Light scattering and transmission electron microscopy studies reveal a mechanism for calcium/calmodulin-dependent protein kinase II self-association
Abstract
Calmodulin (CaM)-kinase II holoenzymes composed of either α or β subunits were analyzed using light scattering to determine a mechanism for self-association. Under identical reaction conditions, only αCaM-kinase II holoenzymes self-associated. Self-association was detected at a remarkably low enzyme concentration (0.14 µm or 7 µg/mL). Light scattering revealed two phases of self-association: a rapid rise that peaked, followed by a slower decrease that stabilized after 2–3 min. Electron microscopy identified that the rapid rise in scattering was due to the formation of loosely packed clusters of holoenzymes that undergo further association into large complexes of several microns in diameter over time. Self-association required activation by Ca2+/CaM and was strongly dependent on pH. Self-association was not detected at pH 7.5, however, the extent of this process increased as reaction pH decreased below 7.0. A peptide substrate (autocamtide-2) and inhibitor (AIP) designed from the autoregulatory domain of CaM-kinase II potently prevented self-association, whereas the peptide substrate syntide-2 did not. Thus, CaM-kinase II self-association is isoform specific, regulated by the conditions of activation, and is inhibited by peptides that bind to the catalytic domain likely via their autoregulatory-like sequence. A model for CaM-kinase II self-association is presented whereby catalytic domains in one holoenzyme interact with the regulatory domains in neighboring holoenzymes. These intersubunit–interholoenzyme autoinhibitory interactions could contribute to both the translocation and inactivation of CaM-kinase II previously reported in models of ischemia.
Abbreviations used
-
- BAPTA
-
- 1,2-bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
-
- BSA
-
- bovine serum albumin
-
- CaM
-
- calmodulin
-
- CaM-kinase II
-
- Ca2+/CaM-dependent protein kinase II
-
- DTT
-
- dithiothreitol
-
- MES
-
- 2-(N-morpholino)ethanesulfonic acid
-
- PIPES
-
- piperazine-N,N′bis-(2-ethanesulfonic acid)
-
- SDS–PAGE
-
- sodium dodecyl sulfate–polyacrylamide gel electrophoresis
Ca2+/calmodulin-dependent protein kinase II (CaM-kinase II) is a ubiquitous multifunctional Ser/Thr protein kinase implicated in regulating diverse cellular processes, including gene transcription, calcium homeostasis, receptor function, cytoskeletal alterations, synaptic transmission and synaptic plasticity (Hanson and Schulman 1992; Braun and Schulman 1994). The enzyme is maintained in an inactive state through inhibitory interactions between the autoregulatory domain (LeVine and Sahyoun 1987; Kwiatkowski and King 1989) and both the ATP (Colbran et al. 1989) and substrate-binding domains (Payne et al. 1988). Following Ca2+/CaM binding, the enzyme undergoes autophosphorylation, an intersubunit intraholoenzyme reaction (Mukherji and Soderling 1994) that is thought to precede substrate phosphorylation (Kwiatkowski et al. 1988).
CaM-kinase II isolated from forebrain is a holoenzyme composed of 8–12 subunits (450–650 kDa). Although multiple isozymes of CaM-kinase II (α, β, γ and δ) arise from distinct gene products or by alternative splicing (Hanson and Schulman 1992), the α (50 kDa) and β (60 kDa) subunits are the predominant isoforms in forebrain (Bennett et al. 1983). The α isoform is the dominant subunit in forebrain (3 : 1, α : β), producing both mixed subunit holoenzymes (Vallano 1989) and homomers composed entirely of α subunits (Brocke et al. 1999). CaM-kinase II is highly concentrated in brain, representing 1–2% of total cellular protein within the cortex and hippocampus (Erondu and Kennedy 1985). The enzyme is found in both soluble and particulate fractions (Kelly and Vernon 1985; Sahyoun et al. 1985a) and has been reported to comprise as much as 20–40% of the protein in neuronal cytoskeletal preparations (Sahyoun et al. 1985b), and the postsynaptic density (PSD; Miller and Kennedy 1985; Kennedy 1993). The subcellular targeting of CaM-kinase II is a dynamic process (Shen and Meyer 1999; Shen et al. 2000) that is differentially influenced by the α and β isoforms (Shen et al. 1998). Although the mechanism producing the subcellular compartmentalization of CaM-kinase II is unknown, the interaction of the α isoform with synapsin I (Benfenati et al. 1996) and the NR2B subtype of the NMDA receptor (Strack et al. 2000) are blocked by peptides that correspond to the autoregulatory domain of CaM-kinase II, indicating that the catalytic domain may serve a role in subcellular targeting of this isoform.
CaM-kinase II was initially called the major postsynaptic density protein, due to its high content in the PSD; however, this content appears to be dramatically enhanced by ischemia-induced conditions associated with decapitation (Suzuki et al. 1994; Aronowski and Grotta 1996; Margrie and Rostas 1997). The redistribution and inactivation of CaM-kinase II from the cytosolic to the particulate fractions during ischemic conditions is well documented (Kochhar et al. 1991; Aronowski et al. 1992; Hanson et al. 1994; Kolb et al. 1995; Shackelford et al. 1995). The extent of these alterations in CaM-kinase II correlates well to the loss of neuronal function leading to cell death following ischemic damage (Hanson et al. 1994).
Although the PSD has been previously implicated as a target for soluble CaM-kinase II translocation following an ischemic insult (Suzuki et al. 1994; Aronowski and Grotta 1996), a recent study identified 100-nm clusters contaminating PSD preparations using transmission electron microscopy and rotary shadowing (Dosemeci et al. 2000). Biochemical characterization of these clusters demonstrated that they were composed predominantly of αCaM-kinase II. These clusters likely contribute to the content of CaM-kinase II previously ascribed to particulate subcellular structures, such as the PSD. In addition, these authors reported that the presence of CaM-kinase II clusters in cultured neurons was increased following exposure to mitochondrial uncouplers in the absence of glucose (Dosemeci et al. 2000). Exposure of neurons to metabolic poisons likely produce similar intracellular changes as observed during ischemic conditions, including increases in intracellular Ca2+ and ATP depletion and possibly decreased pH. During cerebral ischemia, the intracellular pH decreases to 6.2 within minutes following loss of blood flow (Silver and Erecinska 1992) and ATP pools are rapidly depleted (Folbergrova et al. 1990). These changes in ATP and pH are also associated with elevated intracellular Ca2+. Thus, although the mechanism leading to the formation of CaM-kinase II clusters is unknown, the formation of these clusters may underlie the observed redistribution of CaM-kinase II from soluble to particulate fractions in neurons following ischemic stress.
