Regional Differences in Striatal Dopamine Uptake and Release Associated with Recovery from MPTP-Induced Parkinsonism
An In Vivo Electrochemical Study
Abbreviations used : DA, dopamine ; 5-HT, 5-hydroxytryptamine or serotonin ; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine ; redox, reduction/oxidation ; TH, tyrosine hydroxylase.
Abstract
Abstract : This study directly assessed striatal dopamine (DA) uptake rates and peak release in response to KCl in normal, symptomatic, and recovered 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated cats using in vivo electrochemistry. DA uptake rates measured after direct application of known concentrations of DA to the striatum were slowed significantly in both dorsal and ventral striatum in symptomatic cats compared with rates recorded in normal animals. DA uptake rates remained significantly slowed in recovered cats and were not significantly different from the rates recorded in symptomatic animals. In symptomatic cats, both DA uptake rates and the signal recorded in response to KCl stimulation were significantly decreased from normal in all dorsal and ventral striatal regions sampled. Reduction/oxidation (redox) ratios recorded in response to KCl stimulation suggested DA to be the predominant electroactive species. In spontaneously recovered MPTP-treated cats, recordings in the ventral striatum subsequent to KCl stimulation again suggested DA to be the predominant electroactive species released, and peak levels were significantly higher than those recorded in symptomatic animals. In the dorsal striatum of recovered cats, redox ratios recorded subsequent to KCl stimulation suggested serotonin rather than DA to be the predominant electroactive species released. Peak levels of release in the dorsal striatum were not significantly greater than those recorded in symptomatic animals. These results suggest that in spontaneously recovered MPTP-treated cats, there is partial recovery of ventral striatal DAergic terminals, persistent loss of dorsal striatal DAergic terminals, and a down-regulation of DA transporter number/function throughout the striatum. These processes may contribute to volume transmission of DA in the striatum and promote functional recovery.
Cats made parkinsonian by administration of the dopamine (DA) neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) spontaneously recover gross sensorimotor function by 6 weeks after MPTP exposure (Schneider et al., 1986 ; Schneider and Rothblat, 1991). Spontaneous functional recovery in MPTP-treated cats appears to involve compensatory neurochemical responses of the nigrostriatal DA system rather than extensive morphological changes. In cats spontaneously recovered from MPTP-induced parkinsonism, there has been no evidence of recovery of substantia nigra pars compacta neurons (Schneider and Markham, 1986) or extensive sprouting or reinnervation of the striatum (Rothblat and Schneider, 1994).
As in other models of MPTP-induced parkinsonism, there is a heterogeneous loss of the striatal DAergic innervation in cats exposed to this toxin. In symptomatic MPTP-treated cats, DA levels were decreased by 94-99% in all striatal regions sampled, with the dorsal lateral caudate nucleus more affected than the ventral medial caudate/nucleus accumbens (Schneider et al., 1986 ; Schneider and Rothblat, 1991). In spontaneously recovered animals, DA levels were still decreased by 95% in the dorsal striatum, but depleted by only 82% in the ventral medial caudate and by only 34% in the nucleus accumbens (Schneider and Rothblat, 1991). Using in vivo microdialysis, we found a significant recovery of extracellular fluid levels of DA in the dorsal lateral striatum in symptomatic cats despite 95% tissue DA depletion in the same area (Schneider et al., 1993). This increase in extracellular fluid DA levels in the dorsal lateral caudate corresponded to the time course of functional recovery as well as recovery of dorsal striatal electrophysiological parameters (Rothblat and Schneider, 1993).
In addition to increased local synthesis and release of DA by surviving DAergic neurons and terminals after lesion of the nigrostriatal pathway, as shown by Hefti et al. (1985) and others (Zigmond et al., 1984), there may also be enhanced diffusion of released DA away from its site of release and increased availability of DA in the extracellular fluid due to extensive lesion-induced loss of presynaptic DA uptake sites (Altar and Marion, 1989 ; Castaneda et al., 1990). Using in vivo microdialysis, we showed that in recovered MPTP-treated cats and in normal cats with DA uptake inhibition, DA released in the ventral medial caudate can be detected some time later in the dorsal lateral caudate (Schneider et al., 1994). In recovered cats, local release of DA in the dorsal lateral caudate contributed comparatively little to the extracellular fluid DA levels detected in that area. These findings, as well as those from other laboratories (Doucet et al., 1986 ; Garris et al., 1997), suggest that under certain conditions, volume transmission may contribute to the availability of DA in striatal regions almost devoid of their intrinsic DAergic innervation.
