Serine-23 Is a Major Protein Kinase A Phosphorylation Site on the Amino-Terminal Head Domain of the Middle Molecular Mass Subunit of Neurofilament Proteins
Abbreviations used : LC/MS/MS, liquid chromatography-tandem mass spectrometry, MS/MS, tandem mass spectrometry ; NF-H, NF-L, and NF-M, high-, low-, and middle-molecular-mass subunit of neurofilament proteins, respectively ; PTH, phenylthiohydantoin.
Abstract
Abstract : We have shown previously that phosphate groups on the amino-terminal head domain region of the middle molecular mass subunit of neurofilament proteins (NF-M) are added by second messenger-dependent protein kinases. Here, we have identified Ser23 as a specific protein kinase A phosphorylation site on the native NF-M subunit and on two synthetic peptides, S1 (14RRVPTETRSSF24) and S2 (21RSSFSRVSGSPSSGFRSQSWS41), localized within the amino-terminal head domain region. Ser23 was identified as a phosphorylation site on the 32P-labeled α-chymotryptic peptide that carried >80% of the 32P-phosphates incorporated into the NF-M subunit by protein kinase A. The synthetic peptides S1 and S2 were phosphorylated 18 and two times more efficiently by protein kinase A than protein kinase C, respectively. Neither of the peptides was phosphorylated by casein kinase II. The sequence analyses of the chemically modified phosphorylated serine residues showed that Ser23 was the major site of phosphorylation for protein kinase A on both S1 and S2 peptides. Low levels of incorporation of 32P-phosphates into Ser22, Ser28, and Ser32 by protein kinase A were also observed. Protein kinase C incorporated 32P-phosphates into Ser22, Ser23, Ser25, Ser28, Ser32, and a threonine residue, but none of these sites could be assigned as a major site of phosphorylation. Analyses of the phosphorylated synthetic peptides by liquid chromatography-tandem mass spectrometry also showed that protein kinase A phosphorylated only one site on peptide S1 and that ions with up to four phosphates were detected on peptide S2. Analysis of the data from the tandem ion trap mass spectrometry by using the computer program PEPSEARCH did not unequivocally identify the specific sites of phosphorylation on these serine-rich peptides. Our data suggest that Ser23 is a major protein kinase A-specific phosphorylation site on the amino-terminal head region of the NF-M subunit. Phosphorylation of Ser23 on the NF-M subunit by protein kinase A may play a regulatory role in neurofilament assembly and/or the organization of neurofilaments in the axon.
Neurofilaments, the neuron-specific intermediate filaments, are a major constituent of the axonal cytoskeleton. The three subunits of neurofilaments with apparent molecular masses of 70, 145-160, and 200 kDa (commonly referred to as NF-L, NF-M, and NF-H for low-, middle-, and high-molecular-mass subunit of neurofilament proteins, respectively) are highly phosphorylated, particularly the NF-M and NF-H subunits (Julien and Mushynski, 1982 ; Carden et al., 1985). Work from this laboratory and others during the past several years has established that the neurofilament subunits carry phosphate groups on both their head (Sihag and Nixon,, 1989, 1990, 1991) and tail (Julien and Mushynski, 1983 ; Carden et al., 1985 ; Geisler et al., 1987 ; Lee et al., 1988 ; Xu et al., 1992) domains. Previous studies involving retinal ganglion cell neurons as a model system have demonstrated that the phosphate groups on the amino-terminal head domain of NF-M and NF-L subunits in vivo are added by second messenger-dependent protein kinases, whereas the carboxyl-terminal tail domain phosphate groups are added by second messenger-independent protein kinases (Sihag and Nixon, 1989, 1990 ; Nixon and Sihag, 1991). The studies carried out to affect directly the activities of protein kinase A or protein kinase C by microinjection of the catalytic subunit of protein kinase A or inhibitors and activators of protein kinase A or protein kinase C into lamprey neurons further strengthen the findings that neurofilament proteins are in vivo substrates for these kinases (Hall and Kosik, 1993).
Phosphorylation of the NF-L subunit, which forms the “core” of the neurofilaments, by protein kinase A or protein kinase C in vitro has been shown to prevent assembly of NF-L into filamentous structures (Gonda et al., 1990 ; Hisanaga et al., 1990). Although the relevance of polymerization/depolymerization of the filaments formed by only the NF-L subunit is unclear, the rapid turnover of phosphate groups from Ser55, the major protein kinase A phosphorylation site, during axonal transport supports the view that the phosphorylation of the amino-terminal head domain in vivo may play a role in the early events of neurofilament assembly (Sihag and Nixon, 1991). Recently, it has also been reported that phosphorylation of the amino-terminal head domain of NF-L and NF-M subunits by protein kinase A may regulate the assembly/disassembly of heterooligomeric intermediates into filaments (Sihag, 1996 ; Streifel et al., 1996).