Clusters formed of predominantly αCaM-kinase II under ATP depleting conditions in neurons (Dosemeci et al. 2000) bare a striking morphological resemblance to clusters of CaM-kinase II formed from purified forebrain enzyme during ischemia-like conditions in vitro (Hudmon et al. 1996). In the latter study, CaM-kinase II holoenzymes were shown to associate with one another to form sedimentable complexes, a process we termed self-association. Self-association was dependent upon ischemia-like conditions of limiting ATP and acidic pH and was accompanied by a time-dependent inactivation of the enzyme (Hudmon et al. 1996). It appeared that self-association could account for both the translocation and inactivation of CaM-kinase II observed during cerebral ischemia (Hudmon et al. 1996).
In the current study, the mechanism and isoform specificity of CaM-kinase II self-association was examined using light scattering and transmission electron microscopy (TEM). The results show that self-association is isozyme specific as recombinant α holoenzymes undergo self-association whereas β holoenzymes do not. Ca2+/CaM binding is essential for this process, and peptides mimicking the autoregulatory domain potently block self-association. Consistent with these data is a model for self-association whereby interactions form between the catalytic and regulatory domains of α subunits within different holoenzymes. This proposed mechanism is analogous to the intrasubunit interactions within the holoenzyme that maintain the enzyme in an autoinhibited state. However, subunit interactions leading to self-association would occur between neighboring holoenzymes under the appropriate conditions as defined in the present study.
Experimental procedures
Materials
Na+-ATP and [γ-32P]-ATP (3000 Ci/mmol) were purchased from Amersham Pharmacia Biotech., Inc. (Piscataway, NJ, USA). Na+-ADP was purchased from Sigma (St Louis, MO, USA). Low molecular weight standards and Tween-20 were obtained from Bio-Rad (Hercules, CA, USA). Recombinant chicken CaM was expressed and purified as described previously (Putkey and Waxham 1996). The autocamtide-2 (KKALRRQETVDAL) and syntide (PLRRTLSVAA) peptides were synthesized on an automated peptide synthesizer (Applied Biosystems, Foster City, CA, USA) and purified by reverse phase HPLC. The AIP peptide (KKALRRQEAVDAL) and syntide-2 (PLARTLSVAGLPGLL) were purchased from Calbiochem (San Diego, CA, USA).
Baculoviral protein expression and purification of CaM-kinase II
Recombinant α and β isozymes of CaM-kinase II were produced using the baculovirus expression system (Kolb et al. 1998) and were purified as described (Putkey and Waxham 1996) to near homogeneity. Both the α (34 ± 5.2 µmol/min/mg) and β (31 ± 1.5 µmol/min/mg) enzymes possessed similar specific activities. In addition, α and β holoenzymes were multimeric, with calculated total masses of 663 and 599 kDa, respectively (Kolb et al. 1998). The enzyme was quantified using Bradford and BCA (Pierce, Rockford, IL, USA) methods with bovine serum albumin (BSA) as the standard.
Self-association reactions
Standard self-association conditions were 50 mm MES/HEPES, pH 6.5, 0.4 mm DTT, 0.5 mm CaCl2, 2 µm CaM, 10 mm MgCl2, 10 µm ATP, 90 mm KCl, 0.1 mg/mL BSA and 0.1% (v/v) Tween-20. Unless indicated otherwise, the autophosphorylation reactions were initiated with the addition of αCaM-kinase II (20 µg/mL) to reactants that had been pre-warmed at 30°C for 1 min. The time-zero point is taken immediately following addition of the enzyme unless otherwise indicated.
Light scattering
A PTI QuantaMaster fluorimeter was used with the detector positioned at 90° from the light-source. The excitation and emission wavelengths were set at 340 nm, and a slit width of ∼ 1 nm was used. The data sampling rate was four points per second with the sample continuously mixed with a magnetic stir bar. All solutions were filtered before use. The reaction volume (1 mL) was maintained at 30°C via a circulating water bath in all experiments, except those shown in Fig. 3, where the reaction temperature was decreased to 22°C to reduce the reaction rate. Standard reaction conditions are described in the self-association section above. Unless indicated otherwise, the light scattering was measured for 2 min, and then 100 µL of enzyme solution was added to initiate the reaction. The enzyme was diluted immediately before each experiment in enzyme dilution buffer (1% Tween-20, 200 mm KCl and 1 mg/mL BSA), which was maintained at 30°C. The value of the time-dependent light scattering is reported as the difference between the final CPS (count per second) and the CPS before the enzyme was added. The rates reported in Fig. 3 were determined by measuring the initial slope of the curve following addition of the enzyme. All other light-scattering experiments were quantified by measuring the extent of self-association when the light scattering had reached a stable final value (after 5 min from the start of the reaction or as indicated). A plot of enzyme concentration vs. final values in light scattering was linear over a wide range of enzyme concentrations (data not shown).
Sedimentation/western blot analysis
Aliquots of enzyme were subjected to sedimentation analyses essentially as described in Hudmon et al. 1996. Briefly, the sample of reaction mixture (10 µL containing 200 ng of enzyme) was diluted into 50 µL ice-cold spin buffer (50 mm HEPES pH 7.4; 15 mm EDTA) and subjected to centrifugation at 4°C in a microcentrifuge (14 000 g) for 30 min. The enzyme in the supernatant and pellet was collected and suspended in SDS–PAGE sample buffer. Western blotting was performed using a monoclonal antibody (2D-5) to the α subunit, an alkaline phosphatase conjugated secondary antibody (Promega, Madison, WI, USA), and the substrates NBT/BCIP (Promega) to detect the protein.
