Subpopulations of Cryptocephalus beetles (Coleoptera: Chrysomelidae): geographically close but genetically far
Abstract
Abstract. The leaf beetles Cryptocephalus coryli, C. decemmaculatus and C. nitidulus are of conservation concern and are included on the UK Biodiversity Action Plan. The distinctiveness of the disjunct remaining populations of these beetles was compared to that of more continuously distributed Cryptocephalus species. This was carried out with a view to defining evolutionary significant units (ESUs) in the rare species. A portion of the cytochrome b gene, an intergenic spacer and partial tRNA was analysed from 93 specimens of Cryptocephalus beetle (Coleoptera: Chrysomelidae). Considerable sequence divergence was apparent in all the species, even at an intersite scale when the distances between sampled localities were very small (< 1 km). Intrapopulation, intersite and interpopulation divergence observed in the rare species was reflected in the species that have a more continuous distribution, implying that dispersal ability in these species is poor and gene flow can be impeded by relatively trivial barriers to dispersal. The evidence suggests that the disjunct populations of the rare Cryptocephalus species can, tentatively, be considered as ESUs. This has important implications for management strategies and reintroductions.
INTRODUCTION
Determining the extent of genetic differentiation among extant populations is a fundamental facet of conserving rare species. Conservation programmes are conducted at the population level (Goldstein et al., 2000) and it is often the assumption that these populations must be diagnosibly distinct for protection to be justified (Goldstein et al., 2000). The analysis of intraspecific genetic variation in a rare species may lead to the identification of genetically distinct extant populations (Richter et al., 1994; Travis et al., 1996). This genetic distinctiveness implies limited gene flow between populations and possible reproductive isolation (Soltis & Gitzendammer, 1999). Molecular systematics allows, in part, the identification of evolutionary significant units (ESUs) (Ryder, 1986; Woodruff, 1989; Amato, 1991; Vogler & DeSalle, 1994). An ESU is a population unit that merits separate management and has a high priority for conservation (Ryder, 1986). Recognition of these ESUs enables conservation efforts to be concentrated on populations that may be in need of special protection, and also allows the formulation of management strategies that are tailored for particular populations or groups of populations (Moritz, 1994; Haig, 1998; Soltis & Gitzendammer, 1999; Johnson & Jordan, 2000).
An understanding of the genetic differentiation within rare species is also important if reintroductions are to be attempted. The mixing of individuals from a divergent population with another population could result in the adaptive potential and unique evolutionary trajectory of the sink population being impaired. These translocations could result in introgression and its associated homogenizing effects (Johnson & Jordan, 2000). Many of the rare insects on the Biodiversity Action Plan (BAP) will need to be introduced to sites in order to meet the plan objectives. No reintroductions should be carried out in the absence of studies to identify divergent populations.
In this study intraspecific divergence in eight species of Cryptocephalus Muller (Chrysomelidae) beetle were examined. The genus has some 1500 species worldwide (Erber, 1988) and 19 of these are found in the United Kingdom. Three of the species in this study are listed on the UK Biodiversity Action Plan (UKBDG, 1999). C. coryli Linnaeus and C. nitidulus Fabricius are classed as endangered and C. decemmaculatus Linnaeus is vulnerable (Hyman & Parsons, 1992). C. coryli and C. nitidulus have shown a large decline in the United Kingdom over the past 50 years (Fig. 1a,c) (all distribution data from M. Cox, pers. comm.) (Hyman & Parsons, 1992). C. decemmaculatus has also exhibited a decline (Fig. 1b) but has never been a widespread species (Stott, 1929; Allen, 1970). The BAP targets for the three species above all include reintroductions as a means of establishing populations in the historic range of each. Typically, five populations have to be reintroduced by 2005. The other five species used in the study (C. bipunctatus Linnaeus, C. hypochaeridis Suffrian, C. labiatus Linnaeus, C. moraei Linnaeus and C. parvulus Muller) are more widespread in the United Kingdom (Fig. 2a–d).