In order for volume transmission of DA to occur, there needs to be a continued loss or down-regulation of DA transporter sites, particularly in the areas of greatest DA release. An examination of [3H]mazindol binding to DA uptake sites in symptomatic and recovered MPTP-treated cats showed either no change or less binding to DA uptake sites, particularly in the ventral striatum of recovered cats compared with levels observed in symptomatic animals (Frohna et al., 1995). This was observed despite increased DA levels and tyrosine hydroxylase (TH)-positive fiber density in the ventral striatum in recovered animals. These data suggest a mechanism for recovery that may involve partial recovery of ventral striatal DAergic terminals to synthesize and release DA together with a down-regulation of local DA uptake sites. The combination of increased synthesis and release of ventral striatal DA and low DA uptake may lead to normalization of striatal physiology and gross behavioral recovery. The present study was designed to examine mechanisms of regional striatal DA uptake and release in normal, symptomatic, and recovered MPTP-treated cats using in vivo electrochemical techniques.
MATERIALS AND METHODS
Subjects and MPTP administration
Adult male or female cats (2.8-5.0 kg of body weight) were used. All procedures were performed in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Thomas Jefferson University Institutional Animal Care and Use Committee. Nine cats were studied when normal, seven cats were studied when symptomatic for parkinsonism, and 11 cats were studied when spontaneously recovered from parkinsonism. To produce a parkinsonian motor disorder, cats were administered MPTP-HCl (5.0-7.5 mg/kg i.m., once daily) for 7-10 days until stable motor deficits were observed. Animals were rated twice weekly on a modified Parkinson symptom rating scale (Table 1), beginning the day after the last injection of MPTP. Symptomatic animals were assessed 7-10 days after the last MPTP injection, and recovered animals were assessed 6 weeks after MPTP administration.
| Locomotion and movement | ||
| 0 | = | Normal walking and movements are fluid and consistent. |
| 1 | = | Some slowing, walking and movements are inconsistent. Minor abnormalities are present. |
| 2 | = | Mild to moderate impairments. There is decreased spontaneous locomotion and other movements. Hind limbs may be splayed and dragged when walking. |
| 3 | = | No effective locomotion or other movements. Animal stays in same general position for ~5 min or more. |
| Posture | ||
| 0 | = | Normal ; stands and sits in a normal fashion. |
| 1 | = | Limbs mildly flexed when standing. Hind limbs possibly slightly splayed. |
| 2 | = | Definite flexed posture with hind limbs splayed. |
| 3 | = | Grossly abnormal ; hunched posture when sitting ; all limbs flexed or splayed. |
| Sensory response | ||
| Tactile (brushing of hair and whiskers, mild to moderate punctate stimulation of face) | ||
| 0 | = | Readily orients toward or away from light stimulation of face. |
| 1 | = | Responds to light stimulation of face in <50% of trials (light brushing or punctate stimulation). |
| 2 | = | Only responds when stimulation is potentially noxious. |
| 3 | = | No response to any stimulation. |
| Visual (movement of small object, (e.g., string, across visual field ; a trial consists of two or three passes of object across visual field) | ||
| 0 | = | Follows all passes on most trials. |
| 1 | = | Responds initially to stimulus but does not follow every pass. Habituates rapidly. |
| 2 | = | Responds to initial pass of stimulus in <50% of trials. |
| 3 | = | No response. |
| Auditory (light stimulation | = | light shake of keys ; strong stimulation = rapid, loud shaking of keys) |
| 0 | = | Turns toward light stimulation almost 100% of time. |
| 1 | = | Responds to light stimulation <50% of the time, but responds to strong stimulation >50% of time. |
| 2 | = | Responds to strong stimulation <50% of time. |
| 3 | = | Does not respond to any stimulation. |
| Step-down time | : | Time to remove front paws from platform located 12 inches above the ground. |
In vivo electrochemistry
For in vivo electrochemical studies, cats were anesthetized with isoflurane (1.0-2.5%) and placed in a stereotaxic frame, and the skull overlying the striatum was removed. In vivo electrochemistry was performed according to previously described procedures (Gerhardt and Palmer, 1987). Micropipette/electrode assemblies were prepared by attaching a double-barrel micropipette to a carbon-fiber electrode. The tips of the micropipettes were 15-30 μm in diameter and positioned 150-250 μm from the tip of the electrode. One barrel of the pipette contained 200 μM DA and 100 μM ascorbic acid in saline. The other barrel contained 120 mM KCl and 2.5 mM CaCl2. The active electrode consisted of three carbon fibers sealed in a glass capillary (fiber diameter 30 μm ; exposed length 120-320 μm). The carbon fibers were coated with Nafion and calibrated in vitro to provide selectivity for DA over ascorbic acid of greater than 1,000 : 1. Only those electrodes that achieved or exceeded these specifications were used in this study. All electrodes exhibited linearity for calibration with concentrations of 2.0-10.0 μM DA, with correlation coefficients between 0.997 and 1.000. The signal-to-noise ratio of the electrodes was 3 : 1 resulting in a detection limit of 50 nM DA.