Transmission electron microscopy
Purified recombinant αCaM-kinase II was examined at the ultrastructural level during the time-course of a light scattering experiment by placing aliquots of enzyme reactions (6 µL) on freshly prepared carbon-coated formvar grids by the drop method. The excess specimen was removed by wicking with bibulous paper and was washed three times with 10 µL of stain (0.25% methylamine tungstate), each time wicking with bibulous paper. Methylamine tungstate stain was freshly prepared and utilized throughout the study (Stoops et al. 1991). Transmission electron microscopy was performed on a JOEL JEM 1200 operating at 100 kV.
Results
Light-scattering measurements of αCaM-kinase II self-association
Light scattering is sensitive to the shape and size of protein in solution, and was used successfully to monitor the dynamics of glutamine synthetase (a protein similar in mass to CaM-kinase II) polymerization previously (Yanchunas et al. 1994). We adopted similar light-scattering parameters to examine the dynamics of CaM-kinase II self-association. Recombinant α holoenzymes were used in our initial experiments to characterize changes in light scattering under conditions previously shown to induce self-association of forebrain holoenzymes (Hudmon et al. 1996). A typical time course of the reaction of αCaM-kinase II self-association showed a rapid initial increase in light scattering followed by a decline to a constant final value (Fig. 1A). To correlate the changes in light scattering with enzyme solubility using the sedimentation analysis used in our previous study (Hudmon et al. 1996), samples were removed at the indicated times (Fig. 1A, a–e), and the ratio of soluble vs. self-associated enzyme was determined as described in the Methods section. The western blot (Fig. 1B), before addition of Ca2+/CaM (point a in the light-scattering trace), showed that the enzyme was initially soluble. During the initial rise in light scattering, immediately after the addition of Ca2+/CaM (b), a portion of the enzyme was detected in the pellet. At 0.5 min (c), 2 min (d) and 5 min (e) time points, all detectable enzyme was in the pellet. The migration difference (50–54 kDa) of the α subunit observed in the western blot was characteristic of CaM-kinase II following autophosphorylation under these conditions (Hudmon et al. 1996). Changes in light scattering correspond well to the sedimentation analyses previously used to detect self-association. However, the light-scattering analysis reveals changes in the enzyme complexes (peak decreasing to a constant final value) over time that were not discernable using sedimentation analysis.
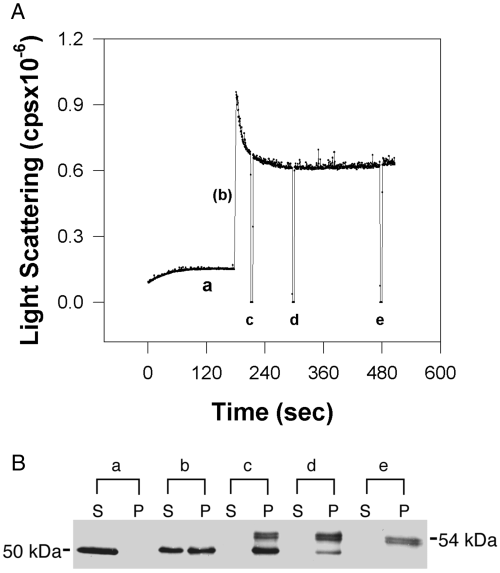
Changes in light scattering correlate with sedimentable enzyme. (A) Light scattering data were collected in a fluorimeter with the excitation and emission wavelengths set at 340 nm as described in the Methods section. αCaM-kinase II was activated under the following standard conditions: 10 mm PIPES, pH 6.5, 0.4 mm DTT, 0.5 mm CaCl2, 2 µm CaM, 10 mm MgCl2, 10 µm ATP, 20 mm KCl, 0.1 mg/mL BSA, and 0.1% Tween-20. The 3 min baseline was recorded with the recombinant αCaM-kinase II (20 µg/mL) present in the mixture, before the reaction was initiated by the addition of a 10X stock of Ca2+/CaM. (A) The letters (a–e) on the light-scattering trace show when aliquots were taken and subjected to sedimentation/western blot analysis as described in the Methods section. The deflections of light scattering in the trace are associated with opening the chamber door of the fluorimeter during sample collection. (B) Western blot showing the ratio of enzyme in the supernatant (S) and pellet (P). After baseline collection, samples were collected immediately before the addition of Ca2+/CaM (a), within 10 s (b), and at 30 s (c), 2 min (d), and 5 min (e) following the addition of Ca2+/CaM.
Changes in light scattering reflect different stages in the self-association of αCaM-kinase II holoenzymes
Changes in the appearance of αCaM-kinase II during the process of self-association were correlated to the light-scattering trace by removing aliquots of the reaction mix and examined by transmission electron microscopy (TEM). Using self-association conditions similar to those in Fig. 1, samples were removed for TEM examination (Fig. 2) during (a) the initial rise (within 20 s) (b) the trailing edge of the peak (45 s), and (c) at a constant value of light scattering (5 min). These time points collected for TEM analysis correspond approximately to points b, c and e on the light-scattering trace in Fig. 1. The initial increase in light scattering appears to be due to the association of individual holoenzymes into loosely packed clusters as shown in Fig. 2(A). Holoenzymes oriented within the cluster so that their ring-like shape 10–20 nm in diameter are visible (arrowheads in inset of micrograph B) and appear similar to the morphology of CaM-kinase II holoenzymes identified previously (Woodgett et al. 1983; Kolodziej et al. 2000). The clusters were sparse on the micrographs and of variable shape and size, precluding a thorough assessment of their average features. For reference, the diameter of the cluster in Fig. 2(A) across its longest dimension was ∼430 nm. Over time, these clusters begin to associate with one another and condense as they adopt a more rounded appearance with sharp outer boundaries (micrographs B and C). A lower magnification micrograph of the 5 min time point shown in micrograph D shows a complex, several microns in diameter, composed of dozens of clusters. The clusters shown in Fig. 2(D) range from 344 nm to 77 nm, with an average diameter across their longest dimension of 182 nm ± 59 nm (mean ± SD; n = 24). Only clusters with distinct boundaries were included in the analysis.