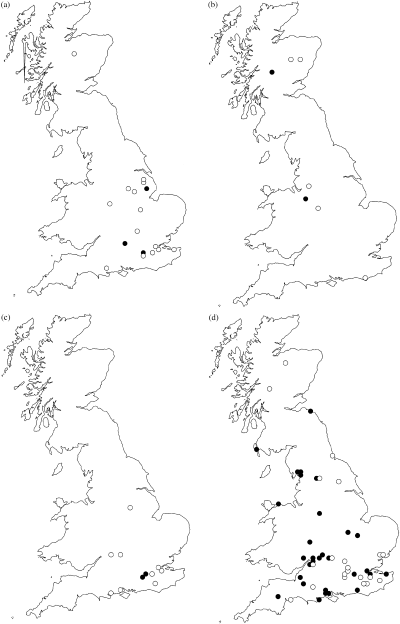
Past and present distribution of Cryptocephalus species (filled circles = extant populations; open circles = populations presumed extinct). (a) C. coryli; (b) C. decemmaculatus; (c) C. nitidulus; (d) C. bipunctatus. All maps produced using dmap (©Alan Morton).
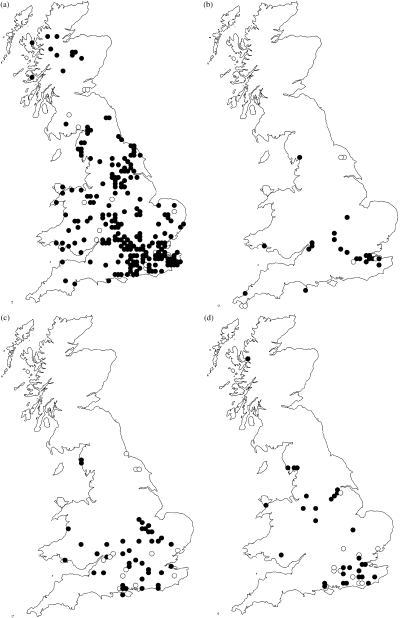
Past and present distribution of Cryptocephalus species (filled circles = extant populations; open circles = populations presumed extinct). (a) C. labiatus; (b) C. hypochaeridis; (c) C. moraei; (d) C. parvulus. All maps produced using dmap (©Alan Morton).
All the British species are widely distributed through the Western Palearctic and beyond (Warchalowski, 1991). Of the above species, all except C. moraei are essentially oligophagous. Host plants of the more catholic species include Betula spp., Corylus avellana and Crateagus monogyna, Hieracium spp. and Helianthemum nummularium while C. moraei only feeds on Hypericum perforatum.
The objective of our study was to examine the mtDNA variation between remaining disjunct populations of the three species of conservation concern (Cryptocephalus coryli, C. nitidulus and C. decemmaculatus). Comparisons were also made with the more widespread and common species. These exhibit a more continuous distribution, but were sampled from populations separated by, in most cases, distances similar to the rare species. Thus, we address the question of whether genetic distinctiveness within a species that exhibits a disjunct distribution is similar to that observed within a more continuously distributed species. This comparison and sampling strategy enables inferences to be made regarding the processes that are responsible for any observed interpopulation differences. Ecologically significant characters such as ecological requirements and demographic characteristics (Crandall et al., 2000) are also considered as a compound means of identifying ESUs. Using this information in combination with genetic differences should allow informed recommendations to be made regarding the management and reintroduction of populations of the endangered and vulnerable Cryptocephalus species.
MATERIALS AND METHODS
Field sampling
Adults, eggs or larvae of eight species of Cryptocephalus beetle were collected during June 2000 from several localities in England and Scotland (Fig. 3 and Table 1). Samples were collected in such a manner that intrapopulation, subpopulation (i.e. specimens from discrete populations separated by only trivial distances) and interpopulation differentiation could be assessed. The more common species were sampled from populations separated by distances comparable to the distances between the extant populations of the rare species. Unfortunately, populations of a common Cryptocephalus species separated by distances similar to the distance between the sampled C. decemmaculatus populations were not located. Wherever possible eggs of C. coryli, C. decemmaculatus and C. nitidulus laid by gravid females held captive in the field were collected instead of adults to lessen the effect of sampling pressure on these sensitive populations. In these instances, six–10 eggs were taken from five females from each site sampled. No siblings were used in the study. For the more common species, adults were taken from each population. They were taken back to the laboratory alive and stored at −80 °C before processing.
