Rapid transferrin efflux from brain to blood across the blood–brain barrier
Abstract
The brain efflux index method is used to examine the extent to which transferrin effluxes from brain to blood across the blood–brain barrier (BBB) following intracerebral injection. Whereas high-molecular-weight dextran is nearly 100% retained in brain for up to 90 min after intracerebral injection in the Par2 region of the parietal cortex of brain, there is rapid efflux of transferrin from brain to blood across the BBB. The efflux of apotransferrin is 3.5-fold faster than the efflux of holo-transferrin. The brain to blood efflux of apotransferrin is completely saturable by unlabeled transferrin, but is not inhibited by other plasma proteins. These studies provide evidence for reverse transcytosis of transferrin from brain to blood across the BBB. As circulating transferrin is known to undergo transcytosis across the BBB in the blood-to-brain direction, these studies support the model of bidirectional transcytosis of transferrin through the BBB in vivo.
Abbreviations used
-
- BBB
-
- blood–brain barrier
-
- CSF
-
- cerebrospinal fluid
-
- IgG
-
- immunoglobulin G
-
- TCA
-
- trichloroacetic acid
-
- Tf
-
- transferrin
-
- TfR
-
- transferrin receptor
There are two models of transferrin (Tf) transport between blood and brain. The transcytosis model proposes that circulating Tf undergoes receptor-mediated endocytosis into the brain capillary endothelial cell, which forms the blood–brain barrier (BBB) in vivo, via the endothelial transferrin receptor (TfR) (Fishman et al. 1987; Pardridge et al. 1987). The holo-transferrin then undergoes exocytosis into brain interstitial fluid followed by receptor-mediated endocytosis into brain cells via the TfR expressed at brain-cell plasma membranes. Inside brain cells, the iron is separated from the holo-transferrin, and the apo-transferrin undergoes retro-endocytosis back to brain interstitial fluid followed by ‘reverse transcytosis’ of the apo-transferrin from brain interstitial fluid back to blood via transport across the brain capillary endothelial cell. Conversely, the retro-endocytosis proposes that circulating holo-transferrin undergoes receptor-mediated endocytosis into the brain capillary endothelial cell, and the iron is separated from the transferrin within the endothelial compartment (Taylor et al. 1991; Morris et al. 1992; Roberts et al. 1993; Bradbury 1997). The apo-transferrin then undergoes immediate retro-endocytosis back to blood across the luminal membrane of the brain capillary endothelial cell, and the iron moves from the intra-endothelial compartment to the intracellular compartment of brain cells by unknown mechanisms.
The transcytosis model requires that the TfR is present on the abluminal membrane of the brain capillary endothelial cell in order to mediate reverse-transcytosis of the apo-transferrin from brain to blood. Confocal microscopy studies with isolated rat brain capillaries have demonstrated that the TfR is present on both the luminal and abluminal membranes on the brain capillary endothelial cell (Huwyler and Pardridge 1998). However, to date, there have been no experiments reported that examine the extent to which transferrin may move from the brain compartment to blood via reverse-transcytosis across the BBB. Transferrin undergoes receptor-mediated transcytosis from the blood to tissue compartment in both bone marrow (Soda and Tavassoli 1984) and brain (Skarlatos et al. 1995). Therefore, if transferrin undergoes reverse-transcytosis from brain to blood, then the BBB transferrin transcytosis pathway is bidirectional. Although bidirectional transferrin transcytosis has been demonstrated in cell-culture models (Cerneus et al. 1993), this process has not, to date, been demonstrated at the BBB in vivo.
The kinetics of transferrin efflux from brain to blood across the BBB may be quantitated with the brain efflux index method (Kakee et al. 1996). The present studies examine the kinetics of export from brain to blood of [125I]holo- or [125I]apo-rat transferrin.
Materials and methods
Materials
Sodium iodine ([125I]; 17.4 Ci/mg) and dextran ([3H]; 182 mCi/g) were purchased from Amersham Pharmacia Biotech (Arlington Heights, IL, USA). The dextran had a molecular weight of 50 000–90 000 Da (mean 70 000 Da). Rat apo-transferrin was purchased from the Cappel Division of ICN Pharmaceuticals (Aurora, OH, USA). Mouse IgG2a (κ) UPC 10 myeloma ascites was purchased from Organon Teknika (Durham, NC, USA) and the mouse IgG2a was purified by Protein G sepharose affinity chromatography as described previously (Yoshikawa and Pardridge 1992). Chloramine T and all other reagents were obtained from Sigma Chemical Company (St Louis, MO, USA).