High-speed chronoamperometric electrochemical measurements were made continuously at 5 Hz and averaged to 1 Hz using an IVEC-10 system (Medical Systems Corp., Great Neck, NY, U.S.A.). The applied oxidation potential was +0.50 V for 100 ms (versus an Ag/AgCl reference electrode), and the resting potential was 0.0 V for 100 ms. The oxidation and reduction currents were digitally integrated during the last 80 ms of each 100-ms pulse. At the start of each experiment, pipette/electrode assemblies were stereotaxically positioned in one of the striatal regions to be sampled. Recordings were obtained from three different anterior-posterior locations (A : 16.0, 17.0, and 18.0) in each animal. The following striatal subregions were sampled : dorsal lateral caudate (L : 6.5, D : 6.5) ; dorsal medial caudate (L : 3.5, D : 6.0) ; ventral medial caudate (L : 3.5, D : 3.0) ; and nucleus accumbens (L : 3.5, D : 1.0), according to the atlas of Berman and Jones (1982).
For the determination of KCl-stimulated release of electro-active species and DA uptake rates, the micropipette/electrode assembly was lowered to the desired tissue location and left in place for at least 15 min before proceeding with the experiment. When a stable baseline was achieved, peak levels of KCl-stimulated release were determined, in most cases, before DA uptake rates were measured. Previous studies in this laboratory showed that priming the striatum with KCl before measuring DA uptake had no effect on DA clearance profiles (unpublished observations). Pressure (2-12 psi for 2-8 s) was applied to the micropipette containing KCl/CaCl2, and the peak electrochemical signal was recorded along with the reduction/oxidation (redox) ratio. Redox ratio was used as an indicator of the electroactive species released (Luthman et al., 1993). After at least 15 min and restabilization of baseline, pressure and time settings were adjusted to apply a greater amount of KCl. This sequence was repeated several times until a “washout” effect was observed consequent to the fluid volume of KCl administered (Luthman et al., 1993). The peak electrochemical signal recorded before this washout effect was obtained was taken as the index of peak release of the electroactive species for that region.
DA uptake rate measurements were obtained by measuring clearance of pressure-ejected DA (2-12 psi for 1-15 s) at peak levels of either 10 or 3 μM DA. The pipette contained 200 μM DA in 0.9% NaCl with 100 μM ascorbate. This concentration applied in volumes of 100-500 nl produced extracellular DA levels ranging from 3 to 10 μM. The amount of DA ejected to produce a desired peak DA level was also dependent on specific pipette/electrode assembly parameters. Three to five applications of DA were made at each striatal location. DA uptake was calculated as the slope of the descending portion of the recorded curve between 20 and 60% of peak amplitude (Cass et al., 1995).
At the conclusion of the experiment, each animal was killed with an overdose of sodium pentobarbital (150 mg/kg i.v.), and the brain was removed and placed in cold 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 24-48 h, followed by submersion in cold 30% sucrose in 0.1 M phosphate buffer for at least 48 h.
Histology
Serial frozen sections (35 μm) through the striatum used for electrochemistry were cut for confirmation of electrode placements and verification of the DAergic lesion. Some sections were processed for TH immunoreactivity as described previously (Schneider and Markham, 1986). In brief, sections were incubated in primary antibody (Pel Freeze ; rabbit anti-TH, 1 : 500 at 4°C for 48 h), followed by incubations in biotinylated secondary antisera and ABC reagent (Vector Laboratories) according to the manufacturer's instructions. Diaminobenzidine (0.05%) in 0.03% H2O2 was used as the chromogen. Striatal electrode placements were located visually during tissue sectioning and verified against stereotaxic coordinates used during in vivo studies.
Data analysis
Behavioral ratings were analyzed by nonparametric statistics (Kruskal-Wallis nonparametric analysis of variance with Dunn's test of post-hoc pairwise comparisons). Peak stimulated DA release and DA uptake rates were compared using analysis of variance, followed by post-hoc t tests using the Bonferroni correction. Analysis of variance used striatal subregions as the within-subjects variable and group (normal, symptomatic, recovered) as the between-subjects variable.