Isolation of in vivo phosphorylated peptides from the amino-terminal head domains of NF-M and NF-L for the determination of phosphorylation sites has been technically difficult. Among the possible reasons are (a) a very low mass ratio of phosphorylated to unphosphorylated peptides owing to rapid turnover of the phosphate groups as the neurofilaments enter the axons, (b) the poor recoveries of peptides during proteolytic digestion and isolation, or (c) the sensitivity of the protein sequencing methods to detect phosphoamino acids (Sihag and Nixon, 1991). Recently, Cleverley et al. (1998) used the more sensitive mass spectrometry techniques to identify Ser1 and Ser46 as in vitro protein kinase A phosphorylation sites on the amino-terminal head domain region of NF-M. However, attempts to identify the in vivo phosphorylation sites on the amino-terminal head domain region of neurofilament protein subunits by mass spectrometry were unsuccessful (Betts et al., 1997).
In this study, we have used native neurofilaments and synthetic peptides corresponding to the amino-terminal region of NF-M to identify the phosphorylation sites and the putative protein kinases that may phosphorylate the specific sites in vivo. Our data provide the initial evidence that Ser23 is a major specific site of phosphorylation for protein kinase A on the amino-terminal head domain of NF-M. Our results also show that both protein kinase A and protein kinase C are capable of incorporating phosphate groups into several minor sites on the amino-terminal head region of NF-M. A portion of this work has been presented in preliminary form (Sihag et al., 1997).
MATERIALS AND METHODS
In vitro phosphorylation of NF-M and synthetic peptides corresponding to the amino-terminal head domain
Neurofilament-enriched cytoskeleton preparations from mouse spinal cord were prepared in the presence of a coktail of protease inhibitors and phosphorylated by an endogenous second messenger-independent protein kinase, protein kinase A or protein kinase C, as described previously (Sihag et al., 1988 ; Sihag and Nixon, 1989). For phosphorylation of neurofilament-enriched cytoskeleton preparations from 100 mg of fresh tissues, 500 units of protein kinase A catalytic subunit (Sigma Chemical Co.) or 0.4 unit of brain protein kinase C (Boehringer Mannheim) was used. All phosphorylation assays were carried out at 37°C for 5 min in 0.8 ml of assay mixture containing 50 μCi of [γ-32P]ATP. In the protein kinase A or protein kinase C assay mixtures, 150 μg/ml heparin was included to inhibit the endogenous second messenger-independent protein kinases (Sihag and Nixon, 1989).
Most of the studies presented here were carried out by using two synthetic peptides, S1 (14RRVPTETRSSF24) and S2 (21RSSFSRVSGSPSSGFRSQSWS41), corresponding to the in vivo phosphorylated amino-terminal head domain region of NF-M (Sihag and Nixon, 1990), which were synthesized by using 9-fluorenylmethoxycarbonyl-N-methylpyrrolidone chemistry (Anaspec). Peptide S1 exhibited evidence of Arg14 deletion during sequencing, which did not interfere in the assignment of phosphorylated residues. The peptides were phosphorylated by protein kinase A, protein kinase C, or casein kinase II under two different assay conditions. Assay mixture I (400 μl) contained 40 nmol of peptide, 10 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, 200 arbitrary units of protein kinase, and 0.1 mM ATP (40 nmol) containing 25 μCi of [γ-32P]ATP in 20 mM HEPES, pH 7.4. For protein kinase C phosphorylation, 100 μg/ml phosphatidylserine, 10 μg/ml diolein, and 1.2 mM CaCl2 were included in the assay. An arbitrary unit is defined as the amount of protein kinase A, protein kinase C, or casein kinase II required to incorporate 1 pmol of phosphate/min into kemptide (LRRASLG), H1 histone, or dephosphorylated α-casein, respectively. In some assays, the ATP concentration was increased to 1 mM, and the amount of each protein kinase used was doubled to 400 arbitrary units (assay mixture II). The assays were carried out at 37°C for 15 min.
Gel electrophoresis and autoradiography
The phosphorylated axonal cytoskeleton proteins were separated on 24-cm-long 7% polyacrylamide gels as described previously (Sihag and Nixon, 1989). Gels were stained with Coomassie Brilliant Blue R-250, dried under vacuum, and exposed to Kodak XAR-5 film for autoradiography (Sihag and Nixon, 1989). In one experiment, the α-chymotryptic digest of 32P-labeled NF-M was separated on 18% polyacrylamide gels (acrylamide/bis, 32 : 1) electrophoresed in Tris-Tricine buffer (pH 9.5) as described (Schägger and von Jagow, 1987). The low-range-molecular-mass markers (2.5-31 kDa) were obtained from Promega. The peptides were electrotransferred to PVDF membranes (pore size, 0.2 μm ; Bio-Rad) as described (Sihag and Cataldo, 1996), and the 32P-labeled peptides were detected by autoradiography.