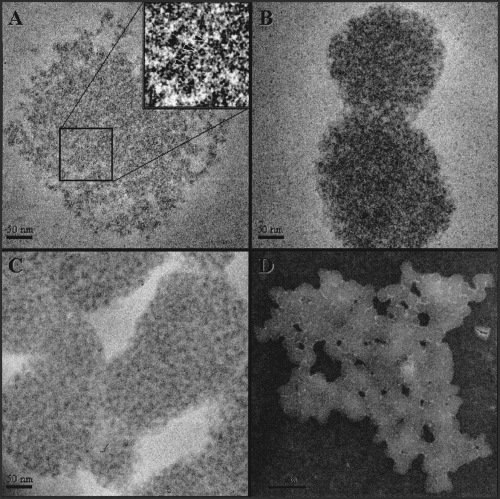
Structural changes of αCaM-kinase II related to the light scattering. CaM-kinase II was activated under standard conditions in the absence of 0.1% Tween-20 and BSA. The micrographs labeled (A–D) show the images of αCaM-kinase II that were collected at 20 s (A), 45 s (B) and 5 min (C), respectively. The magnification of A–C was 50 000 × with the scale bars corresponding to 50 nm. The arrowheads in the enlarged inset in panel A denote three particles within the complex which are oriented so as to display their ring-like shape. The size of these rings (∼ 100 Å in diameter) is consistent with the central core structure of CaM-kinase II holoenzymes (see text for more details). Close inspection reveals that the complexes are composed of these ring-like structures in various orientations. The contrast of the images have been inverted and enhanced to improve their display. The micrograph labeled (D) is a lower magnification (10 000 ×) of the sample collected at 5 min (C), and the scale bar corresponds to 1 µm.
The TEM data provides a morphological explanation for the two phases seen in the light-scattering curves. The initial increase in light scattering occurs as soluble holoenzymes form loosely organized clusters. These clusters condense and associate with one other, forming complexes of several microns in diameter that represent the final scattering value. Thus, the decrease in light scattering leading to the constant final value can be attributed to both a decrease in the total number of clusters in solution (even though they are larger) and/or to the condensation of the clusters.
The concentration dependence of CaM-kinase II self-association
Using self-association conditions similar to those employed in 1, 2, the rate and extent of light scattering was determined over a range of enzyme concentrations (0.07–0.4 µm) (Fig. 3A). The average values (n = 3) for the initial rates are summarized in Fig. 3(B). CaM-kinase II self-association was detected at a protein concentration as low as 0.14 µm (7 µg/mL). As enzyme concentration increased, the light-scattering traces became more complex in shape, exhibiting a transient peak that decreased to a constant value after 1–2 min. The change in initial rate as a function of enzyme concentration (Fig. 3B) is consistent with an intermolecular self-association reaction between holoenzymes.
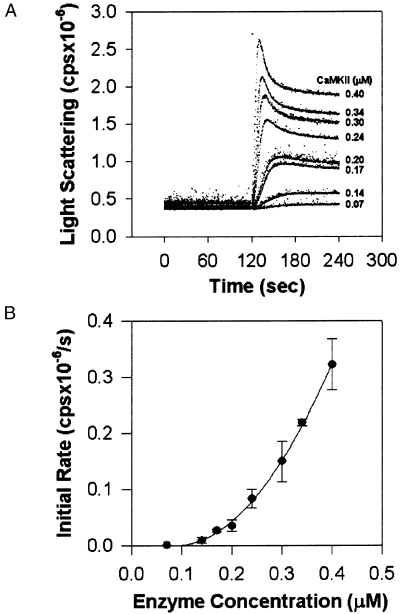
The effect of enzyme concentration on the rate of self-association. Recombinant αCaM-kinase II was varied at the indicated concentrations in standard self-association conditions as defined in the legend for Fig. 1. The reactions were initiated by addition of the enzyme after a 2-min baseline was recorded. Experiments were performed at 22°C to decrease the rate of the reaction. The traces for a representative experiment are provided in panel A. The group data (mean ± SD; n = 3) for initial rate measurements shown in panel B. The data in panel B were fit to the equation F = y0 + A x [enzyme]2.
ΒCaM-kinase II does not undergo self-association
Our experiments indicate that recombinant α holoenzymes mimic the self-association process observed for CaM-kinase II isolated from forebrain composed of both α and β subunits (Hudmon et al. 1996). To test whether self-association was a generalized feature of CaM-kinase II holoenzymes, holoenzymes composed of only β subunits were examined for self-association. βCaM-kinase II did not self-associate when subjected to conditions that produced robust self-association of αCaM-kinase II (Fig. 4).
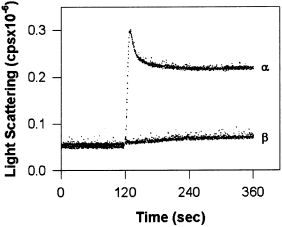
βCaM-kinase II is resistant to self-association. A 2-min baseline was collected in standard reaction conditions, and either α or βCaM-kinase II (20 µg/mL) was added to the mixture to initiate the reaction. The traces for one experiment are shown, however, these results are representative of several experiments.
Increases in pH and ionic strength decrease self-association
Self- association of forebrain CaM-kinase II was dependent on pH (Hudmon et al. 1996). However, in that study, a constant ionic strength was not maintained as pH was varied. Therefore, it was possible that the observed effect was due to changes in ionic strength rather than hydrogen ion concentration.
To examine these two possibilities, the influence of ionic strength on self-association was first examined. The reaction buffer (10 mm PIPES at pH 6.5) was supplemented with KCl to provide increases in ionic strength (either 60, 90, 140, 190 or 240 mm) at three different concentrations of enzyme (0.2, 0.4 and 0.8 µm). The averaged data for final values of light scattering (Fig. 5) indicated that the extent of enzyme self-association was decreased by increasing ionic strength. The half-maximal dependence of ionic strength on self-association was influenced by enzyme concentration and increased from approximately 150 mm to 190 mm and 240 mm at enzyme concentrations of 0.2, 0.4 and 0.8 µm, respectively.