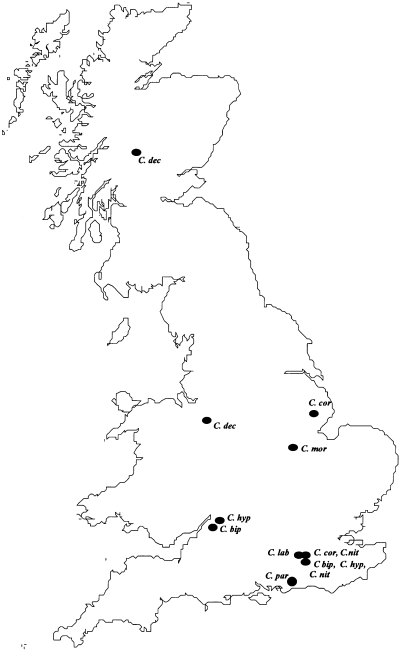
Localities for collections of eight Cryptocephalus species (C. bip = Cryptocephalus bipunctatus; C. cor = C. coryli; C. dec = C. decemmaculatus; C. hyp = C. hypochaeridis; C. lab = C. labiatus; C. mor= C. moraei; C. nit = C. nitidulus and C. par = C. parvulus).
| Species | Collection sites | Grid Ref | Individuals sampled |
|---|---|---|---|
| C. coryli | Kirby Moor (Lincs.) A | TF234623 | 5 |
| Kirkby Moor (Lincs.) B | TF227636 | 5 | |
| Headley Warren (Surrey) A | TQ190539 | 5 | |
| Headley Warren (Surrey) B | TQ189540 | 5 | |
| Wybunbury Moss (Cheshire) A | SJ698502 | 5 | |
| C. decemmaculatus | Wybunbury Moss (Cheshire) B | SJ696503 | 5 |
| Camghouran (Perthshire) | NN595563 | 5 | |
| C. nitidulus | White Downs (Surrey) A | TQ125496 | 5 |
| Headley Warren (Surrey) C | TQ193533 | 5 | |
| Headley Warren (Surrey) A | TQ190539 | 3 | |
| C. bipunctatus | Stinchcombe Hill (Gloucs.) A | ST738981 | 3 |
| Stinchcombe Hill (Gloucs.) B | ST734986 | 3 | |
| White Downs (Surrey) B | TQ116488 | 3 | |
| C. hypochaeridis | Ranmore Common (Surrey) A | TQ135500 | 3 |
| Ranmore Common (Surrey) B | TQ139505 | 3 | |
| Rodborough Common (Gloucs.) | SO8402 | 3 | |
| Wisley Common (Surrey) | TQ0658 | 3 | |
| C. labiatus | Wybunbury Moss (Cheshire) A | SJ698502 | 3 |
| Wybunbury Moss (Cheshire) B | SJ696503 | 3 | |
| C. moraei | White Downs (Surrey) C | TQ116488 | 3 |
| White Downs (Surrey) D | TQ114482 | 3 | |
| Mokery Wood (Lincs.) | SK950185 | 3 | |
| C. parvulus | Lavington Common (W. Sussex) | SU951192 | 3 |
| Wybunbury Moss (Cheshire) A | SJ698502 | 3 | |
| Wybunbury Moss (Cheshire) B | SJ696503 | 3 |
DNA extraction, PCR and sequencing
DNA for subsequent amplification was extracted from an egg, adult head, thorax or legs. The DNA was extracted using standard protocols (Juan et al., 1995). The mitochondrial cytochrome b gene was amplified using PCR. The total volume of the reaction mixtures was 25 µL. Amplification used the primers described by Simon et al. (1994): CB-N-10920, 5′-TATGTTTACCTTGAGGACAAATATC-3′ and N1-N-11841, 5′- ATCATAACGAAACCGAGGTAATGTACC-3′. The PCR mix contained: 14.9 µL H20, 2.5 µL Taq buffer, 3 µL MgCl2, 0.1 µL (0.5 units) Taq polymerase, 0.25 µL dNTPs (20 mm), 1.75 µL (10 pm/µL) of each primer, 0.25 µL BSA and 2 µL of template (Taq buffer, MgCl2 and Taq polymerase all Promega). Temperature cycling was performed using a Biometra Trioblock. Cycling involved an initial denaturation of 92 °C for 5 min then 36 cycles of denaturation at 92 °C for 1 min, annealing at 50 °C for 1 min 30 s and extension at 72 °C for 1 min 30 s. A final elongation step of 72 °C for 10 min completed the DNA amplification. PCRs were checked by electrophoresis in 1% agarose gel containing ethidium bromide. The length of the amplified region was compared to the bands on a ‘100 base — pair ladder’ (Promega, Düren, Germany).
