Inhibition of Calcium-Dependent NMDA Receptor Current Rundown by Calbindin-D28k
Abbreviations used : BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N',N' -tetraacetic acid ; CaBP, calbindin-D28k ; β-gal, β-galactosidase ; HEK 293, human embryonic kidney 293 ; TBS, Tris-buffered saline ; TSP, total soluble protein.
Abstract
Abstract : NMDA receptors are regulated by several different calcium-dependent processes. To determine if the presence of the intracellular calcium-binding protein calbindin-D28k can influence the calcium regulation of NMDA receptor activity,human embryonic kidney 293 cells were co-transfected with cDNAs for NMDA receptor subunits and cablinding. Recordings were made using the nystatin perforated patch technique to preserve intracellular contents. When compared with control cells (transfected with cDNA enconding β-galactosidase in place of calbindin), the presence of calbindin had no effect on either calcium-dependent inactivation or the calciumsensitive, time-dependent increase in glycine-independent desensitization of NMDA receptor-mediated currents. However, the development of calcium-dependent rundown of peak glutamate-evoked current was slowed significantly in calbindin versus β-galactosidase cotransfected cells. This result was true for cells transfected with either NR1/NR2A or NR1/NR2B subunits, although calbindin was relatively less effective at inhibiting rundown in NR1/NR2B-expressing cells. NMDA peak current rundown has been attributed to calcium-induced depolymerization of the actin cytoskeleton. Therefore, our results indicate that although calbindin may not influence calcium-dependent regulatory processes occurring very near the NMDA receptor channel, it appears to be more effective at buffering local elevations in intracellular calcium at the actin cytoskeleton.
NMDA receptors, a subgroup of the ligand-gated ion channel class of glutamate receptors, play a key role in several neuroplastic mechanisms, including long-term potentiation and long-term depression in the hippocampus (Bear and Malenka, 1994). Activation of these receptors is also thought to be involved in mechanisms underlying cell death associated with neurodegenerative diseases and stroke (Choi, 1992, 1995 ; Bonfoco et al., 1995). One characteristic of NMDA receptors vital to both its neuroplastic and neurodegenerative functions is its relatively high calcium permeability (Choi, 1992 ; Bear and Malenka, 1994). In turn, calcium ions negatively modulate NMDA receptor function by activating several intracellular processes, including calmodulin-dependent inactivation, calcineurin-dependent increases in glycine-independent desensitization, and peak current rundown due to actin depolymerization (Rosenmund and Westbrook, 1993a,b ; Tong and Jahr, 1994 ; Tong et al., 1995 ; Ehlers et al., 1996 ; Zhang et al., 1998).
NMDA receptors are heteromeric proteins made up of NR1 subunits combined with any of either NR2A, NR2B, NR2C, or NR2D (Hollmann and Heinemann, 1994). The NR2 subunits show distinct patterns of expression in the CNS and are thought to serve a regulatory role in receptor function, modulating such properties as voltage-dependent Mg2+ block, single-channel conductance, and macroscopic kinetics of glutamate-evoked current responses (for review, see McBrain and Mayer, 1994). Sensitivity to calcium-dependent inactivation and current rundown may also be determined by NR2 subunit composition (Krupp et al., 1996 ; Medina et al., 1996). Thus, calcium-dependent regulation of NMDA receptor function may vary across neuronal populations according to subunit composition. As well, this process may be modified by the presence of intracellular proteins that can rapidly bind calcium with high affinity.
Calbindin-D28k (CaBP), a calcium-binding protein of the EF-hand family, is expressed abundantly in the CNS, where it is found in distinct neuronal populations and is believed to function as a buffer against increases in cytosolic free calcium (Baimbridge et al., 1992 ; Chard et al., 1993). The concentration of CaBP within cerebellar Purkinje cells has been estimated to be as high as 0.1-0.2 mM based on data obtained by radioimmunoassay (Baimbridge et al., 1982), a quantitative immunocytochemistry method (Davenport et al., 1990), and a fura-2 titration method (Fierro and Llano, 1996). As each molecule of CaBP can bind up to four calcium ions, the total calcium-buffering capacity of Purkinje cells accounted for by the presence of CaBP is on the order of 0.4-0.8 mmol/L of cytoplasm. Evidence for CaBP's precise role in neuronal functioning, however, is limited. In one study of mice carrying a null mutation of the CaBP gene, synaptically activated calcium signals recorded in cerebellar Purkinje neurons were enhanced, and the animals exhibited ataxia (Airaksinen et al., 1997). Other studies indicate that CaBP can alter firing properties of rat supraoptic neurons and interfere with calcium-dependent inactivation of L-type calcium channels, suggesting that this protein can effectively buffer submembrane free calcium (Köhr and Mody, 1991 ; Li et al., 1995 ; however, see Lledo et al., 1992 ; Chard et al., 1993). Whether expression of CaBP may be neuroprotective is less clear ; some studies have shown a correlation between high levels of this protein and resistance to excitotoxic neuronal death (Mattson et al., 1991 ; Iacopino et al., 1992 ; Burke and Baimbridge, 1993 ; Goodman et al., 1993 ; Diop et al., 1995), whereas others have found no correlation or even increased vulnerability (Freund et al., 1991 ; Möckel and Fischer, 1994 ; Klapstein et al., 1998).