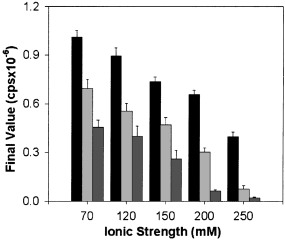
The effect of ionic strength on self-association. Three concentrations of αCaM-kinase II (0.2, 0.4, and 0.8 µm; 10, 20 and 40 µg/mL respectively) were activated in standard conditions at the indicated final ionic strength. The group data for the final light-scattering values are shown (mean ± SD; n = 3). The black, light gray and dark gray bars represent the results using 0.8, 0.4 and 0.2 µmαCaM-kinase II, respectively.
Is the pH sensitivity previously observed for self-association simply a function of ionic strength? To answer this question, the pH dependence of self-association was determined in reactions of a constant ionic strength. The contribution to ionic strength of the MES, PIPES and HEPES buffers at each pH was calculated as described by Ellis and Morrison (1982). The averaged data indicated that at pH values above 7.0, little self-association occured (Fig. 6A). However, at pH values of 7.0 and below, self-association increased with decreasing pH as reported previously when sedimentation was used as the criteria (Hudmon et al. 1996). To confirm the importance of pH in inducing CaM-kinase II self-association, we performed a pH shift experiment in real time using light scattering (Fig. 6B). The enzyme was added to an autophosphorylation reaction mix at pH 7.5, a reaction condition that does not produce self-association. After 30 s, the pH was decreased to 6.5 by the addition of MES/HEPES buffer. The addition of enzyme at 120 s induced a slight change in the baseline of the light scattering trace due predominantly to the presence of detergent in the added sample (Fig. 6B, trace b). A significant increase in light scattering occurs immediately following the drop in pH to 6.5 (trace b, arrow), although the extent of self-association is reduced somewhat relative to standard reactions (compare traces a and b, Fig. 6). Clearly, a drop in pH supports self-association and seems to be a key regulator of the process.
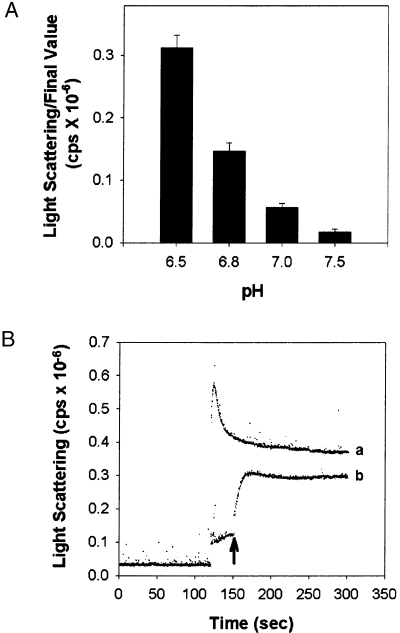
The reaction pH regulates self-association. The final ionic strength of the reaction at the indicated pH values was made to 70 mm using KCl. αCaM-kinase II (0.4 µm) was activated as described except that 50 mm MES/HEPES was used as the buffer with the pH as indicated (pH 6.5, 6.8, 7.0, 7.5). The group data for the final light-scattering values are shown in Panel A (mean ± SD; n = 3). Panel B shows that a drop in pH is sufficient to induce self-association. The same reaction conditions as that for panel A were utilized except the initial pH was at 7.5. The trace in (a) shows the extent of light scattering following activation at pH 6.5 in standard conditions. Trace (b) shows reactions started at 25 mm MES/HEPES (pH 7.5) by the addition of αCaM-kinase II (20 µg/mL) and Tween-20 (0.01% final), note a small rise in baseline. After an additional 30 s (trace b), the pH of the reaction was reduced to 6.5 by the addition of an appropriate amount of low pH buffer (25 mm MES/HEPES).
Enzyme inactivation prevents self-association
The decrease in light scattering observed in Fig. 6 (compare traces a and b) may have been due to enzyme inactivation under these conditions (Hudmon et al. 1996). Reactions at pH 7.5 do not produce self-association, although a time-dependent inactivation does occur as autophosphorylation increases (Hudmon et al. 1996). To differentiate the impacts of autophosphorylation and enzyme inactivation in preventing self-association, the reaction conditions were modified to inactivate the enzyme in the absence of autophosphorylation. Enzyme inactivation was accomplished by incubating αCaM-kinase II with Ca2+/CaM at pH 6.5 for increasing times and then self-association was initiated by the addition of Mg2+/ATP. The inset in Fig. 7 shows the time course of inactivation of αCaM-kinase II under these conditions. The light-scattering trace in Fig. 7 demonstrates the consequence of this inactivation on self-association. Self-association is reduced after 30 s (addition of Mg2+/ATP at the first arrow) and completely prevented after 2 min (addition of Mg2+/ATP at second arrow) of incubation under these conditions. These time points correspond to a 30% and 70% decrease in the initial activity, respectively. These data indicate that inactivation of the enzyme is correlated with prevention of self-association.