Transferrin radioiodination
The apo- or holo-transferrin was radio-iodinated with [125I]-iodine and chloramine T as described previously (Skarlatos et al. 1995), to a specific activity of 4.0 μCi/μg and a trichloroacetic acid (TCA) precipitability of 98–99%.
Iron loading of transferrin
The rat apo-transferrin (1 mg/mL) was mixed with an equal volume of 1 mg/mL ferric ammonium citrate in 0.01 m NaHCO3 at room temperature (23°C) for 4 h. The free iron was then removed by dialysis overnight at 4°C against PBS (pH = 7.4) using 12 000-Da molecular weight cut-off dialysis tubing, where PBS = 0.01 m NaH2PO4/0.15 m NaCl. The concentration of the holo-transferrin was measured by absorbance at 454 nm using an extinction coefficient of 5000/m (Evans and Williams 1978). The spectrophotometric results demonstrated that the commercial transferrin was > 95% apo-transferrin and that following iron loading and dialysis, > 95% of the transferrin was iron-charged or holo-transferrin.
Brain efflux index method
[125I]Apo- or holo-transferrin or [3H]dextran was dissolved in physiologic buffer containing 122 mm NaCl, 25 mm NaHCO3, 10 mm d-glucose, 3 mm KCl, 1.4 mm CaCl2, 1.2 mm MgSO4, 0.4 mm K2HPO4 and 10 mm HEPES (pH = 7.4). Adult male Sprague–Dawley rats (Harlan Breeders, Indianapolis, IN, USA), weighing 250–300 g, were anesthetized with intraperitoneal ketamine (50 mg/kg) and xylazine (4 mg/kg). A volume of 0.3 μL of isotope solution in physiologic buffer containing 0.05 μCi of transferrin or dextran plus varying amounts of unlabeled protein was injected into the Par2 region of cortex. The solutions were injected via a 1-μL Hamilton syringe, which was left in place for 4 min after injection. The rat was killed at 0.25–90 min after injection and the ipsilateral hemisphere was removed. The injection of the parietal cortex was performed under stereotaxic guidance with the following coordinates: 0.2 mm anterior and 5.5 mm lateral to bregma and 4.5 mm deep. Prior work has shown there is no efflux of solute from the Par2 region of parietal cortex via cerebrospinal fluid (CSF), and that solute efflux from this area occurs only via transport across the BBB (Kakee et al. 1996). The Par2 region is 4.2 mm from the CSF surface, and < 1% of the solute injected in this region is found in the contralateral brain (Kakee et al. 1996). To determine the amount of isotope injected in the brain, control rats were killed by halothane inhalation and isotope was injected immediately prior to decapitation and measurement of brain radioactivity.
The fractional brain radioactivity at each time point was fitted to a mono-exponential decay curve by derivative-free non-linear regression analysis without weighting using Program Ar of the BMDP Statistical Software package developed by the UCLA Biomedical Computing facility.
In order to determine saturability or cross-competition of transferrin efflux from brain, additional studies were performed in which the injection solution containing [125I]apo-transferrin also contained 80 or 2400 μg/mL of unlabeled apo-transferrin or 2400 μg/mL of a control plasma protein, IgG2a.
In order to determine the extent to which the transferrin was metabolized in brain during the experimental period, the amount of brain radioactivity that was precipitable by TCA was determined at 30, 60 or 90 min after injection. The brain tissue was homogenized in a tissue grinder in cold PBS solution containing 2.5% bovine serum albumin. An equal volume of cold 20% TCA was added and the solution was re-homogenized and centrifuged at 3000 g for 10 min at 4°C. The supernatant and pellet were counted for radioactivity and the per cent TCA precipitability was determined.
Data are presented as mean ± SD, and statistical differences were determined by analysis of variance with Bonferroni correction using the 7d program of the BMDP Statistical Software package.
Results
The rate of efflux of high-molecular-weight [3H]dextran from brain to blood during the 90-min period was minimal as shown in Fig. 1. Non-linear regression analysis of the efflux curve indicated the half-time (t1/2) of dextran export from brain to blood was 16 ± 7 h (Table 1). In contrast, apo-rat transferrin was rapidly exported from brain to blood, whereas the rate of export of holo-rat transferrin was intermediate (Fig. 1). The t1/2 of holo-transferrin and apo-transferrin export from brain to blood were 170 ± 15 and 49 ± 4 min, respectively (Table 1). Whereas the intercept of the linear regression curve for the dextran was 100 ± 2%, the intercepts of the holo-transferrin and the apo-transferrin curves were 90 ± 2 and 84 ± 3%, respectively (Table 1), indicating there was an early rapid efflux of these molecules from brain.