RESULTS
Behavior
All cats administered MPTP developed a severe parkinsonian motor disorder (Fig. 1). Symptomatic animals, assessed 7-10 days after the last MPTP injection, had significantly impaired locomotor abilities, sensory orienting responses, and abnormal posture (Fig. 1). In addition, step-down latency, a measure of akinesia, was increased significantly in symptomatic animals (27.5 ± 2.7 s) compared with normal animals (2.8 ± 1.1 s, p < 0.0001). Spontaneously recovered animals, assessed 6 weeks after the last MPTP injection, had significantly less impairments than symptomatic animals and were not significantly different from normal animals (Fig. 1). Furthermore, all cats assessed when recovered showed significant functional improvements compared with when they were symptomatic. Step-down latencies in recovered animals (2.5 ± 1.1 s) were similar to those recorded in normal animals and significantly faster than when these animals were symptomatic (28.1 ± 2.7 s, p < 0.0001).
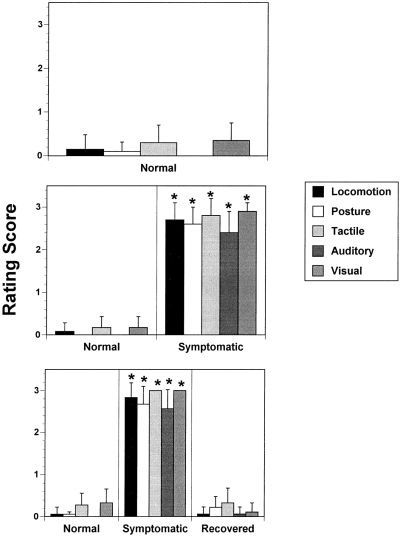
Behavioral rating scores (means ± SEM) from animals used in electrochemical studies as normal (top), parkinsonian (middle), and spontaneously recovered (bottom) MPTP-treated cats. Animals assessed when symptomatic had significant impairments (higher rating scores) compared to when they were normal (*p < 0.0001). Animals studied when recovered had significant motor deficits when initially symptomatic (high rating scores ; *p < 0.0001 vs. pre-MPTP scores), but at the time of the electrochemical studies, they had ratings that were not significantly different from normal. Missing bars in the ratings of normal animals indicate a score of 0 ± 0.
Recording sites
All electrochemistry measures used for analysis were obtained from histologically verified sites within the dorsal lateral, dorsal medial, and ventral medial caudate and nucleus accumbens (Fig. 2). As described previously (Schneider and Markham, 1986), TH immunohistochemistry showed significantly decreased TH-positive staining throughout the striatum in symptomatic animals with an increase in TH-positive staining in the ventral striatum in recovered cats, but no increase in TH-positive staining in the dorsal lateral caudate in the same animal (Fig. 3).

Diagrams of locations of electrochemical recordings obtained from the striatum of normal (A), symptomatic (B), and recovered (C) cats. Shaded areas represent the approximate striatal regions from which KCl-stimulated release and DA uptake measures were taken based on depth coordinates and histological verification. Vertical lines represent individual recording tracts based on histological reconstruction. DL, dorsal lateral caudate ; DM, dorsal medial caudate ; VM, ventral medial caudate ; N.ACC, nucleus accumbens.

A-C : Photomicrographs of TH-positive immunohistochemical staining in the dorsal lateral caudate of normal (A), symptomatic (B), and recovered (C) cats. Note the significant loss of TH-positive fibers in symptomatic animals with little recovery of TH-positive fiber density in recovered cats. D-F : Photomicrographs of TH-positive staining in the nucleus accumbens of normal (D), symptomatic (E), and recovered (F) cats stained for TH. TH staining is also significantly decreased in the nucleus accumbens in symptomatic animals. However, in contrast to the dorsal lateral caudate, there appears to be significant recovery of TH-positive fibers in recovered cats. Scale bar = 50 μm.
In vivo electrochemistry : DA uptake
DA uptake rates were recorded at three anterior-posterior levels, separated by ~1.0 mm, in each striatal subregion in each cat. As there were no statistically significant differences in uptake rates at different anterior-posterior locations within an area (e.g., dorsal lateral caudate), the data obtained from different anterior-posterior locations in a particular area were combined for analysis. Two levels of extracellular DA were used to measure uptake kinetics, a low (3 μM) and a high (10 μM) level. This was done because uptake rates may vary as a function of the amount of extracellular DA present (Zahniser et al., 1996).
In normal animals, striatal DA uptake rates followed a pattern predicted by previously reported [3H]mazindol binding studies (Frohna et al., 1995). That is, there were no statistically significant differences between DA uptake rates in dorsal lateral or dorsal medial caudate or between ventral medial caudate and nucleus accumbens. However, DA uptake rates measured after application of 3 or 10 μM DA were significantly faster (p < 0.05) in the dorsal striatum (0.061 ± 0.012 μM/s and 0.112 ± 0.023 μM/s, respectively) than in the ventral striatum (0.044 ± 0.009 μM/s and 0.070 ± 0.018 μM/s, respectively). In addition, DA uptake rates in all striatal regions were significantly faster (p < 0.05) in the presence of high extracellular DA levels (10 μM) than low extracellular DA levels (3 μM).