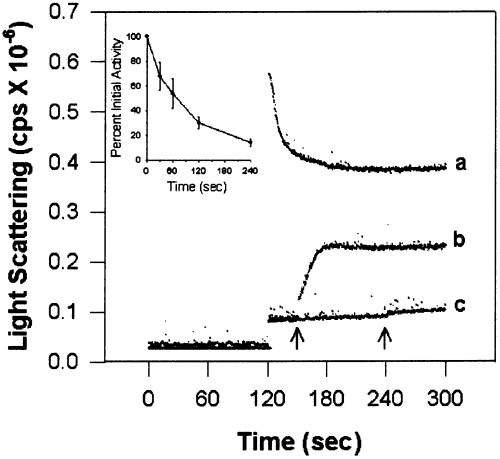
Enzyme inactivation prevents self-association. αCaM-kinase II incubated in standard conditions in the absence of Mg2+/ATP produces both a time dependent inactivation (see inset) as well as a time-dependent loss in the ability to self-associate following the addition of Mg2+/ATP. The trace labeled (b) is a representative experiment and shows that partial self-association is still observed following addition of Mg2+/ATP after 30 s (see arrow). For reference, the trace in (a) shows the extent of light scattering following activation at pH 6.5 in standard conditions. The trace labeled (c) demonstrates that self-association does not occur if the Mg2+/ATP is added after 2 min of incubation (see arrow). Time-dependent inactivation was determined in a second stage reaction as described in the Methods section. The activity was normalized to the time-0 point (mean ± SD; n = 3) following enzyme incubation in standard conditions in the absence of Mg2+/ATP.
Ca2+/CaM binding is essential to induce self-association
The binding of Ca2+/CaM is thought to disinhibit CaM-kinase II by exposing sites in the catalytic domain for substrate and ATP binding, thus permitting subunits within holoenzymes to undergo both autophosphorylation and substrate phosphorylation. A requirement for Ca2+/CaM would suggest that at least one of the partners required for subunit contacts for self-association is not exposed in the native or autoinhibited holoenzyme. In Fig. 8, CaM levels were varied while maintaining saturating Ca2+. Ca2+/CaM was essential for inducing CaM-kinase II self-association and exhibits a half-maximal value of 0.4 µm CaM. Because of the multisubunit nature of the holoenzyme, a relevant question is how many subunits within each holoenzyme require binding of Ca2+/CaM for self-association to occur? At 0.4 µm enzyme, self-association exhibits a strong sensitivity to [CaM] between 0.3 and 2 µm (Fig. 8). The KD reported for Ca2+/CaM binding to CaM-kinase II is approximately 50 nm (Meyer et al. 1992). At 400 nmα subunit and CaM, one would expect roughly 280 nm Ca2+/CaM–CaM–kinase II (subunit) complex, with 120 nm free Ca2+/CaM and free enzyme. Assuming 12 subunits per holoenzyme (Kolodziej et al. 2000), one would infer that half-maximal self-association requires approximately eight subunits bound with Ca2+/CaM. Thus, Ca2+/CaM is essential for the initiation of self-association, however, robust self-association is still observed when Ca2+/CaM is not saturating the holoenzyme.
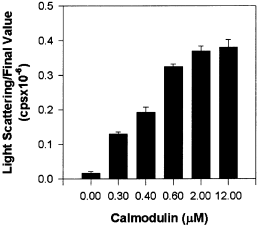
The influence of CaM concentration on self-association. αCaM-kinase II (20 µg/mL) was activated in standard reaction conditions, however, CaM concentration was varied from 0 to 12 µm as indicated. The group data for the final light-scattering values are shown (mean ± SD; n = 3).
Peptide substrates and peptide inhibitors block self-association
These data indicate that self-association requires that subunits be activated by Ca2+/CaM-binding, possibly by exposing catalytic domains through disruption of their interactions with regulatory domains. This hypothesis was explored using synthetic peptides that bind to and occupy the catalytic cleft following activation. Self-association was first assessed in the presence of increasing concentrations of the peptide substrate autocamtide-2. The sequence of autocamtide-2 was derived from the autoregulatory domain surrounding Thr286 and has a Km of 0.4 µm (Ishida and Fujisawa 1995). Increasing concentrations of autocamtide-2 decreased the extent of self-association in a dose-dependent fashion (Fig. 9). Approximately 1.6 µm autocamtide-2 was required for half-maximal inhibition of self-association. Autocamtide-2 is phosphorylated by the enzyme and phosphorylation would decrease its binding affinity, thus decreasing the peptide's inhibitory potency. Therefore, we also examined the dose-dependent effects of a similar peptide, AIP, which contains an Ala substituted for the phosphorylated Thr residue (Ishida and Fujisawa 1995). As anticipated, AIP was a more effective inhibitor of self-association than autocamtide-2, exhibiting half-maximal inhibition at 0.2 µm. In contrast, the peptide substrate syntide-2 (Km = 8.5 µm (Ishida and Fujisawa 1995) did not prevent self-association at concentrations as high as 100 µm (data not shown). The fact that syntide-2 cannot block self-association indicates: (i) that the inhibition seen with autocamtide-2 and AIP is specific, and (ii) that autocamtide-2 and AIP bind to the catalytic domain in a distinct manner from syntide-2. These data strongly support a role for the catalytic domain in self-association, but not the substrate binding site. One obvious target for binding to the catalytic domain is the autoregulatory domain. Specifically, residues around Thr286 in the autoregulatory domain are implicated by these peptide competition studies. In summary, we propose a model for self-association based partly on the mechanism for autoinhibition; interactions between the catalytic domain of one subunit with the autoregulatory domain in another subunit are formed between neighboring holoenzymes in the presence of Ca2+/CaM to produce self-association.
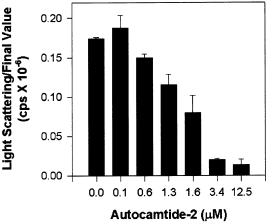
Autocamtide-2 block self-association. αCaM-kinase II (0.4 µm) was activated under standard conditions with the addition of the indicated concentrations (0–12.5 µm) of autocamtide-2 peptide. The group data for the final light-scattering values are shown (mean ± SD; n = 3).
Discussion
Using electron microscopy and light scattering, we observed that self-association of CaM-kinase II in vitro is a multiphasic process. Electron microscopy showed that holoenzymes initially bind to each other to form loosely packed clusters that condense and undergo further association over time. The TEM images also indicate that self-association does not involve a destruction of the enzyme's ring-like morphology. The threshold for detecting self-association was approximately 0.14 µmαCaM-kinase II under standard reaction conditions, indicating that this process occurs at remarkably low protein concentration. The typical enzyme concentration (0.4 µm) utilized in our experiments is well below the estimated concentration of 19–37 µm for αCaM-kinase II in forebrain homogenates (Kennedy et al. 1990). Increases in ionic strength decreased self-association indicating that the protein interactions between holoenzymes are, in part, ionic in nature. Increases in enzyme concentration decreased the sensitivity to ionic strength, with robust self-association still observed at enzyme concentrations 20–40 fold below levels reported for brain at physiological ionic strength.
