Characterization and Distribution of Ferritin Binding Sites in the Adult Mouse Brain
Abbreviations used : BSA, bovine serum albumin ; H-chain and L-chain, heavy chain and light chain, respectively ; rH-ferritin, human recombinant H-chain ferritin
Abstract
Abstract : Studies on iron uptake into the brain have traditionally focused on transport by transferrin. However, transferrin receptors are not found in all brain regions and are especially low in white matter tracts where high iron concentrations have been reported. Several lines of research suggest that a receptor for ferritin, the intracellular storage protein for iron, may exist. We present, herein, evidence for ferritin binding sites in the brains of adult mice. Autoradiographic studies using 125I-recombinant human ferritin demonstrate that ferritin binding sites in brain are predominantly in white matter. Saturation binding analyses revealed a single class of binding sites with a dissociation constant (KD) of 4.65 × 10-9M and a binding site density (Bmax) of 17.9 fmol bound/μg of protein. Binding of radiolabeled ferritin can be competitively displaced by an excess of ferritin but not transferrin. Ferritin has previously been shown to affect cellular proliferation, protect cells from oxidative damage, and deliver iron. The significance of a cellular ferritin receptor is that ferritin is capable of delivering 2,000 times more iron per mole of protein than transferrin. The distribution of ferritin binding sites in brain vis-à-vis transferrin receptor distribution suggests distinct methods for iron delivery between gray and whi
Iron is an essential nutrient that is not only required for carrying oxygen throughout the bloodstream but is also a vital component of enzymes necessary for oxidative respiration. The brain has the highest rate of oxidative metabolism of any organ (Wrigglesworth and Baum, 1988) ; thus, it requires relatively high quantities of iron. Indeed, the concentration of iron in some brain regions is higher than in any other organ except the liver (Hallgren and Sourander, 1958). Dietary iron deficiencies during early postnatal development can result in both mental disorders and severe motor impairments that can persist into adulthood (Pollit and Kim, 1988). Iron is a required cofactor for synthesis of several brain-specific enzymes and neurotransmitters including dopamine, serotonin, epinephrine, norepinephrine (Youdim, 1990), and γ-aminobutyric acid (Hill, 1985). The process of myelination seems to be particularly dependent on the availability of iron especially during the critical growth period (Larkin and Rao, 1990). Indeed, white matter tracts consistently contain higher concentrations of iron than gray matter areas (Rajan et al., 1976 ; Connor and Menzies, 1995).
Although iron is an essential nutrient, it can also be a potent neurotoxin. Free ferrous (Fe II) iron is particularly destructive, because it can react with hydrogen peroxide (H2O2) via the Fenton reaction to produce a hydroxyl radical. Hydroxyl free radicals can attack lipids, proteins, and nucleic acids. Therefore, the brain must rigorously maintain iron homeostasis and minimize free iron. There are a number of common neurological disorders in which brain iron accumulation has been noted. Neuritic plaques, the hallmark of Alzheimer's disease, contain iron as a significant component and iron encrustation of blood vessels is a common observation in Alzheimer's diseased brain (Connor and Menzies, 1995). In Parkinson's disease, iron accumulation in the substantia nigra is a consistent finding and considered part of the pathogenesis of the disease (Sofic et al., 1988). Thus, it is necessary to uncover the extant mechanisms for iron delivery to the brain. We report here a novel system, for possible iron delivery to the brain, that uses ferritin.
Ferritin is a high molecular weight protein (480,000 molecular weight) that is composed of heavy chain (H-chain) and light chain (L-chain) subunits which aggregate (in varying ratios) to form a mature protein totaling 24 subunits. The H-chain subunit confers ferroxidase activity to ferritin, enabling ferritin to oxidize ferrous (Fe II) iron to ferric iron (Fe III). The 24 subunits arrange to form a sphere with a hollow center capable of storing up to 4,000 atoms of iron (Harrison and Arosio, 1996). Traditionally considered an intracellular protein, evidence is increasing that ferritin may be released from cells (Simpson et al., 1991 ; Madani and Linder, 1992 ; Beaumont et al., 1995 ; Girelli et al., 1995 ; Tran et al., 1997). Ferritin is also present in normal human serum (Addison et al., 1972) and CSF (Hallgren et al., 1980). Although the iron content of serum ferritin is quite low, it should be noted that iron-rich ferritin is rapidly cleared from serum (Siimes and Dallman, 1974), suggesting ferritin may function as an iron transporter.