To determine whether the calcium buffering capacity of CaBP can influence calcium-dependent inhibition of NMDA receptor function, we overexpressed CaBP in human embryonic kidney 293 (HEK 293) cells co-transfected with NMDA receptors. We used patch-clamp recording with nystatin perforated patch to examine glutamate-evoked whole-cell current properties. This approach permitted analysis of the effect of CaBP on NMDA receptors of known subunit composition, expressed in a homogeneous cell population that differed only by the presence or absence of calbindin. We found that CaBP slows onset of peak current rundown, with a more sustained effect on receptors composed of NR1/NR2A than NR1/NR2B. Our results suggest that the potential of CaBP to afford protection against excitotoxic neuronal injury may depend upon neuronal NMDA receptor subunit composition.
MATERIALS AND METHODS
Cell culture and transfection
HEK 293 cells were maintained in an incubator at 37°C, 5% CO2 in minimal essential medium (GIBCO, Grand Island, NY, U.S.A.) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, U.S.A.), L-glutamine, and sodium pyruvate (GIBCO). Transfection was via calcium phosphate precipitation (Chen and Okayama, 1987) of plasmid cDNA using a total of 16 μg of cDNA per 10-cm plate at a ratio of 1 : 1 : 2 for NR1/NR2/CaBP. In control cells, cDNA encoding β-galactosidase (β-gal) was substituted for CaBP to account for any possible effects of protein overexpression on NMDA receptor synthesis or assembly. Cells were transfected for ~8 h in a 3% CO2 incubator, then washed with fresh medium supplemented with 1 mM DL-2-amino-5-phosphonopentanoic acid or 100 μM memantine (both from RBI, Natick, MA, U.S.A.) (Raymond et al., 1996 ; Chen et al., 1997), and plated onto glass coverslips in 35-mm culture dishes (Falcon, Franklin Lakes, NJ, U.S.A.).
Western blot analysis
Approximately 40 h after the start of transfection, HEK 293 cells transiently transfected with CaBP were washed once with warm phosphate-buffered saline, scraped from the plates into ice-cold buffer, centrifuged (600 g, for 5 min), and stored at -20°C. After thawing on ice, cell pellets were resuspended in 20 mM Tris (pH 7.4), 100 mM NaCl, and 0.1 mM CaCl2, and boiled for 5 min. Cell debris were removed by centrifugation, and the soluble protein concentration in the supernatant was estimated by A280. Total soluble protein (TSP ; 30 μg/lane) from each transfection was loaded and separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Gels were then transferred to nitrocellulose membranes, and western blot analysis was performed as outlined below.
Estimation of CaBP concentration in transfected cells
The concentration of CaBP in transfected cells was estimated using a western slot-blot method (Hersham et al., 1993). Transfected cells, grown on 10-cm tissue culture plates, were dislodged by trypsinization in 10 mM phosphate-buffered saline (pH 7.4). Live cells were counted using trypan blue exclusion, and replicate volumes containing 106 cells were centrifuged at 600 g for 3 min at room temperature. Cell pellets were stored at -20°C. To calculate transfection efficiency, duplicate 50,000-cell aliquots were replated and grown for 5 h before fixation and immunocytochemical staining for CaBP, as previously described (Buchan and Baimbridge, 1988). For slot-blot analysis, cell pellets were resuspended in 500 μl of 50 mM Tris-buffered saline (TBS) containing 1 mM EDTA, boiled for 5 min, and centrifuged in a microfuge for 10 min. The supernatant was collected and concentration of TSP determined by A280. Aliquots (200 μl) containing from 0.05-20 μg of TSP were applied to a nitocellulose membrane using a Bio-Rad slot-blot apparatus according to the manufacturer's instructions. For standards, we used recombinant human CaBP (range 0-1,000 ng) and TSP extracted from a rat cerebellar homogenate.