PCR products were purified using a clean-up kit (Macherey–Nagel, Madison, USA). The amplified regions were then sequenced using an automated sequencer (ABI 377 sequencer, Perkin Elmer, University of Oxford sequencing facility) with the amplification primer N1-N-11841.
Data analysis
Ninety-three cytochrome b sequences were obtained. These were edited in BioEdit (Hall, 1999) and then aligned using the Clustal W multiple alignment program (Thompson et al., 1994 in BioEdit). DNA–DNA distance matrices were then produced (Felsenstein, 1993) to examine the level of genetic differentiation present. Each species was analysed separately so that interpopulation, subpopulation and intrapopulation differentiation could be examined. In order to assess the relationship of genetic differentiation with geographical distance linear distances were calculated between the sampled sites of the eight species. Population genetic structure was analysed using an analysis of molecular variance (amova in arlequin 2.0; Schneider et al., 2000). The relationship between unique haplotypes was described using a minimum spanning network with the sequences as nodes of a tree instead of the terminal tips of a tree. Networks are useful when many of the sequences may be derived from the same ancestral genotype. The algorithm used was minspnet, a program within arlequin 2.0 (Schneider et al., 2000).
RESULTS
The mitochondrial cytochrome b sequences obtained (≈ 550 base pairs (bp) long) for the eight species of Cryptocephalus beetle yielded interesting intraspecific variation. This variation consisted of point mutations most of which were present in an intergenic spacer (27–36 bp long) between the end of the cytochrome b gene and the serine tRNA. Sequences can be obtained from GenBank with the accession codes AF536566–AF536658.
The open circles in 4-12 represent individuals. Genetic differentiation between these individuals was high and all were separated by numerous substitutions. The branch lengths in the networks are proportional to the number of substitutions. The reason for these large differences is that the sequenced mitochondrial cytochrome b fragment encompassed a very variable intergenic spacer. Clear distinctions of subpopulations could be seen in most of the species, but there were exceptions. For example, the Cryptocephalus nitidulus population in the Flying Bomb Field of Headley Warren is related more closely to the population sampled on the White Downs than the other Headley Warren population in the Downs Field (Fig. 5). The pattern of differentiation between the C. coryli populations suggests that the gene flow between the Kirby Moor and Ostler's plantation populations is extremely limited. Long branch lengths between geographically proximal populations suggest that the homogenizing effects of migration are nonexistent or occur very rarely. For many of the species the branch lengths at the subpopulation level were large, in some cases as large as the branch lengths at the interpopulation level. Many of the species also exhibited abundant substitutions at the intrapopulation level suggesting that even at this scale gene flow is low. It is possible that the branch lengths observed in the widespread species may also be due to the small sample size. For the species with small sample sizes and long branch lengths at the intrapopulation level (C. bipunctatus, C. hypochaeridis and C. parvulus) it is possible that larger samples would have yielded closely linked haplotypic clusters such as those seen in C. decemmaculatus (Fig. 6), C. labiatus (Fig. 9) and C. moraei (Fig. 10).

Minimum spanning haplotype network for Cryptocephalus coryli. D = Downs Field, S = Stainton's Field (both at Headley Warren, Surrey. K = Kirkby Moor Lake, O = Ostlers Plantation (both at Kirkby Moor, Lincs). Open circles = individuals. Branch values proportional to no. of substitutions.
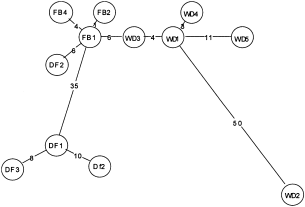
Minimum spanning haplotype network for C. nitidulus. FB = Flying Bomb Field, DF = Downs Field (both in Headley Warren, Surrey). WD = White Downs (Surrey).