Phosphopeptide mapping and phosphoamino acid analysis
Two-dimensional phosphopeptide mapping was carried out as described previously (Sihag and Nixon, 1990). The 32P-labeled NF-M subunit or the synthetic peptides were digested with 10 μg/ml Nα-p-tosyl-L-lysine chloromethyl ketone-α-chymotrypsin (Sigma) in 50 mM NH4HCO3 for 18 h at 37°C. The digests were resolved in two dimensions on 20- × 20-cm 100μm cellulose-coated TLC plates (E. Merck) as described (Sihag and Nixon, 1990). For phosphoamino acid analysis, the α-chymotryptic digest of 32P-labeled NF-M or the 32P-labeled synthetic peptides S1 and S2 was hydrolyzed in 6 M HCl at 100°C for 90 min. Phosphoamino acids were resolved on cellulose-coated TLC plates by electrophoresis at 1,500 V for 1 h (Sihag, 1998). The standard phosphoamino acids were visualized by staining with 0.1% ninhydrin in acetone, and the 32P-labeled amino acids were detected by autoradiography. For quantification of the 32P-phosphates incorporated into the α-chymotryptic peptides or the phosphoamino acids, the autoradiographs were scanned and analyzed by using the NIH Image software program.
Phosphopeptide separation by reversed-phase HPLC
The α-chymotryptic digests of in vitro phosphorylated NF-M were solubilized in 2% acetic acid and loaded onto a C18 reversed-phase column (4.6 mm × 25 cm ; Vydac Separations Group) as described (Sihag and Nixon, 1990). A linear gradient of 2-80% (vol/vol) acetonitrile in 0.08% trifluoroacetic acid was started 2 min after injection and completed in an additional 45 min. The eluting peptides were monitored by UV absorbance at 230 nm. To identify the 32P-labeled peptides, the column fractions were counted for Cerenkov radiation. The major 32P-peptide was then further purified on an analytical C8 reversed-phase column (2.1 mm × 10 cm, RP-300 ; Pierce Chemical Co.) in a 10-60% (vol/vol) linear gradient of acetonitrile 60 min. The 32P-labeled phosphorylated by protein kinase A, protein kinase C, or casein kinase II were also purified as described above. In these experiments, the phosphorylation reaction was inhibited by addition of 2.5 μl of 0.5 M EDTA/100 μ1 of assay mixture, and aliquots of the reaction mixture were then loaded directly onto the C18 column.
Protein sequencing
The HPLC-purified phosphopeptide fractions were (a) applied in 30-μl aliquots to Biobrene (Applied Biosystems)-treated glass fiber filters or (b) dried and derivatized with ethanethiol (Meyer et al., 1986) before sequencing. The sequence analysis was carried with a pulsed-liquid sequencer (model 477A ; Applied Biosystems) equipped with an on-line phenylthiohydantoin (PTH)-amino acid derivative analyzer (model 120A ; Applied Biosystems) using methods and programs supplied by the manufacturer. Data were collected and analyzed on a model 610A data analysis system (Applied Biosystems). In one experiment, the 32P-peptide from native NF-M was sequenced by automated solid-phase Edman degradation method using a Milligen model 6400 prosequencer. In this experiment, one-half of the 32P-labeled peptide was analyzed for amino acid sequence, and the remainder was used for the determination of phosphorylation sites. In brief, the 32P-labeled peptide was attached to an arylamine membrane (Sequelon-AA ; Millipore) and subjected to automated solid-phase Edman degradation (Pappin et al., 1990). The PTH derivatives were then collected directly by using a fraction collector, and the radioactivity was measured in each fraction.
Mass spectroscopy
Phosphopeptides were analyzed by a Finnigan model LCQ mass spectrometer equipped with an electrospray interface as described (Jaffe et al., 1998). In brief, the mass spectrometer was operated in the “triple play” mode, in which the instrument was set up to acquire (a) a full scan, (b) a ZoomScan (higher resolution, lower mass range scan) of the (M + nH)n+ ions above a present threshold, and (c) a tandem mass spectrometry (MS/MS) spectrum (relative collision energy of 35%) from those ions. The MS/MS spectra were interpreted by using the Finnigan PEPSEARCH program against a database constructed from the mouse NF-M sequence (Levy et al., 1987).
RESULTS
Distinct peptides are phosphorylated on the NF-M subunit by second messenger-dependent and -independent protein kinases
As shown in Fig. 1, the α-chymotryptic two-dimensional phosphopeptide map analyses showed that the endogenous cytoskeleton-associated protein kinase(s) phosphorylated different peptides on the NF-M subunit than those phosphorylated by protein kinase A or protein kinase C. More than 80% of the 32P-phosphates added by protein kinase A or protein kinase C were localized to the same peptide, peptide 1. Peptide 2 was phosphorylated by protein kinase A but very poorly by protein kinase C. Low levels of incorporation of 32P-phosphates on peptide 3 by protein kinase C were also observed. Phosphorylation of the protein kinase A- or protein kinase C-specific peptides was >90% inhibited by 60 μg/ml Walsh inhibitor and 1 μM staurosporine, respectively (data not shown), ruling out the possibility that the peptides C1-3 could have been phosphorylated by either the endogenous protein kinases or protein kinases that might be present as contaminants in the commercially obtained that preparations.