EXPERIMENTAL PROCEDURES
Tissue preparation
Adult C57BL/6 mice were anesthetized with sodium pentobarbital, weighed, and killed by decapitation in accordance with specified International Animal Care and Use Committee guidelines. The brains (including cerebellum) were removed, weighed, and frozen immediately at —25°C in 2-methylbutane. For the binding analysis, one hemisphere of each brain was thawed on ice in 100 volumes of cold 50 mM Tris-HCl, pH 7.4, in the presence of absence of protease inhibitors (10 μM leupeptin and 10 mM phenylmethylsulfonyl fluoride). The presence of the protease inhibitors during membrane isolation or binding analysis did not influence the results. Brains were homogenized for 1 min using a Janke and Kunkel Ultra Turrax T25 tissue homogenizer. The homogenate was centrifuged at 29,000 g for 10 min at 4°C. The resulting supernatant was aspirated and the pellet (representing the membranes) was resuspended with the original volume of buffer and then placed in a 37°C incubator for 30 min to promote the release of endogenous ferritin from the tissue preparation. The membrane homogenate was then recentrifuged at 29,000 g for 10 min at 4°C. The supernatant was removed and the pellet was resuspended in 4 ml of 50 mM Tris, pH 7.4. Protein concentrations were determined by the Bio-Rad assay.
Materials
Human recombinant H-chain ferritin (rH-ferritin) was generated as described previously (Levi et al., 1987). Amino acid sequences of ferritin H-chains are highly conserved (90%) between various species (Harrison et al., 1987) and rH-ferritin has been the standard form of ferritin used in binding studies (Broxmeyer et al., 1986 ; Fargion et al., 1988, 1991a,b, 1992 ; Moss et al., 1992a,b). The recombinant protein has been shown to form the complete ferritin cage and can contain up to 3,200 iron atoms per molecule (Levi et al., 1988). The iron content of the ferritin used in the studies ranged from 77 to 97 iron atoms per molecule of ferritin as determined by a Ferrochem II iron analyzer (ESA, Inc.). Bovine serum albumin (BSA), horse spleen ferritin, and human holo-transferrin were purchased from Sigma (St. Louis, MO, U.S.A.). Na125I was purchased from Amersham (Arlington Heights, IL, U.S.A.).
Radioligand binding
Iodination.
rH-chain ferritin was iodinated via modification of the chloramine-T method (Hunter and Greenwood, 1962) using Na125I. Iodinated protein was separated from free 125I by using a PD-10 G-25 Sephadex column (Pharmacia Biotech, Piscataway, NJ, U.S.A.). A specific activity of 6,000—15,000 dpm/ng of protein was routinely obtained.
Time course study.
Duplicates of membrane preparations from three different mice were incubated at 22°C with 0.2 nM125I-rH-ferritin for up to 180 min. The binding suspension consisted of 50 mM Tris (pH 7.4), 0.1% BSA, 5 mM HCl, and 25 μg of membrane protein preparation with or without the addition of 1 μM unlabeled rH-ferritin in a final volume of 400 μl. Binding was terminated by the addition of 3 ml of ice-cold 50 mM Tris-HCl. Bound radioactivity was isolated by rapid filtration over Whatman glass-fber C filters that had been previously coated in a solution of 5% nonfat dried milk (Blotto) with 0.1 mg/ml horse spleen ferritin. This combination was determined empirically to reduce the nonspecific binding of radiolabeled protein to the filters to 1—3% of the total counts added. The filters were washed 5× with 3 ml of ice-cold 50 mM Tris containing 200 mM NaCl. The filters were dried under a heat lamp before being counted in a Gamma Trac γ-counter. Specific binding was calculated by subtracting binding in the presence of excess unlabeled rH-ferritin (nonspecific binding) from binding without excess unlabeled rH-ferritin present (total binding). Nonspecific binding was measured at five of the 10 concentrations, and linear baseline analysis (GraphPad Prism, San Diego, CA, U.S.A.) was used to calculate nonspecific binding data over the entire concentration range.
Saturation analysis.
A total of four mice were used for these experiments. Each binding experiment was performed in duplicate. Increasing concentrations of 125I-rH-ferritin were added to binding suspensions consisting of the same binding buffer described previously with 25 μg of membrane protein preparation with or without the addition of 1 μM unlabeled rH-ferritin in a final volume of 400 μl. After a 60-min incubation at 22°C binding was terminated, and total, nonspecific, and specific bindings were calculated as described previously.