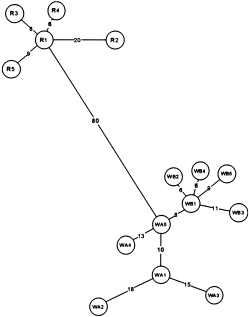
Minimum spanning haplotype network for C. decemmaculatus. R = Camghouran, Perthshire. WA = Wybunbury Moss A, WB = Wybunbury Moss B (both in Cheshire).
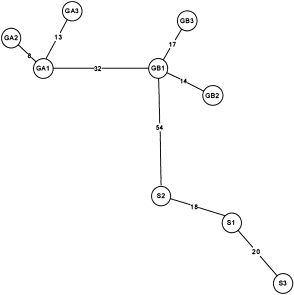
Minimum spanning haplotype network for C. bipunctatus. GA = Stinchcombe Hill A, GB = Stinchcombe Hill B (Both in Gloucs.). S = White Downs (Surrey).

Minimum spanning haplotype network for C. hypochaeridis. SA = Ranmore Common A, SB = Ranmore Common B (both in Surrey). G = Rodborough Common (Gloucs.).
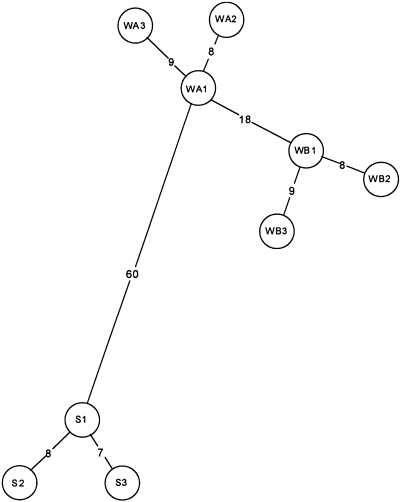
Minimum spanning haplotype network for C. labiatus. WA = Wybunbury Moss A, WB = Wybunbury Moss B (both in Cheshire). S = Wisley Common (Surrey).

Minimum spanning haplotype network for C. moraei. SA = White Downs A, SB = White Downs B (both in Surrey). L = Mokery Wood (Lincs.).
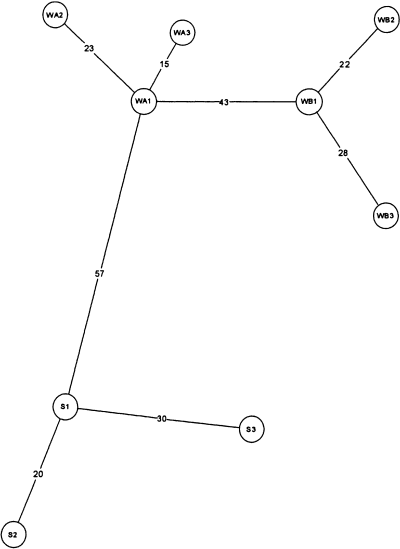
Minimum spanning haplotype network for C. parvulus. WA = Wybunbury Moss A, WB = Wybunbury Moss B (both in Cheshire). S = Lavington Common (W. Sussex).
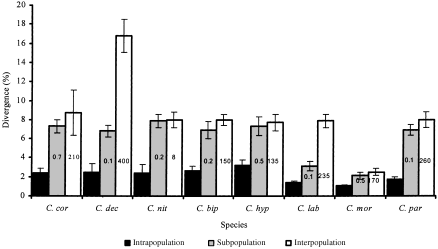
Intrapopulation, subpopulation and interpopulation divergences in eight species of Cryptocephalus beetle (mean ± SD). The first three species have few UK sites, the remaining species are more widespread. Values within columns = distances between subpopulations and interpopulation geographical distances.