In addition to requiring Ca2+/CaM, self-association was highly dependent on reaction pH. At a pH of 7.5 little self-association was evident, but as the pH decreased to values of 7.0 and below, the extent of self-association increased. Thus, pH appears to be a critical triggering step that determines whether interholoenzyme subunit contacts form and/or are stabilized following enzyme activation. The protonation state of His282 in the regulatory domain of CaM-kinase II is sensitive to pH in a range reported here that produced self-association (Smith et al. 1992; Brickey et al. 1994). Protonation of His282 led to an increased exposure of the ATP binding pocket within the catalytic domain and, interestingly, this effect required the previous binding of Ca2+/CaM (Brickey et al. 1994). Apparently, His282 resides in a sequestered environment. Upon Ca2+/CaM binding, protonation of His282 leads to exposure of the catalytic domain by disrupting autoregulatory interactions (Brickey et al. 1994). The pH sensitivity and location of His282 in the autoregulatory domain make this residue a potential trigger in regulating self-association, possibly by relieving steric constraints and exposing domains in the autoregulatory and/or catalytic regions that are not accessible at pH values above 7.0.
Synthetic peptides mimicking the autoregulatory domain (autocamtide-2 and AIP) were potent inhibitors of self-association implicating the catalytic domain as a binding partner for the self-association reaction. Specifically, these experiments implicate the region of the catalytic domain that is normally inhibited by the autoregulatory domain, presumably residues around Thr286, as the interholoenzyme subunit partner to produce self-association (see Fig. 10). The syntide-2 peptide showed no inhibitory effect even at 50-fold over the half maximal concentration observed for AC-2, indicating that the substrate binding pocket is not directly involved in self-association. Ishida and Fujisawa (1995) described similar findings on the ability of these peptides to differentially prevent enzyme inactivation. AIP and autocamtide-2, but not syntide-2, protected CaM-kinase II from time-dependent inactivation following activation by Ca2+/CaM in the absence of ATP and autophosphorylation. Presumably autocamtide-2/AIP and syntide-2 interact with the catalytic domain in unique ways to explain their distinct effects in both inactivation and self-association. The peptide competition experiments reported by Ishida and Fujisawa (1995) suggest mechanistic similarities to the self-association reaction observed here between holoenzymes. Inactivation of the enzyme by Ca2+/CaM treatment prior to a pH drop prevented self-association. It would appear that exposing the enzyme to Ca2+/CaM in the absence of ATP, produces subunit interactions or structural changes within the holoenzyme that compete for binding partners involved in the self-association reaction.
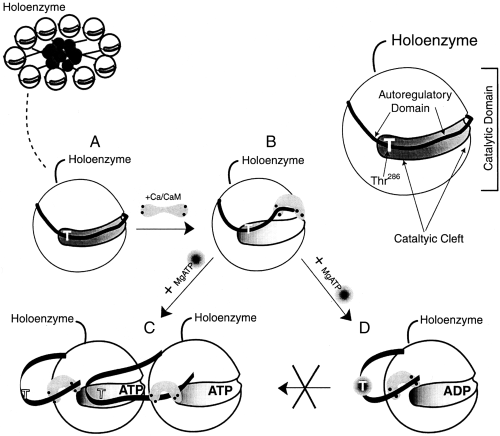
Model for αCaM-kinase II self-association. A simplified diagram for a CaM-kinase II holoenzyme is indicated in the top left of the figure. A catalytic lobe of one subunit from the holoenzyme is diagramed to demonstrate its ribbon like autoregulatory domain lying within the catalytic cleft with Thr286 identified as a T (A). The autoregulatory region is partially displaced from the catalytic domain following Ca2+/CaM-binding (B). If the pH is below 7 and Thr-286 is not phosphorylated, the regulatory domain of the subunit undergoes a unique displacement that is permissive for the binding to the catalytic domain in another holoenzyme (C) leading to self-association. However, if Thr-286 becomes phosphorylated (D) following Mg2+/ATP binding, self-association is prevented even at a pH below 7.
We have shown that recombinant α holoenzymes undergo self-association while β holoenzymes do not. This result suggests that the ratio of α and β subunits within the same holoenzyme, as found in CaM-kinase II preparations from rat brain (Vallano 1989; Brocke et al. 1999), would influence their propensity for self-association. In support of this possibility, CaM-kinase II isolated from the cerebellum (predominantly β subunits) is less susceptible to the process of self-association compared with the forebrain enzyme (predominantly α subunits) (Hudmon and Waxham, unpublished observations). The three-dimensional structure of αCaM-kinase II reveals that the enzyme is dodecameric and that the catalytic and regulatory domains reside in foot-like processes extending out from a central core on tethers (Kolodziej et al. 2000). Inter-holoenzyme contacts between the catalytic/autoregulatory foot-like processes could produce the lattices of holoenzymes visible by electron microscopy. We have determined that the overall three-dimensional architecture of βCaM-kinase II and αCaM-kinase II are similar (manuscript in preparation), indicating that a difference in their subunit organization or content (both are dodecamers) cannot explain the difference in their capacity for self-association.
The relative hydrophobicity, predicted secondary structure, and charge distribution are conserved throughout much of the amino acid sequence of the α and β subunits (Bulleit et al. 1988; Kolb et al. 1998). Variations in their structures are primarily attributed to two variable inserts that make-up the ‘unique’ domain of the β subunit (Bulleit et al. 1988). This domain may be responsible for preventing the self-association of βCaM-kinase II. Interestingly, the region analogous to the variable domain of β in splice variants of Drosophila CaM-kinase II appears to interact with the catalytic domain (GuptaRoy and Griffith 1996). It is possible that additional contacts are established between the variable and catalytic domains that prevent exposure of sites necessary for self-association in the β holoenzymes.