After nitrocellulose membranes had been blocked overnight at 4°C in TBS containing 2% skim milk, 50% horse serum, and 0.2% Tween-20, they were incubated with polyclonal antibodies against CaBP [raised in rabbits against purified bovine cerebellar CaBP (Buchan and Baimbridge, 1988)] at a dilution of 1 : 1,000 in TBS-Tween for 1 h at room temperature. Blots were rinsed twice in TBS-Tween (5 min each), and the secondary antibody (donkey anti-rabbit IgG conjugated with horseradish peroxidase ; Amersham, Arlington Heights, IL, U.S.A.) was applied at a dilution of 1 : 2,000 for 1 h at room temperature. After two additional washes, Amersham's enhanced chemiluminescence system was used to visualize CaBP. The amount of CaBP in cell extracts was determined according to the method of Hersham et al. (1993).
Electrophysiology
Approximately 26-32 h after the transfection was started, HEK 293 cells were transferred to a recording chamber mounted on the stage of an Axiovert 100 inverted microscope (Carl Zeiss, Thornburg, NY, U.S.A.). Patch-clamp recording in the whole-cell mode was performed using the nystatin perforated patch method to maintain intracellular contents (Horn and Marty, 1988). A 100 mg/ml stock of nystatin was prepared fresh daily in dimethyl sulfoxide ; 5 μl of this stock was added to 0.5 ml of the electrode solution (55 mM KCl, 75 mM potassium acetate, 8 mM MgCl2, 10 mM HEPES ; pH 7.3 with (KOH), and the resulting suspension was vortexed and then sonicated for 30 s. When filled with this solution, electrodes had resistances of 1-3 MΩ. Upon sealing the electrode to the cell, the cell was lifted from the glass surface and antibiotic partitioning was allowed to proceed until the series resistance had stabilized between 20 and 30 MΩ. Voltage-clamped currents were recorded using either an EPC 7 (List Electronics, Darmstadt-Eberstadt, Germany) or an Axopatch 200B (Axon Instruments, Foster City, CA, U.S.A.) patch-clamp amplifier. pCLAMP software (Axon Instruments) was used for data acquisition and analysis. The membrane holding potential was -60 mV for all experiments. The recording chamber was constantly perfused with the external recording solution, containing 145 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 11 mM glucose, and 10 mM HEPES and titrated to pH 7.3 with NaOH, to which 30 μM glycine was added just before use. Cells were rapidly exposed to 10 μM glutamate with 30 μM glycine using an ultrafast piezo-driven (Burleigh, Fishers, NY, U.S.A.) theta tube system, as previously described (Chen et al., 1997). Both glutamate and glycine were stored as 10 mM stock solutions at -20°C and diluted just before use.
Materials
Unless stated otherwise, all chemicals were obtained from Sigma (St. Louis, MO, U.S.A.). NR1A (nomenclature of Sugihara et al., 1992) and NR2B cDNAs were gifts from Dr. Nakanishi (Kyoto University, Kyoto, Japan), and NR2A (also called ε 1, from mouse) was a gift from Dr. Mishina (Tokyo University, Tokyo, Japan). These cDNAs were subcloned into a mammalian expression vector with a CMV promoter, as previously described (Raymond et al., 1996). cDNA encoding CaBP was cloned from a human brain cDNA library via PCR. The identity of the PCR product was confirmed by automated DNA sequencing (nucleic acid and protein sequencing unit, University of British Columbia) and found to be identical to the published human CaBP cDNA coding region (Parmentier et al., 1987). The gene was then inserted in the NotI restriction site of pCMVβ (Clontech) following excision of the lacZ gene.
Data presentation
Data are presented as means ± SEM. Statistical tests of significance were via either two-way ANOVA, used to test if two curves were different, or two-way Student's t test to test between population means. Results were deemed to be significant if p < 0.05.