Mean intrapopulation sequence divergence ranged from 1.1% to 3.2% (Fig. 12). The lowest intrapopulation divergences were seen in C. moraei, C. labiatus and C. parvulus. The three species of conservation concern all had similar intrapopulation divergences. Interpopulation divergence within all of the studied species, except in C. labiatus and C. decemmaculatus was almost as great as subpopulation divergence. Subpopulation divergence and interpopulation divergence within C. moraei were also very similar to one another; but both were only marginally higher than intrapopulation divergence. The only other species with a similarly low level of subpopulation divergence was C. labiatus. Apart from C. moraei and C. decemmaculatus the amount of interpopulation divergence observed in this study was relatively consistent. The sampled populations of C. decemmaculatus exhibited a high level of divergence compared with that seen in the other species, but they were also separated by the greatest distance of any of the populations sampled in this study.
Of the total genetic diversity identified in Cryptocephalus coryli more than half (54.6%) can be attributed to variation at the subpopulation level (Table 2). Most variation (91.2%) observed within C. nitidulus was at the same level (Table 2). The negative value obtained for C. nitidulus reflects that this statistic is actually a covariance and negative values can occur when the actual values are close to zero. Most variation (71.9%) within C. decemmaculatus was seen at the interpopulation level. With C. bipunctatus variation was similarly partitioned at the subpopulation and intrapopulation levels (45.9 and 44.4%, respectively). Variation within C. hypochaeridis was spread almost equally over the three levels; 62.8% of the variation observed within C. labiatus was attributable to the variation at the interpopulation level, which was also the case for C. moraei (41.7%). Most variation within C. parvulus was attributable to the variation at the subpopulation level.
| Species | Level | d.f. | Sums of squares | Variance components | % variance |
|---|---|---|---|---|---|
| C. coryli | Interpopulation | 1 | 304.7 | 12.1 | 19.7 |
| Subpopulation | 2 | 366.9 | 33.5 | 54.6 | |
| Intrapopulation | 16 | 252.4 | 15.8 | 25.7 | |
| C. nitidulus | Interpopulation | 1 | 43.7 | −6.7 | −37.7 |
| Subpopulation | 1 | 69.5 | 16.3 | 91.2 | |
| Intrapopulation | 10 | 83.2 | 8.3 | 46.5 | |
| C. decemmaculatus | Interpopulation | 1 | 261.2 | 34.4 | 71.9 |
| Subpopulation | 1 | 31.9 | 4.6 | 9.7 | |
| Intrapopulation | 12 | 105.4 | 8.8 | 18.4 | |
| C. bipunctatus | Interpopulation | 1 | 101.4 | 4.4 | 9.7 |
| Subpopulation | 1 | 83.7 | 21.1 | 45.9 | |
| Intrapopulation | 6 | 122.3 | 20.4 | 44.4 | |
| C. hypochaeridis | Interpopulation | 1 | 166.4 | 21.2 | 34.5 |
| Subpopulation | 1 | 81.7 | 20.7 | 33.6 | |
| Intrapopulation | 6 | 118.0 | 19.7 | 32.0 | |
| C. labiatus | Interpopulation | 1 | 128.9 | 24.2 | 62.8 |
| Subpopulation | 1 | 32.2 | 8.9 | 23.1 | |
| Intrapopulation | 6 | 32.7 | 5.4 | 14.1 | |
| C. moraei | Interpopulation | 1 | 80.6 | 11.4 | 41.7 |
| Subpopulation | 1 | 35.2 | 9.6 | 35.4 | |
| Intrapopulation | 6 | 37.3 | 6.2 | 22.8 | |
| C. parvulus | Interpopulation | 1 | 97.4 | 5.2 | 9.9 |
| Subpopulation | 1 | 79.3 | 17.3 | 49.8 | |
| Intrapopulation | 6 | 101.3 | 23.6 | 40.3 |
DISCUSSION
The levels of divergence encountered in the Cryptocephalus cytochrome b gene were of approximately the same magnitude as comparable mitochondrial genetic elements studied in other insect species (Vogler & DeSalle, 1994; Vogler et al., 1998; Diogo et al., 1999; Sperling et al., 1999; Gomez-Zurita et al., 2000; Szalanski et al., 2000) and in very different taxa sampled from disjunct locations (Johnson, 2000). Although many of these studies have made use of COI or COII, what comparative information does exist suggests that most of the protein coding regions of the mitochondrial genome perform equally well at resolving relationships at varying levels of acuity (Caterino et al., 2000).