In symptomatic MPTP-treated cats, DA uptake rates associated with low (3 μM) and high (10 μM) peak DA levels were significantly slower than in normal animals in all striatal regions sampled (Table 2, Figs. 4 and 5). In the dorsal striatum, DA uptake rates decreased 62-76% from normal values, whereas in the ventral striatum, uptake rates slowed to 51-57% below normal (Table 2). DA uptake rates in the dorsal lateral caudate were not significantly slower than in the dorsal medial caudate. Likewise, ventral medial caudate uptake rates were not statistically different from rates measured in the nucleus accumbens Table 2). In contrast to normal animals, uptake rates in the dorsal lateral caudate in symptomatic animals were not significantly faster than those in either the ventral medial caudate or the nucleus accumbens (Table 2). DA uptake rates were significantly faster in the presence of higher extracellular DA levels in all striatal areas (p < 0.05 ; Table 2).
| Uptake rate (low) | Uptake rate (high) | |
|---|---|---|
| Dorsal lateral caudate | ||
| Normal | 0.067 ± 0.007 (8) | 0.114 ± 0.010 (8) |
| Symptomatic a | 0.016 ± 0.001 (6) | 0.027 ± 0.002 (7) |
| Recovered a | 0.020 ± 0.002 (11) | 0.035 ± 0.002 (10) |
| Dorsal medial caudate | ||
| Normal | 0.054 ± 0.002 (7) | 0.109 ± 0.007 (9) |
| Symptomatic a | 0.021 ± 0.002 (6) | 0.031 ± 0.004 (7) |
| Recovered a | 0.021 ± 0.002 (11) | 0.035 ± 0.004 (9) |
| Ventral medial caudate | ||
| Normal | 0.041 ± 0.003 (8) | 0.069 ± 0.005 (7) |
| Symptomatic a | 0.020 ± 0.004 (6) | 0.032 ± 0.003 (7) |
| Recovered a | 0.018 ± 0.002 (11) | 0.035 ± 0.003 (10) |
| Nucleus accumbens | ||
| Normal | 0.046 ± 0.004 (6) | 0.070 ± 0.009 (6) |
| Symptomatic a | 0.020 ± 0.003 (6) | 0.030 ± 0.004 (7) |
| Recovered a | 0.021 ± 0.002 (9) | 0.037 ± 0.003 (9) |
- a DA uptake rates recorded in all striatal regions in symptomatic and recovered cats (for both levels of extracellular DA) were decreased significantly from normal (p < 0.0001).
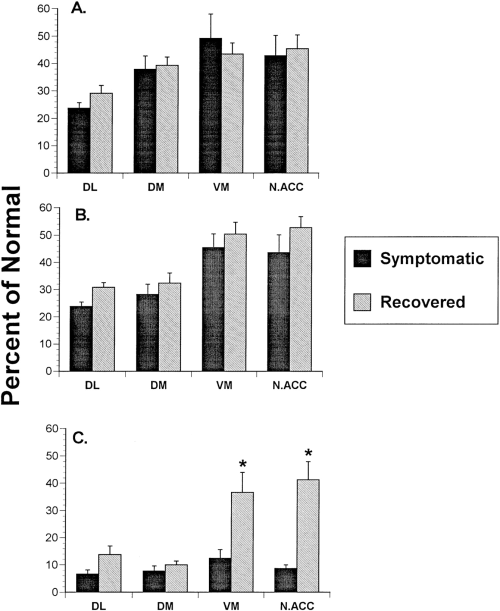
Mean (±SEM) changes in (A) uptake rate at the low DA level (3 μM), (B) uptake rate at the high DA level (10 μM), and (C) peak KCl-stimulated release in symptomatic and recovered MPTP-treated cats expressed as a percentage of levels observed in normal animals. In the ventral striatum, KCl-stimulated DA release increased significantly in recovered cats without an accompanying increase in uptake rate. The electrochemical signals recorded in ventral medial caudate and nucleus accumbens of recovered animals in response to KCl stimulation were the only measures significantly different from those in symptomatic cats (*p < 0.05). DL, dorsal lateral caudate ; DM, dorsal medial caudate ; VM, ventral medial caudate ; N.ACC, nucleus accumbens.