RESULTS
Expression of recombinant CaBP was confirmed by western blot analysis of transfected HEK 293 cell extracts (representative blot shown in Fig. 1A ; n = 4), as well as by immunocytochemical staining of transfected cells (data not shown). Using a western slot-blot analysis (representative blot and standard curve shown in Fig. 1B and C ; n = 4), we estimated that the average concentration of recombinant CaBP in transfected cells, corrected for transfection efficiency, was 32 μg/mg of TSP. This compared with a value of 24 μg/mg of TSP for rat cerebellum. In the latter, only Purkinje cells and climbing fibers contain CaBP, and the value obtained is therefore an underestimate (in proportion to the relative volume of Purkinje cells and climbing fibers to total cerebellar volume) of the actual concentration of CaBP in the cytoplasm of Purkinje cells. The average expression level of CaBP in our transfections is therefore less than that expressed naturally in Purkinje cells, but is probably equivalent to or higher than levels expressed in other neurons (Baimbridge et al., 1992).
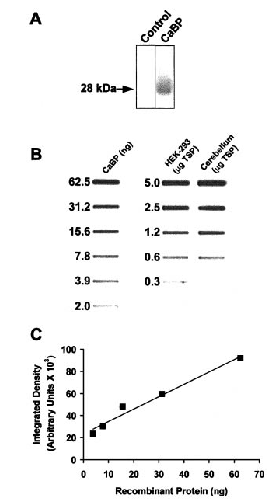
Western blot analysis of protein extracts from transiently transfected HEK 293 cell cultures and estimation of CaBP concentration. A : Extracts from cells transiently transfected with pCMVβ (control lane) or cDNA encoding calbindin (CaBP lane) and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis were probed with anti-CaBP antibody. Note that a prominent band at 28 kDa is present only in the lane loaded with CaBP-transfected cell extracts. B : Quantitative slot-blot analysis of CaBP-transfected HEK-293 cell cultures. Recombinant CaBP standards were blotted along with varying amounts of TSP extracts from transiently transfected HEK-293 cells and (for comparison) rat cerebellum. C : Standard curve generated by densitometric analysis of the range of recombinant CaBP standards.
To determine if buffering of calcium by CaBP can influence NMDA receptor function, we used the perforated patch whole-cell recording technique to compare glutamate-evoked current responses of NMDA receptors in cells co-transfected with CaBP versus β-gal (control). Perforated patch recording was chosen to ensure preservation of endogenous calcium buffering systems, as well as the transfected CaBP. Importantly, cells expressing β-gal versus CaBP showed similar current amplitudes in response to fast application of 10 μM glycine. For cells expressing NR1/NR2A, mean initial peak current amplitudes were 1,257 ± 109 pA (n = 14) versus 1,080 ± 88 pA (n = 17) for co-transfection with β-gal and CaBP, respectively (p = 0.21, t test). Likewise, for NR1/NR2B-expressing cells, mean peak current amplitudes were 709 ± 60 pA (n = 11) versus 741 ± 73 pA (n = 14) for co-transfection with β-gal and CaBP, respectively (p = 0.74, t test).
Calcium-dependent inactivation of NR1/NR2A-mediated currents
In recordings from cells transfected with NR1/NR2A, previous studies have shown that currents evoked by sustained application of glutamate exhibit calcium-dependent inactivation (Krupp et al., 1996 ; Zhang et al., 1998). Using 2-s pulses of 10 μM glutamate at 1-min intervals, together with a saturating concentration of glycine (30 μM) to eliminate glycine-dependent desensitization, we observed desensitization in recordings from HEK 293 cells co-transfected with NR1, NR2A, and either β-gal or CaBP (Fig. 2). The extent of desensitization was measured on the second glutamate application as the ratio of current at the end of the 2-s agonist pulse compared with the peak current response (I2s/Ipeak). By comparing I2s/Ipeak for control cells transfected with β-gal and recorded in normal extracellular calcium (1.8 mM) to that of control cells recorded in low extracellular calcium (0.18 mM), it was clear that a significant portion of this desensitization was calcium-sensitive and consistent with calcium-dependent inactivation (Fig. 2B ; p = 0.0089, t test). However, expression of calbindin apparently did not affect calcium-dependent inactivation, because the extent of inactivation measured in normal extracellular calcium was similar for cells transfected with CaBP compared with control cells (Fig. 2B ; CaBP : 0.685 ± 0.025, n = 11 ; control : 0.691 ± 0.024, n = 13 ; p = 0.8636, t test).