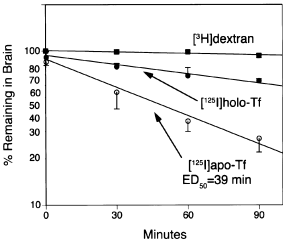
The percentage of radioactivity remaining in brain for up to 90 min after intracerebral injection is shown for [3H]dextran, [125I]apo-transferrin or [125I]holo-transferrin. Data are mean ± SD (n = 3 rats/point). The time at which 50% of the injected apo-transferrin has effluxed from brain to blood is 39 min.
| Molecule | Intercept | Slope (min−1) | t1/2 |
|---|---|---|---|
| [3H]Dextran | 1.00 ± 0.02 | 0.00070 ± 0.00029 | 990 ± 410 |
| [125I]Holo-transferrin | 0.90 ± 0.02 | 0.0041 ± 0.0003 | 170 ± 15 |
| [125I]Apo-transferrin | 0.84 ± 0.03 | 0.0139 ± 0.0010 | 49 ± 4 |
- Determined by non-linear regression analysis of data in Fig. 1.
The [125I]apo-transferrin was metabolically stable during the 90 min experimental period as the TCA precipitability of the brain homogenate was 98.4 ± 0.9%, 98.5 ± 0.1% and 97.8 ± 1.2%, respectively, at 30, 60 and 90 min after injection.
The saturability of the brain to blood efflux of apo-transferrin was assessed by adding increasing concentrations of unlabeled apo-transferrin to the injection solution. The efflux of apo-transferrin from brain to blood was approximately 50% inhibited by 80 μg/mL unlabeled apo-transferrin, and was nearly completely inhibited by 2400 μg/mL apo-transferrin (Fig. 2). In contrast, there was no inhibition of transferrin efflux from brain to blood by the addition of 2400 μg/mL of the IgG2a(Fig. 2).
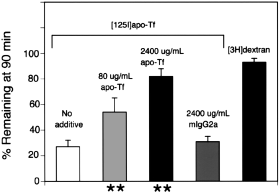
The percentage of radioactivity remaining in brain at 90 min after intracerebral injection of either [3H]dextran or [125I]apo-transferrin is shown for injection solutions containing either no additive, 80 or 2400 μg/mL of apo-transferrin, or 2400 μg/mL of mouse IgG2a. Data are mean ± SD (n = 3 rats/point). **Indicates p < 0.01 difference between no additive.
Discussion
The results of the present investigations are consistent with the following conclusions. First, transferrin undergoes a rapid, mediated efflux from brain to blood via a process that is saturable by transferrin, but not by other plasma proteins (Fig. 2). Secondly, the rate of efflux from brain to blood of apo-transferrin is faster than the efflux of holo-transferrin (Fig. 1).
The TCA precipitability measurements (see Results) indicate the efflux of radioactivity from brain to blood is not caused by the efflux of a radiolabeled metabolite generated from the metabolism in brain of transferrin, and that the efflux of radioactivity from brain to blood represents transport of the intact transferrin molecule. The rapid efflux of transferrin, and the preferential efflux of apo-transferrin relative to holo-transferrin, explains the selective retention of 59Fe by brain, relative to [125I]transferrin, following intravenous injection in rats (Taylor et al. 1991; Morris et al. 1992). The preferential uptake of 59Fe, relative to [125I]transferrin, by rat brain is the principle observation supporting the retro-endocytosis model of transferrin transport in brain. However, the present results show that this observation is also consistent with the transcytosis model. There is very rapid export of apo-transferrin from brain to blood, and there is preferential affinity of the efflux system for apo-transferrin relative to holo-transferrin (Fig. 1). Therefore, the selective retention of 59Fe by brain is consistent with the dissociation of iron from transferrin in brain cells followed by the rapid export of apo-transferrin from brain back to blood via retro-transcytosis across the BBB. The BBB permeability surface area (PS) products of [59Fe] and [125I]transferrin are 0.89 and 0.07 μL/min/g, respectively, over a 36-h period in the rat (Morris et al. 1992). These data indicate 92% of the apo-transferrin has effluxed from brain back to blood during this time period, which is consistent with the rapid rate of efflux reported in Fig. 1.