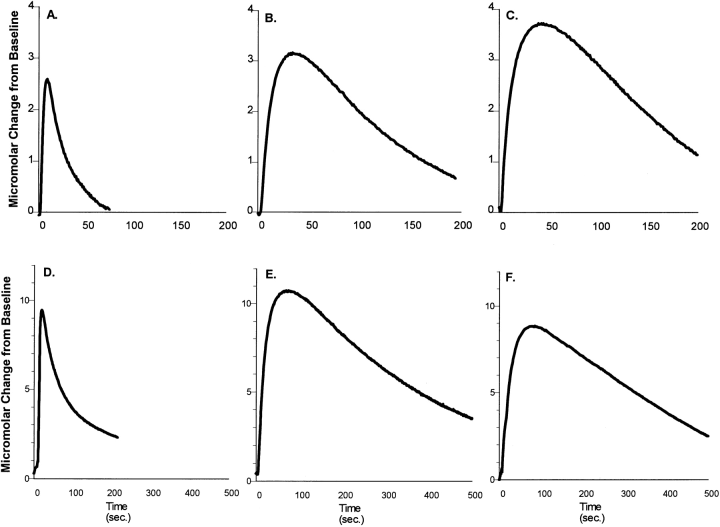
A-C : Representative electrochemical recordings obtained from the dorsal lateral caudate in response to pressure ejection of 200 μM DA in normal (A), parkinsonian (B), and spontaneously recovered (C) cats. Time and pressure settings were adjusted to achieve an extracellular DA level of ~3 μM. Note the slowed DA uptake rates in B and C indicated by the change in shape of the descending portion of the uptake curves in symptomatic and recovered cats. There is no difference between the uptake curves obtained from symptomatic and recovered animals. D-F : Representative electrochemical recordings obtained from the nucleus accumbens in response to pressure ejection of 200 μM DA in normal (D), parkinsonian (E), and spontaneously recovered (F) cats. Time and pressure settings were adjusted to achieve an extracellular DA level of ~10 μM. Again, note the slowed DA uptake rates in E and F indicated by the change in shape of the descending portion of the uptake curves in parkinsonian and recovered cats. There is no difference between the curves obtained from symptomatic and recovered animals.
In both dorsal and ventral striatum in spontaneously recovered MPTP-treated cats, DA uptake rates were very similar to those observed in symptomatic cats (Figs. 4 and 5) and significantly slower than those measured in the same regions in normal animals (Table 2). Uptake rates in the dorsal lateral caudate (69-71% decrease from normal) were not significantly different from those measured in the dorsal medial caudate (61-68% decrease from normal) regardless of the level of DA used to obtain the measures. Likewise, uptake rates in the ventral medial caudate (50-57% decrease from normal) were not significantly different from those measured in the nucleus accumbens (47-55% decrease from normal). As in symptomatic animals, DA uptake rates in the dorsal lateral caudate were not significantly faster than those measured in either the ventral medial caudate or nucleus accumbens (Figs. 4 and 5). DA uptake rates were significantly faster in the presence of higher extracellular DA levels in all dorsal and ventral striatal regions sampled (p < 0.05 ; Table 2).
In vivo electrochemistry : KCl-stimulated release
KCl-stimulated release of elctroactive species in normal animals demonstrated a pattern predicted by measures of postmortem tissue catecholamine levels (Schneider and Rothblat, 1991). The peak signal recorded was significanlty greater in the dorsal lateral caudate versus the ventral medial caudate and nucleus accumbens (p < 0.05 ; Table 3). Redox ratios recorded in all striatal areas (and verified by in vitro calibration) suggested that the predominant electrochemical signal recorded reflected DA release (Table 3, Fig. 6).
| Peak response to KCl | Redox ratio | |
|---|---|---|
| Dorsal lateral caudate | ||
| Normal | 9.37 ± 0.66 (9) | 0.51 ± 0.03 (9) |
| Symptomatic a | 0.62 ± 0.14 (7) | 0.40 ± 0.06 (7) |
| Recovered a | 1.29 ± 0.29 (9) | 0.26 ± 0.03 (9) b |
| Dorsal medial caudate | ||
| Normal | 9.21 ± 0.59 (7) | 0.52 ± 0.03 (7) |
| Symptomatic a | 0.71 ± 0.17 (7) | 0.51 ± 0.07 (7) |
| Recovered a | 0.93 ± 0.13 (10) | 0.29 ± 0.02 (10) c |
| Ventral medial caudate | ||
| Normal | 6.89 ± 0.68 (7) | 0.45 ± 0.02 (7) |
| Symptomatic a | 0.86 ± 0.22 (7) | 0.41 ± 0.05 (7) |
| Recovered a | 2.53 ± 0.49 (9) b | 0.45 ± 0.05 (9) |
| Nucleus accumbens | ||
| Normal | 6.56 ± 0.77 (6) | 0.50 ± 0.03 (6) |
| Symptomatic a | 0.57 ± 0.08 (7) | 0.33 ± 0.06 (7) |
| Recovered a | 2.70 ± 0.44 (7) c | 0.44 ± 0.04 (7) |
- a All peak responses recorded in symptomatic and recovered cats were decreased significantly from peak responses recorded in normal animals.