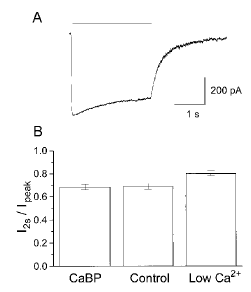
CaBP does not inhibit calcium-dependent inactivation in NR1/NR2A co-transfected cells. A : Nystatin patch recording made in normal calcium (1.8 mM) from a control cell showing current response to a 2-s exposure to 10 μM glutamate (bar indicates time of glutamate exposure). Note the reduction in current seen in the continued presence of the agonist. B : To evaluate the extent of inactivation seen during a glutamate pulse, the current at 2 s was expressed as a ratio of the peak current (I2S/Ipeak). This was determined for CaBP (n = 11) and control cells (n = 13), recorded in 1.8 mM calcium, as well as for control cells in reduced calcium (0.18 mM ; n = 6). Measurements were taken from the second of a pair of agonist pulses, which were separated by 1 min.
Calcium-sensitive increase in glycine-independent desensitization of NR1/NR2A currents
During whole-cell patch-clamp recordings from neurons, the rate and extent of glycine-independent desensitization of NMDA receptor-mediated current increase over time (Sather et al., 1990 ; Tong and Jahr, 1994 ; Tong et al., 1995). A large portion of this progressive increase in desensitization appears to be mediated by a rise in intracellular calcium and activation of the calcium-dependent phosphatase, calcineurin (Tong and Jahr, 1994 ; Tong et al., 1995). In nystatin perforated patch recordings from HEK 293 cells transfected with NR1/NR2A receptors, we observed a calcium-sensitive increase in the rate and extent of desensitization of currents evoked by 15-s pulses of 10 μM glutamate applied at 1-min intervals in the presence of saturating glycine (Fig. 3 ; also compare traces shown in Fig. 3A and 4A2). Prolonged agonist exposure was required to elicit this effect reliably ; we found that with shorter glutamate pulses, many cells were quite slow to develop increases in desensitization, whereas with 15-s agonist applications time-dependent increases in NR1/NR2A current desensitization were observed consistently within the first several agonist pulses. To assess whether calcium buffering by CaBP could influence this process, glutamate-evoked currents were compared in recordings from cells co-transfected with NR1, NR2A, and either β-gal (control) or CaBP. We found that the rate of decline of the steady-state to peak current ratio (ISS/Ipeak) was similar for cells transfected with CaBP compared with control cells (Fig. 3B ; p = 0.1873, two-way ANOVA for between-treatment variance). Therefore, CaBP does not appear to buffer effectively the rise in intracellular calcium that contributes to a progressive increase in the extent of glycine-insensitive desensitization.

CaBP does not inhibit calcium- and time-dependent enhancement of glycine-independent desensitization. A : Nystatin patch recording made in 1.8 mM calcium from HEK 293 cells transfected with NR1/NR2A and β-gal showing current responses to 15-s pulses of 10 μM glutamate. The second, fifth, and tenth in a series of pulses, given at a frequency of 1 pulse/min are shown. To facilitate examining differences in inactivation characteristics, the traces are scaled so the peaks are equal in amplitude. B : Mean steady-state current, expressed as a ratio of the peak current for control cells in normal (1.8 mM ; n = 13) and reduced calcium (0.18 mM ; n = 6) and for CaBP cells in normal (n = 18) and reduced calcium (n = 9).
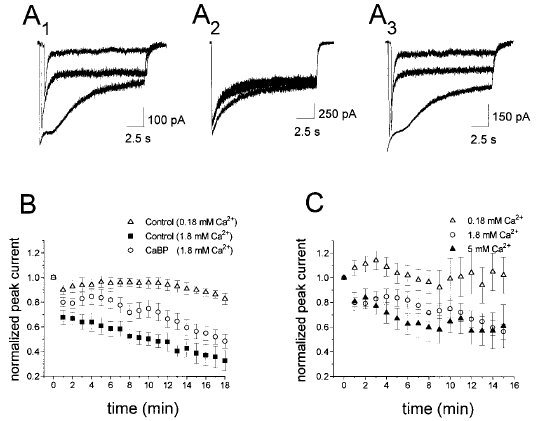
CaBP inhibits calcium-dependent rundown in NR1/NR2A-expressing cells. A : Nystatin patch recordings from HEK 293 cells showing current responses to repeated 15-s exposures to 10 μM glutamate, given at a frequency of once per minute. A1 : Control (β-gal transfected) cells showing the progression of current rundown in 1.8 mM calcium. Traces shown are the first, fifth, and tenth glutamate pulses, and the start of each has been offset along the x-axis to facilitate examination of the peak current. A2 : Control cell recorded in reduced (0.18 mM) extracellular calcium showing that current rundown was inhibited. The first, fifth, and tenth traces are shown. A3 : Recordings from cells co-transfected with CaBP made in 1.8 mM calcium. Traces shown are the first, fifth, and tenth, and the start of each has been offset along the x-axis to facilitate examination of the peak current. B : Mean ± SEM peak current normalized to the current recorded with the first glutamate pulse. The results shown are from control cells in normal (n = 14) and reduced (n = 8) calcium, and from cells expressing CaBP (n = 17). C : Mean ± SEM normalized peak current for CaBP-expressing cells in 0.18 mM (n = 9), 1.8 mM (n = 17), and 5 mM (n = 11) calcium demonstrating the calcium dependence of the CaBP effect on rundown.