Competition.
Duplicates of membrane preparations from three different mice were used in these experiments. Increasing concentrations of unlabeled competitors (rH-ferritin, horse spleen ferritin, and human holo-transferrin) were incubated for 60 min at 22°C with 25 μg of membrane protein in the presence of 0.2 nM125I-rH-ferritin in the same binding buffer described previously. Binding, termination of binding, isolation of membranes, and calculations of specific binding were performed as described above.
Dissociation.
Duplicates of membrane preparations (25 μg of membrane protein) from three different mice were incubated at 22°C with 0.2 nM125I-rH-ferritin for 60 min in the same binding buffer described previously. An aliquot of membrane homogenate was removed, filtered, and washed as described above to determine specific binding at time zero. Dissociation of bound radioligand was initiated by the addition of 1 μM unlabeled rH-ferritin. Aliquots were removed and filtered at specified times to assess dissociation of bound radioligand.
Autoradiography
Brains of adult C57BL/6 mice (n = 6) were removed as described previously and immediately fresh-frozen at —25°C in 2-methylbutane. Coronal and sagittal sections were cut at 10 μm on a cryostat and thaw-mounted on chrome—alum-coated slides. The sections were stored at —20°C for up to 2 weeks with no noticeable reduction in specific binding. To observe the distribution of ferritin binding sites, the sections were incubated for 1 h at 22°C with 5 nM125I-rH-ferritin in a binding buffer consisting of 200 mM sucrose, 50 mM HEPES, 1% BSA, and 10 mM EDTA. To control for nonspecific adsorption of 125I-rH-ferritin, separate sections were incubated with the radiolabeled rH-ferritin in the presence of a 2,000-fold molar excess of unlabeled rH-ferritin. To assess specificity of binding, separate sections were incubated with either 2,000-fold molar excesses of BSA, transferrin, recombinant L-chain ferritin, or horse spleen ferritin. To dissociate nonspecifically bound radioligand, sections were rinsed in four consecutive changes (5 min each) of ice-cold 0.1 M phosphate-buffered saline. After drying, the labeled sections were apposed to Kodak autoradiography film for 4 days at -65°C. Previous studies have demonstrated that the net negative charge of myelin proteins precludes anionic and native ferritins from binding nonspecifically to myelin proteins (Dermietzel et al., 1983 ; Dolapchieva et al., 1990). The rH-ferritin used in these studies was anionic (Levi et al., 1987).
RESULTS
To demonstrate the presence of ferritin binding sites in the brain, binding studies were performed, using iodinated rH-ferritin, on membrane preparations isolated from the brains of adult C57BL/6 mice. Total binding was normally <10% of total dpm added. Specific binding ranged from 80 to 95% of total binding. There was no noticeable difference in specific binding with or without the addition of proteinase inhibitors during membrane isolation or the binding assays. The association rate of 125I-rH-ferritin (0.2 nM) was examined by measuring specific binding at various intervals over a 180-min period. A steady state of binding was reached by 60 min (Fig. 1) with no subsequent reduction in specific binding observed. Thus, incubations for subsequent binding analyses were performed for 60 min to ensure steady state of assay components. Saturation analysis demonstrated specific and saturable binding of 125I-rH-ferritin to mouse brain membrane homogenates (Fig. 2, inset). Although nonspecific binding increased linearly over the entire concentration range, it never exceeded 20%. Scatchard plots of saturation data (n =4) indicated a single binding site with a dissociation constant (KD) of 4.65 ± 0.9 (SE) 10-9M and a receptor density (Bmax) of 17.9 ± 2.8 (SE) fmol bound/μg of protein (Fig. 2).