- b p < 0.05
- c p < 0.01, recovered vs. symptomatic.
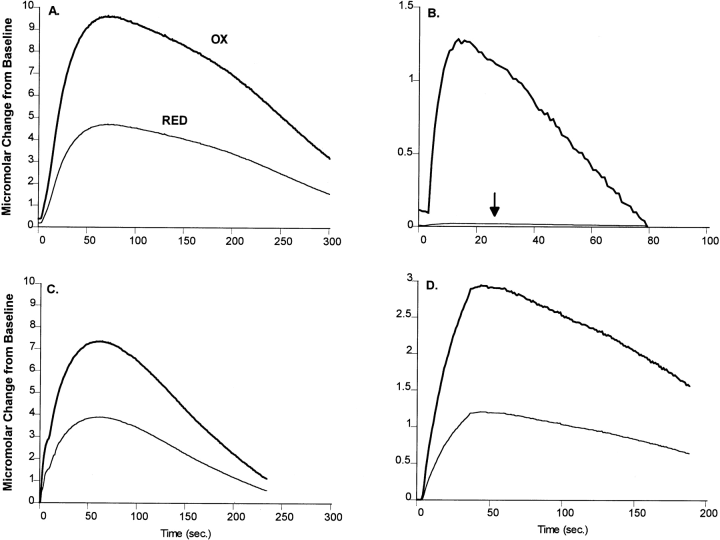
Oxidation (OX) and reduction (RED) curves recorded in response to pressure ejection of 120 mM KCl in the dorsal lateral caudate (A and B) and the nucleus accumbens (C and D) in normal (A and C) and spontaneously recovered (B and D) MPTP-treated cats. The ratio produced by these curves can be indicative of the principal electroactive species measured. The ratios produced by the curves in the normal dorsal lateral caudate (A) and normal and recovered nucleus accumbens (C and D) indicate the electroactive species to be primarily DA. The ratio of the curves produced in the recovered dorsal lateral caudate (B) in a spontaneously recovered cat suggests the electroactive species to be primarily 5-HT-like. The arrow indicates the location of the reduction curve.
The signal recorded from KCl-stimulated release was decreased significantly in all striatal regions in symptomatic cats compared with normal animals (Table 3, Fig. 4). The decreases in the amplitude of the signal per pulse of KCl (compared with normal) were similar in all regions sampled, ranging from 94 ± 4% decrease in the dorsal lateral striatum to 88 ± 8% decrease in the ventral medial caudate. Redox ratios recorded in all striatal regions suggested that the predominant electrochemical species was DA (Table 3).
In spontaneously recovered animals, there were no statistically significant increases in the amplitude of the electrochemical signal recorded in the dorsal lateral and dorsal medial caudate after KCl stimulation compared to symptomatic animals. However, the redox ratios recorded in the dorsal striatum were significantly lower than those recorded in either normal or symptomatic animals (0.28 ± 0.09 in recovered animals vs. 0.52 ± 0.09 in normal animals, p < 0.0001 ; Table 3, Figs. 4 and 6). In the ventral striatum, there were significant increases (up to 4.5-fold) in the peak signal recorded following KCl application, compared to symptomatic animals (Table 3 ; Fig. 4). Redox ratios recorded in the ventral striatum were not significantly different from those measured in either normal or symptomatic animals (Table 3 ; Fig. 6).
DISCUSSION
As described for rodents (Gerhardt et al., 1985), we found regional differences in the normal cat striatum in the peak signal recorded following KCl application and in DA clearance rates. The peak electrochemical signal recorded was higher and DA uptake rates were faster in the dorsal striatum compared to the ventral striatum. The fast uptake of DA from the extracellular space coupled with intrinsically low levels of extracellular DA measured in microdialysate (Schneider et al., 1993) suggests a classical neurotransmitter role for DA in the normal cat striatum (Garris and Wightman, 1994) and a removal of extracellular DA driven primarily by active uptake.
In symptomatic MPTP-treated cats, DA uptake rates in all striatal regions sampled were significantly slowed compared to normal, as would be expected consequent to the extensive loss of DAergic terminals and uptake sites that occurs in these animals (Schneider et al., 1986 ; Frohna et al., 1995). In contrast to normal animals, the clearance of extracellular DA in symptomatic cats appears to be driven primarily by a process of diffusion. Alternatively, slowed uptake might reflect a shift to a predominantly low-affinity uptake system after injury. Although this mode of transport has been demonstrated in the neuroendocrine tuberoinfundibular DA system (Annunziato et al., 1980 ; Demarest and Moore, 1998) ; it has not been shown to exist in the striatum (Garris and Wightman, 1994) and is unlikely to account for the slowed uptake of extracellular DA observed in this study.