NR1/NR2A-mediated current rundown
Peak current mediated by NMDA receptors exhibits a progressive, calcium-dependent decrease in amplitude with repeated agonist application in neuronal whole-cell patch-clamp recordings (Legendre et al., 1993 ; Rosenmund and Westbrook, 1993a,b ; Medina et al., 1996). Using the protocol of 15-s glutamate pulses at 1-min intervals, we demonstrated progressive, calcium-dependent rundown of peak current amplitude in nystatin perforated patch recordings from HEK 293 cells co-transfectd with β-gal and NR1/NR2A (Fig. 4A1, A2, and B). It is interesting that cells recorded in 1.8 mM extracellular calcium and co-transfected with CaBP showed significantly slower progression of peak current rundown than cells co-transfected with β-gal (Fig. 4A1, A3, and B ; p < 0.0001 by two-way ANOVA). As the mean initial peak current amplitude and initial, as well as time-dependent, calcium-sensitive inactivation of agonist-evoked current did not differ significantly between recordings from cells co-transfected with NR1/NR2A and CaBP versus β-gal (see above), the slowing of rundown observed with CaBP expression could be attributed to effects of calcium buffering rather than a difference in net calcium charge transfer. In support of the conclusion that expression of CaBP slows rundown of NR1/NR2A currents due to calcium buffering, we found little difference in progression of rundown in recordings made in low (0.18 mM) calcium from cells co-transfected with NR1/NR2A and CaBP versus β-gal (Fig. 4B and C). Moreover, the rate of progression of rundown recorded from NR1/NR2A/CaBP-expressing cells was more pronounced in 5 mM than in 1.8 mM extracellular calcium, and approached the rate observed for NR1/NR2A/β-gal-expressing cells recorded in 1.8 mM extracellular calcium (Fig. 4B and C).
NR1/NR2B-mediated current rundown
We were interested in comparing the effects of calbindin on receptors composed of NR1/NR2A versus NR1/NR2B, because NR2A and NR2B are the most abundant NR2 subunits expressed in the forebrain (Hollmann and Heinemann, 1994), a region particularly vulnerable to ischemic damage. In nystatin perforated patch recordings from NR1/NR2B-expressing cells co-transfected with β-gal, peak current amplitude evoked by 15-s glutamate pulses every 1 min showed progressive rundown (Fig. 5A1 and B). Like the NR1/NR2A-transfected cells, rundown was largely inhibited when extracellular calcium was reduced from 1.8 to 0.18 mM (Fig. 5A2 and B). It is interesting that recordings from NR1/NR2B-transfected cells expressing CaBP showed a significant delay in progression of current rundown compared with cells expressing β-gal (Fig. 5A3 and B ; p < 0.0002, by two-way ANOVA), although this effect was not as sustained as that observed for NR1/NR2A-transfected cells (compare Figs. 4B and 5B). Therefore, for NMDA receptors composed of either NR1/NR2A or NR1/NR2B, the calcium buffering capacity of calbindin appeared to inhibit progression of calcium-dependent peak current rundown.
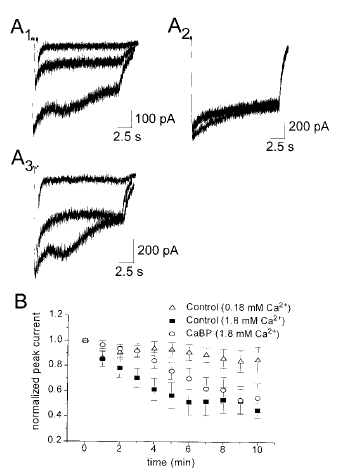
CaBP also inhibits rundown in cells co-transfected with NR1/NR2B. A : Nystatin patch recordings from HEK 293 cells showing current responses to repeated 15-s exposures to 10 μM glutamate, given at a frequency of once per minute. A1 : Control (β-gal) cells showing the progression of current rundown. Traces shown are the first, fifth, and tenth glutamate pulses, and the start of each has been offset along the x-axis to facilitate examination of the peak current. A2 : Control cells recorded in reduced extracellular calcium, showing that current rundown was inhibited. The first, fifth, and tenth traces are shown. A3 : Recordings from cells co-transfected with CaBP. Traces shown are the first, fifth, and tenth in a larger series of traces, and the start of each has been offset along the x-axis to facilitate examination of the peak current. B : Mean ± SEM peak current normalized to the current recorded with the first glutamate pulse. The results shown are from control cells in normal (n = 11) and reduced calcium (n = 14), and from cells co-transfected with CaBP (n = 14).
