its relation to the lack of rejection noted with PSIS is unclear.
PSIS has been used for healing in many different tissues. Our study showed that PSIS had no apparent adverse effects on clean wounds with exposed bone on the distal aspect of dog limbs. Objective data from this study showed no differences in healing between control wounds and wounds treated with PSIS.
Acknowledgment
The authors thank Kersten Johnson and Sherri Hinkle for their technical assistance. All small intestinal submucosa supplied by Cook Veterinary Products, Bloomington, IN.