In symptomatic animals, peak levels of KCl-stimulated release of electroactive species (identified to be primarily DA-like) were decreased significantly in all striatal regions, with no differences in the responses of the dorsal and ventral striatum. These findings confirm previous work that showed that terminal stores of DA measured by postmortem HPLC analyses are extensively and similarly depleted in dorsal and ventral striatum in symptomatic MPTP-treated cats (Schneider and Rothblat, 1991). Redox ratios recorded in the striatum of symptomatic cats were similar to those recorded in normal animals and suggest that the signal measured reflected DA and not another electroactive species. Nafion coating of the electrodes as well as the linear response of the electrodes to DA and selectivity for DA over ascorbate further suggest that the signal measured had an extremely high likelihood of being related to DA (Zahniser et al., 1996). Furthermore, the electrodes used in this study were tested in vitro at the conclusion of the experiment for sensitivity to serotonin (5-HT) versus DA. All electrodes tested in this manner demonstrated a selectivity for DA of at least 2 : 1 compared to 5-HT.
In cats spontaneously recovered from MPTP-induced parkinsonism, DA uptake rates in all striatal regions sampled were significantly slowed compared to normal and were not significantly different from uptake rates measured in symptomatic animals. As in symptomatic animals, clearance of extracellular DA seemed dependent primarily on diffusion rather than active uptake. This suggests that in recovered cats, either there is a persistent loss of striatal DAergic terminals and DA transporter sites or there may be at least a partial recovery of the DAergic innervation accompanied by a down-regulation of transporter expression or a decrease in transporter function. A previous study has shown that there is a substantial decrease in [3H]mazindol binding to striatal DA transporter sites in symptomatic MPTP-treated animals that does not increase significantly in recovered animals (Frohna et al., 1995). In fact, if anything, there was even less [3H]mazindol binding in the ventral striatum in recovered cats compared with symptomatic cats. The present findings support the idea that there is a persistent decrease in DA uptake site density in recovered cats rather than a change in transporter function.
The peak electrochemical signal recorded after KCl application in the ventral striatum in recovered cats was significantly decreased from that in normal animals, but significantly increased from that in symptomatic animals. As in other studies (Luthman et al., 1993), we used the ratios of reduction to oxidation currents at the peak of the oxidation signal as an index of the major species giving rise to the electrochemical signal. Redox ratios were no different from those recorded in normal animals and were indicative of a DA-like signal (Luthman et al., 1993). In vitro calibration of our electrodes confirmed this.
In the dorsal striatum of spontaneously recovered MPTP-treated cats, potassium-evoked overflow of neurotransmitters gave rise to a signal that appeared to be more 5-HT-like than DA-like. Previous studies have reported that the ratios of the reduction to oxidation currents (redox ratios) are different for DA (0.4-0.7), 5-HT (0.0-0.2), and ascorbic acid (0.0) (Luthman et al., 1993). Although redox ratios in the dorsal striatum were all considered DA-like (range of 0.40-0.52) in normal and symptomatic animals, there was a clear shift towards a signal that was primarily more 5-HT-like (range of 0.26-0.29) in recovered animals. At the conclusion of the experiments, redox ratios for 5-HT were determined with our electrodes and found to be in the range of 0.0-0.1, consistent with the redox ratios previously reported for 5-HT (Luthman et al., 1993). The redox ratios recorded most likely reflect a complex signal predominated by 5-HT rather DA. Ongoing studies are attempting to clarify this issue. However, these functional in vivo data support a previous report of increased postmortem tissue levels of 5-HT in the dorsal, but not ventral, striatum of recovered MPTP-treated cats compared to normal animals (Schneider and Rothblat, 1991). It is interesting that in the previous study, 5-HT levels were also elevated significantly in symptomatic animals (Schneider and Rothblat, 1991). In symptomatic cats, 5-hydroxyindoleacetic acid levels were decreased dramatically, suggesting that 5-HT might accumulate in striatal terminals, but may not be in a releasable form. In recovered animals, 5-HT levels were as high as in symptomatic animals, but 5-hydroxyindoleacetic acid levels were normal, suggesting an increased release and utilization of 5-HT (Schneider and Rothblat, 1991).
It is possible that the process of neurochemical compensation for the loss of DA that occurs in recovered MPTP-treated cats may lead to changes in the functional responsiveness of dorsal striatal monoamine terminals. Such a process has previously been suggested to occur in rats that received neonatal lesions of the DA system (Luthman et al., 1993). This also implies that there may be a relationship between functional recovery and increases
Acknowledgements
This work was supported by NIH grant NS 23980.