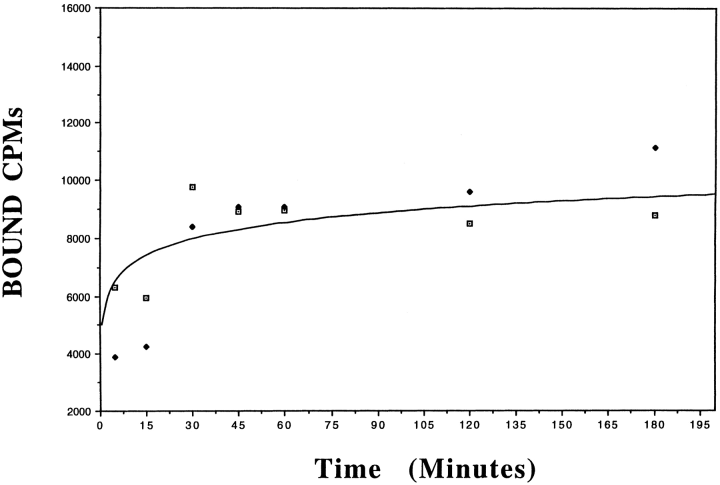
. Time course analysis of specific rH-ferritin binding to mouse brain membrane homogenates. 125I-rH-ferritin (0.2 nM) was incubated with 25 μg of membrane homogenate at 22°C for up to 180 min. The curve shown is representative of kinetic analysis of three separate brain preparations (each performed in duplicate), revealing a steady state of binding that is reached after 60 min. Points shown are duplicates from one experiment.
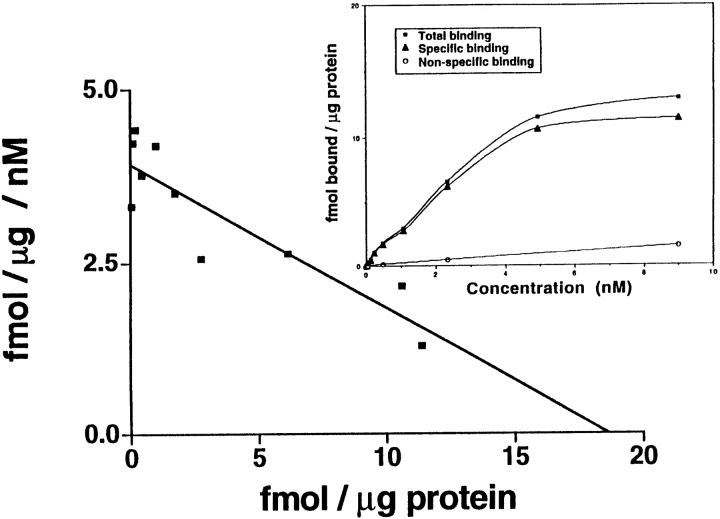
. Scatchard analysis. The saturation curve in the inset shows that the binding of 125I-rH-ferritin to membrane homogenates prepared from the brains of adult C57 black mice is saturable as the concentration of the radioligand increases, whereas nonspecific binding never exceeds 20% of total binding. The saturation and Scatchard analysis shown is a representative analysis taken from one experiment revealing a KD of 4.65 nM and a Bmax of 17.9 fmol bound/μg of protein.
Excess transferrin and horse spleen (L-rich) ferritin did not inhibit binding of the 125I-rH-ferritin to the membrane preparations, whereas excess unlabeled rH-ferritin inhibited >80% of binding of the labeled protein at concentrations of >0.5 μM(Fig. 3). A Hill plot of the competition data revealed a coefficient of 2.5 (not shown).

. Competition analysis. A graph showing that binding of 125I-rH-ferritin (0.2 nM) to mouse membrane homogenates can be inhibited, in a concentration-dependent manner, by incubating with excess amounts of unlabeled rH-ferritin but not with excess unlabeled horse spleen ferritin or human holo-transferrin. The curve shown is a representative analysis taken from one experiment. These data were used to generate a Hill plot (not shown).
Dissociation experiments demonstrate that 125I-rH-ferritin binding to membrane homogenates is reversible (Fig. 4). The dissociation rate constant (k-1) was determined graphically from the presented data to be 0.054 min-1.
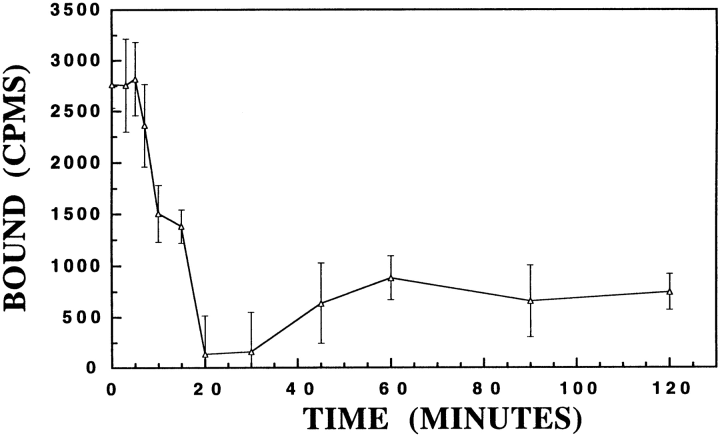
. Dissociation analysis. This graph shows binding of 125I-rH-ferritin (0.2 nM) to mouse membrane homogenates is reversible. After steady-state binding activity had been reached (60 min incubation), the addition of 1 μM rH-ferritin (at time 0) induced the dissociation of radiolabeled ferritin. The dissociation rate constant (K-1) was determined to be 0.054 min-1. Each point is the mean ± SE of duplicates from three separate mice.