The dispersal ability in C. decemmaculatus is extremely poor. Its Wybunbury Moss site contains three discrete populations that have shown no detectable interchange over 2 years of mark–release recapture studies. Some other insect species that exist in subdivided habitats have also been shown to be rather sedentary (Arnold, 1983; Thomas, 1985; Warren, 1987; Legge et al., 1996; Doak, 2000). It appears that species such as these are demographically isolated even when the barriers separating populations are nothing more than relatively small areas of unsuitable habitat (Doak, 2000). Headley Warren, the site in Surrey that was sampled for C. coryli and C. nitidulus, has four distinct Downland fields separated by bands of mature woodland, which can be up to 30 m thick and 20 m tall. C. coryli populations that are separated by apparently trivial distances exhibit considerable levels of sequence divergence. A distance of < 1 km between populations can be as effective at impeding gene flow as much greater distances (> 200 km). This implies that gene flow between geographically close populations may be very limited. Based on the genetic results and inferences made from C. decemmaculatus dispersal it seems as though C. coryli, at the Surrey site has four distinct populations. Other studies have shown that the males of a sedentary species are often far more vagile than the females. This means that populations may be demographically but not genetically separated (Doak, 2000). However, dispersal studies undertaken on C. decemmaculatus have shown there to be no significant difference between male and female dispersal ability. Subpopulations of Cryptocephalus beetles in subdivided habitats may be demographically and genetically isolated.
A population should not be labelled as an ESU based on molecular information alone. Populations of the saturnid moth Cryan's Hemileuca were labelled as evolutionary significant units even though genetic differentiation between this and other Hemileuca species was not found (Legge, 1996). ESU status was applied on the basis of an ecological character, namely the host choice of Cryan's Hemileuca. A difference in an ecological character, such as life history traits, ecological requirements, morphologies or demographic characteristics, may mean that two populations are not ecologically exchangeable (Crandall et al. 2000). This exchangeablility must arise from shared fundamental properties of the populations in question (Templeton, 1994). Of the Crytocephalus species studied here, interpopulation ecological differences consist of subtle host plant and phenological differences. The former is most easily seen in C. coryli where adults have a choice of Betula pendula, Corylus avellana and Crateagus monogyna in the Surrey population, whereas adults of the Lincolnshire population only have the choice of B. pendula and C. monogyna plants. The differences between potential host availability among sites may result in underlying genetic changes initiating in divergence away from the ancestral genotype. Flight period differences can be seen between the disjunct populations of C. decemmaculatus. Eclosion of adults in the Scottish population takes place up to 1 month later than adults in the Cheshire population. This species also exhibits a melanic form (C. decemmaculatus var. bothnicus) that is found at frequencies of up to 50% at the Scottish site (I. Menzies, pers. comm.) and less than 3% at the Cheshire site (pers. obs.). This pattern of increased frequency of melanism with increasing latitude has been shown in other beetle species (de Jong et al., 1998; Majerus & Zakharov, 2000). The dark surface of melanic morphs gives them a thermal advantage over nonmelanics under conditions where (reproductive) activity is limited by the absorption of solar radiation (de Jong et al., 1998). C. bipunctatus also shows a clear ecological interpopulation difference. Gloucestershire populations of this species feed on H. nummularium whereas the host plant of the Surrey populations is B. pendula or C. avellana.
It is hoped that with further work the reasons behind the disjunct distributions of the rare Cryptocephalus species will be understood. Comparative material from Scandinavia and northern France would have to be collected. Currently, two hypotheses exist to explain range contractions leading to the present distributions of rare species. The demographic hypothesis predicts that ranges should implode with final populations persisting near the centre(s) of the historical range (Brown, 1995; Mehlman, 1996; Wolf et al., 1996). The crux of the contagion hypothesis is that populations last impacted by an extinction force persist the longest, i.e. the last populations persist at the periphery of the historical range (Towns & Daugherty, 1994; Lomolino & Channell, 1995, 1998; Channell & Lomolino, 2000). Both of these theories can explain the present disjunct populations of the rare Cryptocephalus species studied here. However, in order to address fully the processes responsible for producing a disjunct distribution postglacial colonization histories have to be considered.