CaM-kinase II autophosphorylation and substrate phosphorylation are stringently coupled to Ca2+/CaM-binding. Binding produces a conformational change that disrupts contacts between the catalytic and autoregulatory domains, exposing the catalytic domain for activation and the autoregulatory domain for autophosphorylation. It is thought that autophosphorylation is required to fully disinhibit the enzyme (Colbran et al. 1989) and it leads to the production of Ca2+/CaM-independent activity. Based on these findings and our present data, Fig. 10 presents a model for self-association, whereby subunit interactions between holoenzymes underlie this process. Specifically, the autoregulatory domain of a subunit in one holoenzyme stably interacts with the catalytic domain of a subunit in a neighboring holoenzyme under conditions of low pH. Due to the multisubunit nature of the CaM-kinase II holoenzyme, additional subunits can also undergo these interactions with the same or other holoenzymes leading to the clusters seen in the EM. The basic interactions of this model are similar to the autoinhibition that normally maintains each subunit in an inactive state within the holoenzyme. Predictions from this model are directly supported by the present data. First, Ca2+/CaM-binding would be essential for exposing subunit contacts necessary for self-association. Second, the formation of these contacts between holoenzymes would be blocked by synthetic peptides that mimic the autoregulatory domain.
It is of interest to note that autophosphorylation is an intersubunit intraholoenzyme reaction mechanism (Mukherji and Soderling 1994). Autophosphorylation occurs when a Ca2+/CaM-bound subunit presents Thr286 within the autoregulatory domain to a neighboring Ca2+/CaM-bound subunit catalytic domain (Rich and Schulman 1998). This mechanism appears to predispose subunits within the holoenzyme to make intersubunit contacts, albeit transiently. Thus, the intersubunit–interholoenzyme interactions that form the basis of our model are a combination of the native autoregulatory interactions (intraholoenzyme–intrasubunit) as well as the autophosphorylation reaction (intraholoenzyme–intersubunit).
A further prediction is that the state of Thr286 autophosphorylation should alter the enzyme's susceptibility to self-association. The specific prediction is that stoichiometric autophosphorylation of Thr286 would prevent self-association (Hudmon et al. 1996). We previously reported that autophosphorylation of forebrain CaM-kinase II alters the enzyme's susceptibility to undergo self-association. This conclusion was drawn from experiments where ATP concentrations around the Km for the enzyme (10 µm) induced self-association, whereas, ATP levels well above this level (500 µm) prevented self-association (Hudmon et al. 1996). We hypothesize that ATP concentration influences the rate of Thr286 autophosphorylation during activation; high ATP maximizes Thr286 autophosphorylation and subsequently minimizes self-association. Thus, as shown in Fig. 10, the process of autophosphorylation would compete with that of self-association, with ATP concentration influencing the outcome of this competition by determining the extent of Thr286 autophosphorylation.
Although somewhat different in size, the enzyme complexes formed following CaM-kinase II self-association in vitro appear remarkably similar in morphology to the CaM-kinase II clusters recently described by Dosemeci et al. (2000) following metabolic poisoning of the neurons. We propose that the mechanism described here for producing clusters in vitro is also responsible for the formation of clusters in neurons. Dosemeci et al. (2000) postulated that one possible function for the formation of these clusters is to sequester enzyme activity during conditions of metabolic stress. The self-association mechanism that we propose would provide a mechanism for inactivation because the interactions described would inactivate the enzyme by a form of intersubunit inhibition between holoenzymes.
Is in vitro self-association, as described here, and the formation of enzyme clusters in neurons as observed by Dosemeci et al. (2000) a potentially reversible process? It currently is unknown whether altering parameters such as pH, ATP and Ca2+ concentration can dissociate clusters of self-associated enzyme, although such experiments are underway. In addition, the possible reversal of cluster formation in neurons was not addressed in the study by Dosemeci et al. (2000). Ischemic-induced translocation of soluble enzyme into the particulate fraction is reversible in vivo if the period of ischemia is kept brief (5 min in the rat model of global ischemia; Aronowski et al. 1992). Thus, following reperfusion, a mechanism must exist in neurons that can reverse the soluble to particlulate translocation detected following ischemia. In fact, the recovery of soluble CaM-kinase II may be crucial for neuronal survival, because the ability to restore a pool of soluble active enzyme is correlated with the extent of neuronal survival in ischemic-sensitive regions of the brain, such as the hippocampus (Aronowski et al. 1992).
An interesting parallel exists between the mechanisms of self-association reported here and the proposed mechanism for CaM-kinase II association with other proteins. Peptides resembling the autoregulatory domain of CaM-kinase II that prevented self-association inhibit the binding of αCaM-kinase II to both the NR2B subunit of the NMDA receptor (Strack et al. 2000) and synapsin I (Benfenati et al. 1996). A reasonable hypothesis is that αCaM-kinase II self-association is an extension of this conserved targeting mechanism of the α isoform with other proteins. The CaM-kinase II clusters (Dosemeci et al. 2000) that form in neurons are then a consequence of enzyme activation and exposure of a physiological targeting mechanism to pathological conditions; in the case of ischemia, this involves sustained intracellular calcium, reduced pH and reduced ATP levels. Our results demonstrating that synthetic peptide inhibitors potently block self-association could offer both a reagent to dissect the role of CaM-kinase II during ischemic damage as well as afford new interventions for neuronal protection. Finally, the role of Thr286 autophosphorylation in regulating self-association is intriguing, and mutagenesis studies currently are underway to examine the role of ATP and Thr286 autophosphorylation in preventing self-association.
Acknowledgements
The authors acknowledge Steve Kolodziej for help in preparing the figure of the electron micrographs. We also recognize Sheela Singla and Dr Ulli Bayer for critical discussions and comments on the manuscript. This study was supported by NIH grants NS26086 (MNW) and HL42886 (JKS). AH and SAK were supported in part by NIH training grant NS07373. SJK was supported in part by the University of Texas Health Science Center at Houston MD/PhD Program.