To determine the distribution of the ferritin binding sites in the adult mouse brains, we used autoradiographic analysis. There was strong binding of 125I-rH-ferritin to all white matter tracts throughout the brain (Fig. 5). This binding was inhibited by the addition of 2,000-fold excess unlabeled rH-ferritin, whereas similar concentrations of transferrin, BSA, and horse spleen ferritin did not inhibit binding of the labeled ferritin (not shown).
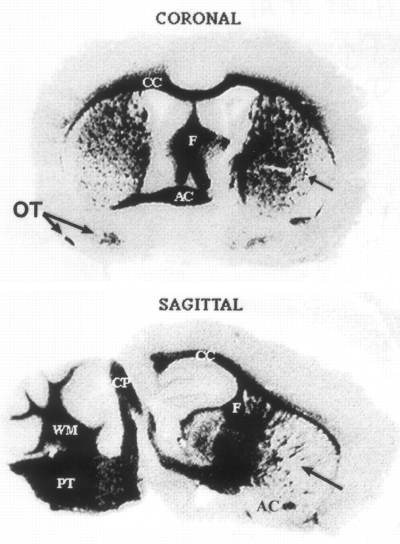
The distribution of the ferritin binding sites was demonstrated by incubating frozen sections (10 μm) of adult mouse brain with 5 nM125I-rH-ferritin for 1 h at 22°C. There is significant binding to white matter tracts throughout the entire brain. CC, corpus callosum ; PT, pontine tracts ; AC, anterior commissure ; F, fornix ; CP, cerebellar peduncles ; WM, white matter of cerebellar folia ; OT, medial and lateral olfactory tract ; arrow, striations through striatum. Note in the coronal section the anterior commissure is clearly present on the left side, whereas the entire corpus striatum can be seen on the right side.
DISCUSSION
These results reveal specific, saturable, and reversible binding sites present in the mouse brain that exhibit characteristics of a membrane receptor for ferritin. The ferritin binding sites are primarily distributed throughout the white matter tracts of the adult mouse brain, which is opposite of transferrin receptor distribution. Transferrin receptors are distributed primarily throughout gray matter (Hill et al., 1985 ; Mash et al., 1990). The cells in the white matter are predominantly oligodendrocytes and these cells contain most of the stainable iron in the brain (Hill and Switzer, 1984 ; Dwork et al., 1988 ; Morris et al., 1992 ; Benkovic and Connor, 1993). Our data may address an existing enigma, i.e., how iron levels are maintained at such high levels in white matter where transferrin receptors are minimally expressed. The implication of ferritin as an additional iron transport mechanism is significant due to the potential for ferritin to deliver a much greater amount of iron to cells than transferrin or lactoferrin (Faucheux et al., 1995). One molecule of transferrin or lactoferrin has the ability to carry two molecules of iron (Crichton and Charloteaux-Wauters, 1987). Theoretically, ferritin can bind up to 4,000 molecules of iron in its core (Harrison and Arosio, 1996), although extracted ferritins contain variable loads of iron ranging from a few hundred atoms up to >3,000 atoms (Levi et al., 1988). Thus, one molecule of ferritin has the potential to deliver 2,000 times the amount of iron to a cell than one molecule of transferrin.
Ferritin has previously been implicated in iron delivery to liver hepatocytes (Sibille et al., 1988). Ferritin receptor expression can be tightly regulated by cellular iron status in both erythroid precursor cells and K562 cells. When K562 cells are iron depleted by exposure to desferrioxamine, there is a twofold increase in rH-ferritin binding (Fargion et al., 1988). In erythroid precursor cells, ferritin receptor numbers were increased by iron depletion and decreased by iron loading (Gelvan et al., 1996). In addition, in this latter study, ferritin uptake was able to down-regulate transferrin receptor expression. This implicates that ferritin receptor and transferrin receptor expression could coregulate the iron status of a cell.