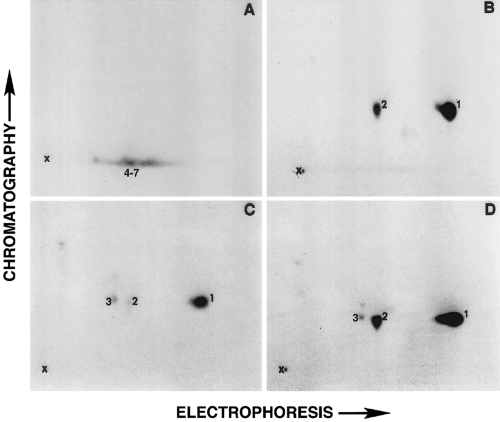
Two-dimensional phosphopeptide map analysis of NF-M. Neurofilament protein-enriched cytoskeletal preparations were phosphorylated in vitro and separated on 7% sodium dodecyl sulfate-polyacrylamide gels. The α-chymotryptic digests of 32P-labeled NF-M bands from Coomassie-stained gels were separated in two dimensions on the cellulose-coated TLC plates as described in Materials and Methods. A-C : α-Chymotryptic phosphopeptide maps of NF-M phosphorylated by endogenous cytoskeleton-associated independent kinase, protein kinase A, and protein kinase C, respectively. D : The two-dimensional map of NF-M when equal cpm values of α-chymotryptic digests used in B and C were mixed.
Isolation and sequence analysis of phosphopeptides from in vitro phosphorylated NF-M
To isolate phosphopeptides for sequence analysis, ~60 μg of NF-M phosphorylated by protein kinase A or protein kinase C was digested in the gel slices with Nα-p-tosyl-L-lysine chloromethyl ketone-α-chymotrypsin, and the α-chymotryptic peptides were separated on a C18 reversed-phase HPLC column as described in Materials and Methods. As expected from the two-dimensional phosphopeptide mapping data (Fig. 1), >80% of radiolabel was recovered in a fraction containing peptide C1. The separation of this 32P-peptide-containing fraction on an 18% polyacrylamide gel in Tris-tricine buffer (Schägger and von Jagow, 1987) showed that the molecular size of the major 32P-peptide was between 3 and 4 kDa (data not shown). When the highly purified 32P-peptide C1 was subjected to automated Edman degradation on a Biobrene-treated glass fiber filter, the sequence of the peptide corresponded to 21RSSFSRVSGSPSSG, which is localized on the amino-terminal head domain of NF-M. Although serine-PTH was observed at the third residue, the presence of phosphoserine was suggested by the >25% increase in the level of S′ (dithiothreitol adducts of dehydroalanine resulting from the β-elimination reaction of serine or phosphoserine) relative to that expected from the serine observed at that residue. There was also a decrease in serine-PTH content at this residue. The decrease in serine-PTH level concomitant with elevated S′ levels has been taken as evidence for phosphoserine (De Jongh et al., 1993). By comparison, when the C1 peptide isolated from NF-M phosphorylated by protein kinase C was analyzed, no significant increases in S′ values were observed for the serine residues. This suggested that the incorporation of phosphate groups into serine residues on C1 peptide by protein kinase C was below the detection level of this method.
It should be noted that, in these experiments, the sequence of the protein kinase A-phosphorylated peptide C1 at the amino-terminal end begins at Ser21 and not Ser25 as reported in an earlier publication (Sihag and Nixon, 1990). The differences observed in the chymotrypsin cleavage sites in these experiments could have resulted from, for example, a change in the concentration of α-chymotrypsin used or simply be due to differences in the properties of α-chymotrypsin supplied by the manufacturer. The presence of contaminating tryptic activity not destroyed by Nα-p-tosyl-L-lysine chloromethyl ketone treatment of α-chymotrypsin could significantly affect the cleavage sites. Also, we have noticed breakdown of peptide C1 during storage, probably due to freezing and thawing. It is therefore very likely that in our earlier experiments, peptide C1 could have been cleaved to generate the two different peptide sequences (Sihag and Nixon, 1990).