The rapidity of the BBB transferrin efflux system is shown by comparison of the rate of efflux of the 70 kDa dextran, which has an efflux t1/2 of 16 ± 7 h, with the rate of efflux of apo-transferrin, which has an efflux t1/2 of 49 ± 4 min (Fig. 1). The efflux t1/2 of high-molecular-weight dextran is not significantly different from the efflux t1/2 for albumin in rat brain, 10–12 h, as reported by Cserr et al. (1981). These observations indicate the rate of efflux of apo-transferrin from rat brain is 20-fold faster than the rate of efflux of physiologically inert molecules such as dextran or albumin. This rapid rate of efflux of apo-transferrin is consistent with a receptor-mediated mechanism. The evidence for receptor-mediated reverse-transcytosis of transferrin from brain to blood across the blood–brain barrier is threefold. First, the transferrin receptor is expressed on the abluminal membrane of the brain capillary endothelial cell (Huwyler and Pardridge 1998). Secondly, the mediated efflux of apo-transferrin is specific for transferrin, and is not inhibited by another plasma protein such as IgG (Fig. 2). Thirdly, the efflux of transferrin from brain to blood is saturable with an ED50 of 80 μg/mL in the injection solution (Fig. 2). As the injection solution undergoes a 30-fold dilution in the brain interstitium immediately upon injection into brain (Kakee et al. 1996; Terasaki 1998), the ED50 is equivalent to 30 nm unlabeled apo-transferrin. However, this is an overestimate of the local transferrin concentration. The effective concentration of apo-transferrin in the brain interstitial fluid is much lower owing to the sink effect of transferrin uptake by brain cells. The transferrin receptor is widely distributed on the neuronal plasma membrane (Mash et al. 1990; Dickinson and Connor 1998; Moos et al. 1999), and would be expected to rapidly bind any transferrin injected into the brain interstitial space. Nevertheless, there is an early rapid component of apo-transferrin efflux from brain to blood. The intercept of the apo-transferrin efflux curve, 84 ± 3% (Table 1), is not significantly different from the brain apo-transferrin content at 15 s after injection, which is 85 ± 4% (Fig. 1). Owing to the early rapid efflux from brain, the ED50(Fig. 1) is less than the t1/2 of apo-transferrin efflux(Table 1), and the intercept of the efflux curve for [125I]apo-transferrin is significantly different from the intercept of the dextran efflux curve, 100 ± 2% (Table 1). This rapid early phase of apo-transferrin efflux from brain to blood may represent immediate reverse-transcytosis across the BBB prior to the binding of the injected transferrin by transferrin receptor on brain cells.
The preferential efflux of apo-transferrin from brain to blood, relative to holo-transferrin, may be caused by a higher affinity for apo-transferrin of the transferrin receptor expressed on the abluminal membrane of the brain capillary endothelial cell. Both apo- and holo-transferrin bind to the transferrin receptor and there may be differential affinity of the receptor for the two forms of transferrin based on conformational changes within the receptor (Lawrence et al. 1999).
In summary, the present studies demonstrate rapid efflux of transferrin from brain to blood via a saturable process. The mediated efflux mechanism must exist on the abluminal membrane of the brain capillary endothelium, because there is no efflux via CSF from the Par2 region of brain (Terasaki 1998). The transferrin receptor is expressed on the abluminal membrane of the brain capillary endothelial cell (Huwyler and Pardridge 1998), and it is hypothesized that the brain to blood efflux of transferrin is mediated by the BBB transferrin receptor. This study provides evidence for ‘reverse-transcytosis’ of macromolecules across the BBB in the brain to blood direction. Because transferrin undergoes rapid receptor-mediated transcytosis from blood to brain (Fishman et al. 1987; Skarlatos et al. 1995), these studies are also consistent with a bidirectional transcytosis process for transferrin at the BBB in vivo. The finding of reverse transcytosis of transferrin via the BBB transferrin receptor parallels other results that indicate a BBB Fc receptor causes the receptor-mediated reverse transcytosis of IgG molecules from brain to blood (Zhang and Pardridge, 2001). Whereas the transferrin receptor mediates bidirectional transcytosis of transferrin in both blood to brain and brain to blood directions, the BBB FcR mediates only unidirectional transcytosis of IgG molecules in the brain to blood direction. There may be other instances of macromolecule secretion from brain to blood via reverse transcytosis across the BBB.
Acknowledgements
This work was supported by NIH grant NS-34698. Daniel Jeong skillfully prepared the manuscript.