DISCUSSION
In this study, we have demonstrated that NR1/NR2A-mediated currents recorded from HEK 293 cells exhibit several forms of calcium-dependent regulation, including inactivation, calcium-sensitive and time-dependent glycine-insensitive desensitization, and peak current rundown. The latter two processes were also observed in recordings from NR1/NR2B-transfected cells. These calcium-dependent processes have been described previously in conventional whole-cell patch-clamp recording studies of recombinant NMDA receptors (Medina et al., 1995, 1996 ; Krupp et al., 1996, 1998). Unlike those studies, our experiments were performed using the nystatin perforated patch recording method to preserve intracellular contents, as in one previous study of calcium-dependent regulation of neuronal NMDA receptors (Kyrozis et al., 1996). Moreover, we have shown that NMDA receptor peak current rundown can be modulated by expression of the calcium-binding protein, CaBP. Although results of a previous study suggest that calbindin can regulate calcium-dependent inactivation of high voltage-activated calcium channels (Köhr and Mody, 1991), ours is the first demonstration, to our knowledge, that NMDA receptor function can be modified by calbindin.
The first form of calcium-dependent regulation of NMDA receptor function we examined, calcium-dependent inactivation, has been shown to be specific for receptors composed of NR1 along with either NR2A or NR2D subunits (Krupp et al., 1996). Structure-function studies examining this form of inactivation have revealed the importance of an intact C terminus of NR1, where data suggest the calcium-binding protein calmodulin competes with α-actinin to trigger inactivation of the receptor (Ehlers et al., 1996 ; Wyszynski et al., 1997 ; Krupp et al., 1998 ; Zhang et al., 1998). Further, it is postulated that recovery from inactivation occurs when α-actinin rebinds the receptor's C terminus as the intracellular calcium levels decline (Wyszynski et al., 1997). The inability of calbindin to reduce this inactivation may reflect the proximity of this calcium-dependent process to the mouth of the NMDA receptor channel where calcium may approach millimolar levels, likely exceeding calbindin concentration and buffering capacity. As well, calbindin may be physically excluded from the binding site, or may not be a fast enough buffer to compete with this process. Consistent with the latter hypothesis, experiments with exogenous calcium buffers have shown that at concentrations in the millimolar range the fast buffer 1,2-bis(2-aminophenoxy)ethane-N,N,N',N' -tetraacetic acid (BAPTA) is more effective than the slow buffer EGTA in suppressing calcium-dependent inactivation (Legendre et al., 1993 ; Krupp et al., 1996) ; data from one study suggest that the on-rate for calcium binding to CaBP is intermediate between BAPTA and EGTA (Roberts, 1993).
In addition to calcium-dependent inactivation, NMDA receptors exhibit two other forms of current decay during agonist application : glycine-dependent and glycine-independent desensitization (Mayer et al., 1989 ; Sather et al., 1990 ; Lester et al., 1993 ; Tong and Jahr, 1994). The former is not calcium-sensitive and can be eliminated by recording agonist-evoked current responses in the presence of saturating glycine concentrations (Mayer et al., 1989), as we did in our experiments. A key feature of the latter form, i.e., glycine-independent desensitization, is that its rate and extent increase over time during whole-cell or outside-out patch recording (Sather et al., 1990 ; Tong and Jahr, 1994). Recent studies have identified two N-terminal regions of the NR2A subunit responsible for this process (Krupp et al., 1998 ; Villarroel et al., 1998). As well, previous work has shown that the time-dependent increase in glycine-independent desensitization is markedly reduced in cells intracellularly perfused with high concentrations of BAPTA or treated with inhibitors of the protein phosphatase calcineurin (Tong and Jahr, 1994 ; Tong et al., 1995). Apparently, a rise in intracellular calcium stimulates calcineurin, resulting in dephosphorylation of NMDA receptors (or closely associated proteins) and enhancement of glycine-independent desensitization (Lieberman and Mody, 1994 ; Tong and Jahr, 1994). As with calcium-dependent inactivation, our results indicate that calbindin does not inhibit the calcium- and time-dependent increase in the extent of glycine-insensitive desensitization, which, again, may be due to the close proximity of the molecular determinants of this process to the channel mouth.