Phosphorylation of synthetic peptides corresponding to the amino-terminal head domain of NF-M
Because most of the phosphates incorporated by protein kinase A and protein kinase C were localized on an ~4-kDa peptide C1 located on the amino-terminal head domain of NF-M, we used two synthetic peptides, S1 (14RRVPTETRSSF24) and S2 (21RSSFSRVSGSPSSGFRSQSWS41), to identify their specific sites of phosphorylation. For determining the relative rate of utilization of these peptides as substrates for various purified kinases, the peptides (40 nmol each) were phosphorylated by 200 arbitrary units of casein kinase II, protein kinase A, or protein kinase C (see assay mixture I). In these experiments, the concentration of ATP and protein kinases was limiting. For example, phosphorylation of peptide S1 by protein kinase A under limiting conditions was ~45% (Table 1), but in assay mixture II conditions it was 100% phosphorylated (Fig. 2A). When the phosphorylated peptides were separated on a C18 reversedphase HPLC column, the phosphorylated and unphosphorylated peptide molecules eluted into different fractions (Fig. 2). The results of the phosphorylation under limiting protein kinase concentrations are summarized in Table 1. These experiments showed that neither of the two peptides was phosphorylated by casein kinase II. Peptides S1 and S2 were phosphorylated 18 and two times more efficiently by protein kinase A than by protein kinase C, respectively. Phosphoamino acid analysis of protein kinase A- and protein kinase C-phosphorylated peptide S1 showed that protein kinase A phosphorylated only serine residues, whereas protein kinase C incorporated ~5% of the phosphates into threonine residues (Fig. 3). Similarly, phosphoamino acid analysis of native NF-M phosphorylated in vitro showed that protein kinase A incorporated 32P-phosphates into serine residues, whereas both serine and threonine residues were phosphorylated by protein kinase C, and the incorporation of 32P-phosphate groups into threonine residues was estimated to be <5% of the total phosphate groups incorporated into NF-M (Fig. 3). Phosphorylation of threonine residues by the neurofilament-associated endogenous kinase(s) dected. The NF-M subunit phosphorylated in vivo is known to incorporate phosphates at both serine and threonine residues (Julien and Mushynski, 1982). These data suggested that threonine residues on NF-M in vivo are phosphorylated by protein kinase C or the second messenger-independent protein kinases.
| Peptide | PKA (nmol) | PKC (nmol) | CKII (nmol) | PKA/PKC relative incorporation |
|---|---|---|---|---|
| S1 | 17.4 | 0.98 | None | 17.9 |
| S2 | 15.5 | 8.2 | None | 1.88 |
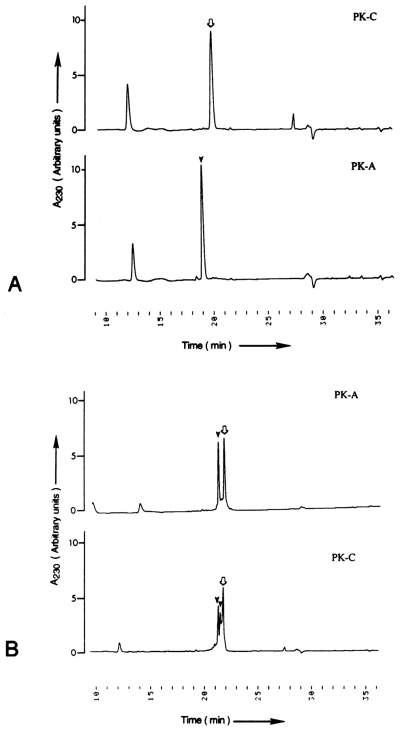
Purification of phosphorylated synthetic peptides on HPLC. The phosphorylated synthetic peptides were isolated on a C18 reversed-phase column. The location of 32P-peptides was determined by Cerenkov radiation of fractions collected at 30-s intervals. A : Complete phosphorylation of peptide S1 in assay mixture II by protein kinase A (PK-A). Peptide S1 was a very poor substrate for protein kinase C (PK-C), and the elution pattern was the same as for the unphosphorylated peptide. B : Partial phosphorylation of peptide S2 by PK-A and PK-C in assay mixture I. The elution patterns of unphosphorylated and phosphorylated peaks are marked with open arrow and solid arrow-heads, respectively.
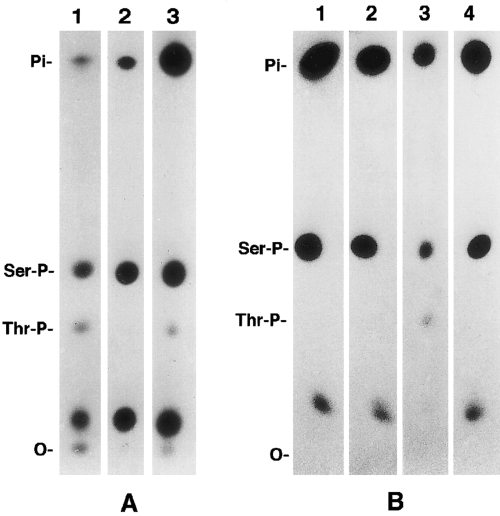
One-dimensional phosphoamino acid analysis of NF-M and synthetic peptides phosphorylated in vitro. The phosphorylated peptides or α-chymotryptic digests of in vitro phosphorylated NF-M were hydrolyzed in 6 M HCl and analyzed by high-voltage electrophoresis on cellulose-coated TLC plates. A : Protein kinase A (lane 2) phosphorylated serine residues, and endogenous independent protein kinase(s) (lane 1) and protein kinase C (lane 3) phosphorylated both serine and threonine residues. B : Protein kinase A phosphorylated serine residues on both peptides S1 (lane 1) and S2 (lane 2), and protein kinase C phosphorylated serine and threonine residues on peptide S1 (lane 3) and serine residues on peptide S2 (lane 4).