C. moraei and C. labiatus exhibited levels of divergence that are difficult to explain satisfactorily. The increased level of homogeneity observed in the sequences from these species suggests a greater degree of subpopulation and interpopulation gene flow in C. moraei and greater subpopulation gene flow in C. labiatus than that observed in the other species. Higher levels of dispersal can prevent selective differentiation among populations resulting in lower levels of total genetic variance (Whitlock 2001). C. moraei is the only monophagous species and its host-plant (Hypericum perforatum) may be more continuous in space and time than the very ephemeral scrub transition habitat or pristine calcareous grassland occupied by the other species in this study. The relative ubiquitous distribution of this species’ host-plant could entail that gene flow is maintained even over large distances. C. labiatus is the most common of the species but occurs in a scrub transition habitat. Low subpopulation divergence in this species suggests gene flow at this scale but the divergence at the interpopulation level is of the same magnitude as the other studied Cryptocephalus species and much higher than C. moraei. Interestingly, only C. moraei and C. labiatus are caught routinely in flight interception traps at Headley Warren, where C. coryli and C. nitidulus also occur (G. Collins, pers. comm.). This suggests that these two species may be more vagile (either actively or passively) than the other studied Cryptocephalus beetles.
This study has highlighted some of the genetic differences that exist between disjunct Cryptocephalus populations. Further, finer-scale sampling of the threatened species would enable the population structure of these species to be resolved at an even smaller scale. For example, C. nitidulus occurs in many isolated populations along approximately 3 km of the White Downs in Surrey. Each population is separated from the next by unsuitable habitat (mature tracts of woodland). Examination of the populations along the whole length of the White Downs would surely yield interesting results. Further research may also enable hypotheses to be formulated regarding the range contraction of the species in question, which is also of fundamental importance in conservation strategies (Simberloff, 1986). Understanding how and why their ranges have contracted may also enable suggestions to be made of which areas to survey for undiscovered populations of the rare Crytocephalus species and the planning of reintroductions (Lomolino & Channell, 1995, 1998).
The level of divergence observed in this study between geographically close populations is reflective of the findings of other studies. Other phytophagous beetle species have been shown to exhibit considerable population structure even at a microgeographical scale (McCauley et al., 1988; McCauley, 1991; Rank, 1992). The results of this study suggest that the populations within the disjunct sites for C. coryli, C. decemmaculatus and C. nitidulus are genetically distinct. Subtle ecological differences also exist although more study is needed in this area. On the basis of these results these disjunct populations can be tentatively termed ESUs. These populations, and their discrete nature warrant their separate management for the maintenance of genetic diversity within the relevant species. Reintroductions of these species should also take into account the presence of distinct lineages even within populations. Reintroduction programmes should aim to maintain this genetic diversity. Introgression, with its homogenizing effects, could arise as a result of these translocations (Johnson & Jordan, 2000). Individuals from a divergent population translocated to another population could severely impede the adaptive potential and unique evolutionary trajectory of the sink population.
The distinctiveness of all the populations of most the populations studied, even at an subpopulation scale suggest that relatively trivial barriers to dispersal, i.e. unsuitable habitat can limit gene flow, effectively, reproductively isolating populations. Further ecological comparisons of the Cryptocephalus populations in question would enable a more robust application of the ESU concept. Some authors have argued that historic levels of gene flow between populations should be maintained (Crandall et al., 2000) but this is a practical impossibility when the organism in question not only depends on an acutely ephemeral habitat, but is also a very poor coloniser. In this situation the only viable means of protection is to maintain the habitats of the extant populations in a manner conducive to their survival and to conduct reintroductions in a manner that preserves genetic variability.
Discrete groups should be managed separately. Failure to recognize discrete populations within rare species as distinct groups can lead to the loss of biodiversity, especially where distinct subpopulations are allowed to go extinct (May, 1990). The findings of this study have implications for the management of the threatened species and for any attempts at translocations and reintroductions.
ACKNOWLEDGMENTS
Many thanks to Peter Hodge for collecting specimens. Thanks also to Cathy Walton and Goldie Bell for reading earlier versions of this manuscript. This research was funded by the Species Recovery Programme (English Nature).