Rundown of ion-channel currents is a common phenomenon seen during whole-cell voltage-clamp recordings. For NMDA receptors, rundown of the peak current response is a calcium-dependent process, and during whole-cell recording, it can be prevented by intracellular perfusion of the actin-stabilizing agent phalloidin, indicating that integrity of the actin cytoskeleton plays a key role (Rosenmund and Westbrook, 1993a,b). Consistent with this conclusion, recent findings from Furukawa et al. (1997) indicate that NMDA-evoked current rundown is reduced in mice lacking gelsolin, a calcium-binding protein that leads to the depolymerization of actin filaments. In our study, we routinely observed rundown of NMDA receptor-mediated peak current responses over time during perforated patch recordings, supporting the idea that this process is not due to loss of a cytoplasmic factor following intracellular dialysis with the recording pipette solution during traditional whole-cell recording. More interestingly, we found that calbindin could reduce rundown of NMDA receptor-mediated currents. Unlike the site of action for calmodulin/α-actinin or calcineurin, calcium-dependent depolymerization of actin filaments is likely to occur at a site sufficiently distant from the NMDA receptor-channel mouth such that the local increase in calcium concentration, following channel activation, may not be supersaturating for gelsolin calcium-binding sites. Under these circumstances, calcium buffering by calbindin could be effective in reducing gelsolin activation and stimulation of actin depolymerization.
A recent study comparing NMDA receptor-mediated channel activity recorded from neurons in CaBP knockout mice versus control mice indicates that lack of calbindin expression has no effect on mean channel open time or on burst, cluster, or supercluster length (Klapstein et al., 1998). Consistent with our findings, the authors conclude that calbindin has no effect on calmodulin- or calcineurin-mediated modulation of NMDA channel activity. Interestingly, Rosenmund and Westbrook (1993a) showed that following rundown NMDA receptor channel open probability is reduced significantly, although mean channel open time is unchanged. As channel open probability was not measured in the recordings from the calbindin-/- mice (Klapstein et al., 1998), that study could not rule out an effect of calbindin on NMDA-evoked current rundown.
Although our results indicate that calbindin inhibits current rundown for NMDA receptors composed of either NR1/NR2A or NR1/NR2B, the effect of calbindin is shorter lived for NR1/NR2B. While our study did not address the molecular mechanisms underlying this difference in CaBP effect, we speculate that subunit-specific interactions with cytoskeletal-associated proteins [e.g., α-actinin-2 (see Wyszynski et al., 1997)] might result in differences in the nature and proximity of NMDA receptor attachment to actin. Under conditions in which control of glutamate release from presynaptic cells is compromised such that extracellular glutamate levels rise, as might occur during stroke or epileptic activity (Choi, 1992), rundown of NMDA receptor-mediated currents would likely occur and would be expected to enhance neuronal survival. However, in neurons expressing calbindin and NMDA receptors containing significant proportions of NR2A, slowing of NMDa receptor-mediated current rundown might result in sustained elevation of calcium influx relative to neurons expressing predominantly NR1/NR2B. Consistent with this idea, studies in immature neurons, which predominantly express NR1/NR2B (Monyer et al., 1994), have shown a good correlation between calbindin expression and resistance to excitoxicity (Burke and Baimbridge, 1993 ; Goodman et al., 1993), whereas findings from some studies in more mature neurons (which tend to express high levels of NR2A) have failed to show such a correlation (Freund et al., 1991 ; Möckel and Fischer, 1994 ; Klapstein et al., 1998). Therefore, in future experiments it would be interesting to determine whether calbindin expression can selectively affect NMDA receptor-mediated current rundown in neurons predominantly expressing NR1 with NR2A versus NR2B.
Acknowledgements
The authors would like to thank Dr. T. H. Murphy for helpful discussions. This work was supported by a Personnel Award from the Heart and Stroke Foundation of B.C. & Yukon to C.J.P., operating grants from the Heart and Stroke Foundation of British Columbia and Yukon and Amyotrophic Lateral Sclerosis Society of Canada (L.A.R.), and an MRc (Canada) operating grant (K.G.B.). L.A.R. is an MRC (Canada) Scholar.