Identification of phosphorylation sites on synthetic peptides
The phosphopeptides separated by reversed-phase HPLC were subjected to automated Edman degradation with or without derivatization with ethanethiol (Meyer et al., 1986) or analyzed by LC/MS/MS. In the underivatized peptides, the presence of phosphoserine was suggested by reduced amounts of the expected serine-PTH and/or elevated levels of S'-labeled (PTH dithiothreitol adducts of dehydroalanine resulting from the β-elimination reaction of serine or phosphoserine) relative to that expected from the serine (S) observed at that residue. Increases in the S'/S ratio observed during protein sequencing have been taken as evidence for phosphoserine (Parten et al., 1994). As shown in Table 2, analysis of the sequencing data from underivatized peptides showed that protein kinase A incorporated most of the phosphates into Ser23 on both peptides S1 and S2, suggesting that Ser23 was the major site of phosphorylation. Low levels of phosphorylation were also detected on Ser28 and possibly Ser22. By comparison, protein kinase C phosphorylation of peptides S1 and S2 did not demonstrate any major site of phosphorylation. Measurable phosphorylation of Ser23 and Ser41 was detected.
| S'/S | ||
|---|---|---|
| Residue | Protein kinase A | Protein kinase C |
| Peptide S1 | ||
| Ser22 | 0.24 | 0.21 |
| Ser23 | 0.64 | 0.31 |
| Peptide S2 | ||
| Ser22 | 0.21 | 0.12 |
| Ser23 | 0.52 | 0.18 |
| Ser25 | 0.12 | 0.10 |
| Ser28 | 0.25 | 0.14 |
| Ser30 | 0.08 | 0.16 |
| Ser32 | 0.11 | 0.21 |
| Ser33 | 0.10 | 0.20 |
| Ser37 | 0.18 | 0.14 |
| Ser39 | 0.06 | 0.19 |
| Ser41 | 0.16 | 0.24 |
Analysis of the protein sequencing data from protein kinase A-phosphorylated and ethanethiol-derivatized peptides also identified Ser23 to be the major site of phosphorylation. Ser23 was also phosphorylated by protein kinase C, but the extent of phosphate incorporation was one-third of the values observed with protein kinase A (Table 3). For example, when an estimated 330 pmol of phosphopeptide S2 phosphorylated by protein kinase A or protein kinase C was subject to automated Edman degradation, 26,847 and 9,069 S-ethylcysteinyl PTH peak height counts were recovered on Ser23, respectively. Other sites that showed measurable phosphorylation by protein kinase A were Ser22, Ser28, and Ser32, which showed 5.9, 2.1, and 1.9% of phosphate incorporation as compared with Ser23. Protein kinase C phosphorylated Ser22, Ser25, Ser28, and Ser32 at a 30.5, 18.7, 27.3, and 8.7% level as compared with Ser23. In addition, although phosphoamino acid analyses showed that protein kinase C also phosphorylated threonine residues on peptide S1, we could not determine by the Meyer derivative formation method which of the threonine residue(s) was phosphorylated.
| Residue no. | Protein kinase A | Protein kinase C |
|---|---|---|
| 1R | 178 | 45 |
| 2S | 1,607 | 2,772 |
| 3S | 26,847 | 9,069 |
| 4F | 2,843 | 975 |
| 5S | 1,258 | 1,699 |
| 6R | 332 | 387 |
| 7V | 421 | 609 |
| 8S | 567 | 2,474 |
| 9G | 182 | 368 |
| 10S | 183 | 430 |
| 11P | 194 | 299 |
| 12S | 523 | 789 |
| 13S | 385 | 657 |
| 14G | 207 | 307 |
| 15F | 111 | 253 |
| 16R | 118 | 258 |
| 17S | 281 | 448 |
| 18Q | 118 | 325 |
| 19S | 213 | 342 |
| 20W | 89 | 188 |
| 21S | 80 | 42 |
To verify the phosphorylated sites obtained by protein sequencing methods, we analyzed the phosphorylated synthetic peptides by LC/MS/MS. We examined the positive-mode MS/MS spectra of the phosphopeptides for the appearance of a dominant ion resulting from the neutral loss of 98, 49, 32.7, or 24.5 mass units from singly (H3PO4), doubly (H3PO4/2), triply (H3PO4/3), or quadruply (H3PO4/4) charged ions, respectively, for each phosphoserine or phosphothreonine residue in the peptide. It is known that on loss of H3PO4, the original serine or threonine is converted to a dehydroalanine or dehydroamino-2-butyric acid residue. Our results showed that protein kinase A-phosphorylated peptides S1 and S2 showed ions with one and one to four phosphate groups, respectively. Similar analysis of protein kinase C-phosphorylated peptides S1 and S2 demonstrated the presence of ions with one and one to four phosphate groups, respectively. The data for a triple play analysis of the triply charged ion of peptide S2 are shown in Fig. 4. As is evident from the PEPSEARCH search (Table 4), phosphate groups could not be assigned to specific sites with an acceptable degree of certainty. The Cn, which is the normalized correlation score from the PEPSEARCH search output of the second through the eighth choice, was not significantly different (>0.1) than the first choice (Yates et al., 1995). These data did not meet the Cn constraints normally required for an unequivocal identification of phosphorylation sites. A similar result was obtained from the analysis of peptide S1 (data not shown). For comparison, the results obtained by using mass spectrometry and the data from the protein sequencing are summarized in Table 5.