In addition to iron delivery, ferritin receptors could provide other functions. The ferroxidase activity of H-ferritin (the preferred subunit chain for the receptor) allows it to store oxidized iron, thereby preventing iron from participating in the Fenton reaction. Thus, ferritin can serve as an antioxidant. Indeed, addition of ferritin to endothelial cell cultures has been shown to protect these cells from iron-induced oxidative damage (Balla et al., 1992).
Ferritin, specifically the H-chain, may have a regulatory role in cellular development through suppression of cellular proliferation in several cell types, including lymphocytes (Fargion et al., 1991a), erythroid precursor cells (Fargion et al., 1992), and granulocyte macrophages (Broxmeyer et al., 1986). In addition, the expression of ferritin receptors has been shown to be cell cycle dependent (Fargion et al., 1988 ; Moss et al., 1992a). In both molt-4 and HL-60 cells, specific binding of H-ferritin was maximal during DNA synthesis (S-phase).
As a protein involved in iron delivery, there would be a requirement that ferritin be released from cells if not actively secreted. Serum ferritin levels normally range between 12 and 300 μg/L (Lipschitz et al., 1974), which suggests variable release (and possibly uptake) rates. Increases in serum ferritin can be detected during inflammatory responses (Lipschitz et al., 1974), cancer (Aungst, 1968 ; Worwood et al., 1974), and changes in body iron stores (Addison et al., 1972). In two recent reports, a mutation in the iron-responsive element, the regulatory site on ferritin mRNA, results in a 15—38-fold increase in ferritin levels in the blood (Beaumont et al., 1995 ; Girelli et al., 1995). In a hypotransferrinemic mouse strain that has <1% of the normal circulating levels of transferrin, there is a 14× increase in ferritin in plasma (Simpson et al., 1991). These data clearly indicate that serum ferritin levels are regulated and can be increased when regulatory mechanisms are dysfunctional or perhaps in a compensatory fashion when the major systemic iron transporter (transferrin) is decreased. The increase in serum ferritin in the hypotransferrinemic (Hp) mice may be responsible for the iron delivered to the oligodendrocytes in these animals (Dickinson and Connor, 1995). Clearly, iron can be delivered to the brain and oligodendrocytes in the absence of circulating transferrin (Simpson et al., 1991 ; Dickinson et al., 1996). The source of ferritin for the brain ferritin receptor is uncertain at this time. Nothing is currently known about transport of ferritin into brain. Local synthesis of ferritin within the CNS has been suggested (Keir et al., 1993) and ferritin is found in the CSF (Hallgren et al., 1980). Thus, the source of ferritin for the brain ferritin receptor could be the choroid plexus, thereby circumventing the need for ferritin to be transported across the blood-brain barrier. This is an area for future investigation.
Our data clearly indicate that there is a saturable, specific, and reversible ferritin binding protein in the mouse brain. However, competition and Hill plot analyses suggest nonunity of binding. Possible explanations for these results include cooperativity, heterogeneity of ligand and/or receptor, aggregation of ligand, and differing affinities for the radiolabeled ligand versus the nonradiolabeled ligand. Because ferritin consists of varying ratios of subunits, we interpret the competition and Hill plot analyses as being influenced by the ferritin in our preparation. Due to the heterogeneity of ferritin, there could also be heterogeneity of the ferritin receptor. Indeed, the hepatic ferritin receptor binds both H-chain and L-chain ferritin subunits with equal affinity (Mack et al., 1983 ; Adams et al., 1988a,b), whereas the ferritin receptor for other cell types prefers H-chain-rich ferritins (Fargion et al., 1991b ; Moss et al., 1992b). In brain, the H-chain subunit of ferritin is more abundant than L-chain subunits, but the ratio of H/L is dependent on brain region, cell type, and health status (Connor and Menzies, 1995). The iron status of ferritin may also influence the binding activity.
In conclusion, we have identified a specific, saturable, and reversible binding site for ferritin in the brains of mice that is localized specifically to white matter tracts. The distribution of ferritin binding is directly opposite that of transferrin and may indicate a novel, possibly selective, iron-regulatory system for cells in white matter. The possibilities that ferritin uptake may serve a protective role by sequestering excess iron in endosomes or that ferritin uptake may regulate cell proliferation and differentiation must also be considered.
Acknowledgements
Acknoledgement : This research was supported by the National Institutes of Health (NS22671 and NS34280) and the Jane B. Barsumian Trust Fund. We thank Dr. P. Santambrogio for purifying the recombinant ferritin.