Full-scan MS/MS spectrum of the triply charged ion from peptide S2. Following phosphorylation by protein kinase A, peptide S2 was analyzed by a Finnigan model LCQ mass spectrometer equipped with an electrospray interface. The mass spectrometer was operated in the “triple play” mode as described in Materials and Methods. The full-scan MS/MS spectrum of the triply charged ion is dominated by four ions at m/z 833.7, 800.8, 768.1, and 735.8, consistent with 1-4 × 32.7 (H3PO43) from the triply charged parent ion at 866.25.
| No. | Rank | C n | Ions | Sequence |
|---|---|---|---|---|
| 1 | 1 | 1.000 | 39/80 | (T)RSS*FSRVS*GSPSSGFRS*QSWS* |
| 2 | 2 | 0.991 | 40/80 | (T)RSS*FSRVS*GSPSSGFRS*QS*WS |
| 3 | 3 | 0.928 | 38/40 | (T)RS*SFSRVS*GSPSSGFRS*QSWS* |
| 4 | ||||
| 4 | 4 | 0.922 | 34/80 | (T)RSSFS*RVS*GSPSSFGRSQS*WS* |
| 5 | 5 | 0.920 | 39/80 | (T)RS*SFSRVS*GSPSSGFRS*QSWS* |
| 6 | 6 | 0.919 | 38/80 | (T)RSS*FSRVSGS*PSSGFRS*QSWS* |
| 7 | 7 | 0.913 | 35/80 | (T)RSSFS*RVS*GSPSSGFRS*QSWS* |
| 8 | 8 | 0.910 | 39/80 | (T)RSS*FSRVSGS*PSSGFRS*QS*WS |
| 9 | 9 | 0.850 | 36/80 | (T)RSS*FSRVS*GSPSSGFRSQS*WS* |
| Protein sequencing | |||
|---|---|---|---|
| Peptide | LC/MS/MS | Derivatized a | S'/S |
| Protein kinase A | |||
| S1 | 1 b | 1 (Ser23*) | 1 (Ser23*) |
| S2 | 4 b | 4 (Ser22, Ser23*, Ser28, Ser32) | 1 (Ser23*, Ser28) |
| Protein kinase C | |||
| S1 | 1 b | 1 (Ser23) | 1 (Ser23) |
| S2 | 4 b | 5 (Ser22, Ser23, Ser25, Ser28, Ser32) | 1 Ser(41) |
- a The phosphopeptides were derivatized before Edman degradation as described (Meyer et al., 1986).
- b Specific sites could not be identified unequivocally (Yates et al., 1995).
DISCUSSION
Our results show that Ser23 is a specific protein kinase A phosphorylation site on the amino-terminal head domain region of NF-M subunit of neurofilaments as determined by in vitro phosphorylation of native NF-M and synthetic peptides S1 and S2 (corresponding to amino acid residues 14-24 and 21-41, respectively, on the amino-terminal head domain) by purified protein kinases. Ser23 was phosphorylated by protein kinase C at several times lower efficiency than by protein kinase A but not by casein kinase II or neurofilament-associated second messenger-independent protein kinases. In addition to Ser23, protein kinase A also phosphorylated at very low levels Ser22, Ser28, and Ser32 on the synthetic peptides, but phosphorylation of these sites on the native NF-M was not confirmed. Protein kinase C phosphorylated Ser22, Ser23, Ser25, Ser28, Ser32, and at least one of the threonine residues on the synthetic peptides, but none of the residues was identified as a major site of phosphorylation.
An examination of the NF-M subunit protein sequence showed that the amino-terminal head domain is enriched in basic residues interspersed with hydrophobic residues and is very rich in serine and threonine residues (Levy et al., 1987). These structural properties of the amino-terminal head domain meet the basic requirements for a good protein kinase A or protein kinase C substrate (Pearson and Kemp, 1991). For example, Ser23 and Ser28 meet the consensus sequence requirements to be phosphorylation sites for protein kinase A as well as protein kinase C. There are 10 serine and two threonine residues on the amino-terminal region corresponding to amino acid residues 14-41, but Ser23 was found to be the most preferred to site for both of these kinases (Table 3). Although Ser23 was a substrate for protein kinase C, the data presented here support the view that Ser23 may be a specific protein kinase A phosphorylation site in vivo. First, the phosphorylation of Ser23 on the synthetic peptides by protein kinase A was stoichiometric. Phosphorylation by protein kinase A showed about three times more incorporation of phosphate groups into Ser23 on peptide S2 than that by protein kinase C (Table 3). Furthermore, the phosphorylation of Ser23 by protein kinase A on peptide S1 was as much as 10 times faster than on peptide S2. It is possible that if a longer phosphorylation reaction time was allowed, protein kinase C could stoichiometrically phosphorylate Ser23. If so, it may be reasonable to suggest that under certain physiological conditions, Ser23 could be phosphorylated by protein kinase C. Second, the phosphorylation of Ser23 on native NF-M phosphorylated by protein kinase C could not be detected, even though equal amounts of peptide C1 obtained from native NF-M phosphorylated by the same number of arbitrary units of protein kinase C and protein kinase A were subjected to automated Edman degradation. Also, the conclusion that Ser23 is a major protein kinase A phosphorylation site is consistent with the results predicted from the amino acid sequence of the amino-terminal head domain residues 14-41 (Pearson and Kemp, 1991).
As discussed above, no serine residue could be identified as a possible specific site of phosphorylation for protein kinase C in vivo on the head domain region between amino acid residues 14 and 41. It is known that threonine residues are phosphorylated in vivo on the NF-M subunit (Julien and Mushynski, 1982). The phosphoamino acid analysis data showed that the threonine residues on native NF-M or peptide S1 were phosphorylated by protein kinase C and not by protein kinase A (Fig. 3). It is interesting that Thr18 also meets the consensus sequence requirements to be a protein kinase C phosphorylation site. However, because threonine residues on NF-M were also phosphorylated by the cytoskeleton-associated second messenger-independent protein kinases, it cannot be reasoned that the phosphate groups on threonine residues in vivo may be added by protein kinase C.
Mass spectrometry is finding increasing use in the identification of protein phosphorylation sites. In addition to protein sequencing of derivatized or underivatized peptides by automated Edman degradation, we used LC/MS/MS to identify the specific sites of phosphorylation. Both protein kinase A and protein kinase C showed phosphorylation of one site on peptide S1. On peptide S2, we identified ions with up to four phosphorylated serine residues each after phosphorylation with protein kinase A or protein kinase C. This confirmed the results obtained by automated Edman degradation of the protein kinase A- or protein kinase C-phosphorylated peptides derivatized with ethanethiol (Table 5). However, owing to the presence of multiple serine and/or threonine residues on the peptides, the specific sites of phosphorylation could not be unequivocally assigned using the PEPSEARCH program against a database constructed from the mouse NF-M sequence (Levy et al., 1987). By contrast, in a MALDI (matrix-assisted laser desorption/ionization) and nanoelectrospray mass spectrometry study of a tryptic digest of in vitro phosphorylated NF-M, Cleverley et al. (1998) identified Ser1 and Ser46 as protein kinase A phosphorylation sites. The tryptic peptide containing Ser23 was not covered in this study. However, the failure to find a given phosphorylation site by mass spectrometry does not rule out the possibility that the site is phosphorylated. For example, we identified by automated Edman degradation of the ethanethiol-derivatized peptide S2 that Ser28 is phosphorylated by protein kinase A, but Cleverley et al. (1998) did not detect phosphorylation of this site. Also, attempts to identify the in vivo amino-terminal head domain phosphorylation sites on NF-M by using mass spectrometry were unsuccessful (Betts et al., 1997). A possible explanation for the failure to detect in vivo amino-terminal head domain phosphorylation sites may be that the turnover of phosphate groups on the amino-terminal head domain of NF-M is relatively rapid as compared with the carboxyl-terminal domain (Sihag and Nixon, 1990, 1993). As a result, only a small percentage of the total NF-M molecules carries phosphate groups on the amino-terminal head domain, making the detection difficult even with the extremely sensitive mass spectrometric techniques.
It has been previously shown that the amino-terminal head domain phosphorylation sites on NF-L subunit may regulate the assembly/disassembly of neurofilaments in vivo (Sihag and Nixon, 1989 ; Gonda et al., 1990 ; Hisanaga et al., 1990 ; Sacher et al., 1994). However, the possible role of the amino-terminal head domain phosphorylation sites on NF-M is much less understood. Streifel et al. (1996) reported that phosphorylation of purified NF-L and NF-M subunits by protein kinase A caused an inhibition of the assembly of heterooligomeric intermediates into filaments. The phosphorylation of the amino-terminal head regions of NF-L and NF-M subunits in native neurofilaments by protein kinase A was also found to promote disassembly of neurofilaments into heteropolymeric oligomers by chaotropic salts (Sihag, 1996). These findings have prompted us to speculate that phosphorylation of Ser23 by protein kinase A may play a role in neurofilament organization.
The data presented here strongly suggest that Ser23 is most likely an in vivo protein kinase A-specific phosphorylation site and provide further support to initial findings that the phosphate groups on the amino-terminal head domain of NF-M are exclusively added by second messenger-dependent protein kinases (Sihag and Nixon, 1990). Identification of the phosphorylation sites on the amino-terminal head domain of NF-M and their putative protein kinase(s) should help in future experiments to determine the role of NF-M subunit in neurofilament structure and function. These results may also help in our understanding of the mechanism and significance of aberrant neurofilament phosphorylation in neurodegenerative diseases.
Acknowledgements
Protein sequence analyses during the early part of this work were carried out by Dr. J. D. Dixon, Boston University. We wish to thank Drs. Thomas Reese and Harish Pant for helpful discussions. This work was supported by the Intramural Program of the National Institutes of Health by the Intramural Program of the National Institutes of Health and grant BNS-9109125 from the National Science Foundation.




