Production of phthiocerol dimycocerosates protects Mycobacterium tuberculosis from the cidal activity of reactive nitrogen intermediates produced by macrophages and modulates the early immune response to infection
Summary
The growth of Mycobacterium tuberculosis mutants unable to synthesize phthiocerol dimycocerosates (DIMs) was recently shown to be impaired in mouse lungs. However, the precise role of these molecules in the course of infection remained to be determined. Here, we provide evidence that the attenuation of a DIM-deficient strain takes place during the acute phase of infection in both lungs and spleen of mice, and that this attenuation results in part from the increased sensitivity of the mutant to the cidal activity of reactive nitrogen intermediates released by activated macrophages. We also show that the DIM-deficient mutant, the growth and survival of which were not impaired within resting macrophages and dendritic cells, induced these cells to secrete more tumour necrosis factor (TNF)-α and interleukin (IL)-6 than the wild-type strain. Although purified DIM molecules by themselves had no effect on the activation of macrophages and dendritic cells in vitro, we found that the proper localization of DIMs in the cell envelope of M. tuberculosis is critical to their biological effects. Thus, our findings suggest that DIM production contributes to the initial growth of M. tuberculosis by protecting it from the nitric oxide-dependent killing of macrophages and modulating the early immune response to infection.
Introduction
Tuberculosis remains a major cause of mortality worldwide (World Health Organization, 2002). Mycobacterium tuberculosis, the aetiological agent of this disease in humans, can infect, replicate and persist inside macrophages. Of the factors that contribute to the resistance of M. tuberculosis to host defence mechanisms, the mycobacterial cell envelope has attracted special attention, because of its unique composition and structure (Daffé and Draper, 1998). One of the remarkable features of the mycobacterial cell envelope is the abundance and variety of long multimethyl-branched fatty acids. Lipids esterified by these complex acyl chains are restricted, with few exceptions, to pathogenic mycobacteria. The families of lipids containing multiple methyl-branched fatty acids in M. tuberculosis include the sulpholipids (for a review, see Goren and Brennan, 1979), the di- and triacylated trehaloses (Lemassu et al., 1991; Besra et al., 1992; Muñoz et al., 1997), the polyacyltrehaloses (Minnikin et al., 1985; Dafféet al., 1988) and the phthiocerol dimycocerosates (Daffé and Lanéelle, 1988). The members of the dimycocerosyl phthiocerol family (DIMs) are the ones that have been most extensively studied in terms of both biosynthesis (for a review, see Kolattukudy et al., 1997) and role in M. tuberculosis virulence in vivo. The observation that a DIM-deficient M. tuberculosis H37Rv mutant replicated less well and elicited fewer lung surface tubercles than a DIM-producing H37Rv strain in guinea pigs suggested for the first time that DIMs play a role in pathogenicity (Goren et al., 1974). It was subsequently found that an avirulent strain of M. tuberculosis coated with DIMs and cholesterol can survive longer in mice than an uncoated strain (Kondo and Kanai, 1976). Recently, two independent signature-tagged transposon mutagenesis (STM) studies resulted in the isolation of M. tuberculosis mutants that were impaired for growth in the lungs of mice. Several of these mutants carried transposon insertions in a region of the genome dedicated to the synthesis and transport of DIMs and were therefore deficient in these processes (Camacho et al., 1999; Cox et al., 1999). drrC and mmpL7 mutants of M. tuberculosis, which are deficient in the transport of DIMs across the cell envelope and consequently accumulate these lipids in their cytoplasm, were equally as attenuated as DIM-deficient mutants. Thus, the location of DIMs in the cell envelope appears to be essential for their role in virulence (Camacho et al., 1999; 2001; Cox et al., 1999). Consistent with this idea, Camacho et al. (2001) demonstrated that DIMs are involved in the permeability of the M. tuberculosis cell envelope. A differential scanning calorimetry study by Barry and coworkers also showed that the cell wall fluidity of a Mycobacterium bovis BCG mutant that is unable to produce DIMs and related species-specific phenolic glycolipids (PGLs) was seriously affected (cited in Sirakova et al., 2001).
Thus, DIMs clearly appeared to be important for the pathogenesis of M. tuberculosis. However, the precise role of these molecules in the course of infection remained unknown. Because of the role of DIMs in the permeability of the cell envelope, DIM-deficient strains may be more sensitive to bactericidal agents. In addition, as noted earlier (Barry, 2001), because of their abundance and their structural role in the cell envelope, DIMs could contribute to pathogenesis indirectly by affecting many cell wall-related processes including the secretion of lipids, proteins and carbohydrates by the tubercle bacillus. This may dramatically affect the host's immune response to infection. The role of DIMs and related molecules in the multiplication of mycobacteria from the TB complex in organs other than the lungs is also unclear. Cox et al. (1999) showed that DIM-deficient strains of M. tuberculosis Erdman were attenuated for growth in the lungs but not in the spleen of mice. However, Kolattukudy and collaborators showed that a DIM/PGL mutant of M. bovis BCG displayed reduced growth in both lungs and spleen of mice (cited in Sirakova et al., 2001). Finally, although both STM studies clearly showed that DIMs were required for the full virulence of M. tuberculosis in the lungs of intravenously infected mice during the acute phase of infection, the contribution of these complex lipids to virulence during the chronic phase of infection has not been investigated. Likewise, the ability of DIM-deficient M. tuberculosis strains to replicate and persist within macrophages in vitro has only been succinctly studied in rest-ing cells on a two time point kinetics (Camacho et al., 1999).
The aim of this study was to characterize the role of DIMs in pathogenesis by addressing some of these issues. We analysed over a 4 month period the multiplication, persistence and tissue damage induced by a DIM-deficient mutant of M. tuberculosis (clinical isolate Mt103) in BALB/c mice infected intranasally. We also analysed the growth and trafficking of the mutant in resting and activated mouse bone marrow macrophages, its replication in activated macrophages specifically impaired in reactive nitrogen intermediate (RNI) production and the secretion of cytokines it induced in macrophages and dendritic cells in vitro. Our data suggest that the reduced ability of the mutant to multiply in vivo results from its increased sensitivity to RNIs produced by macrophages and the enhanced activation of the immune response that it elicits in the very early steps of infection.
Results
Multiplication, persistence and granulomatous response elicited by a DIM-deficient mutant of M. tuberculosis Mt103 in intranasally infected mice
DIM deficiency has been shown to impair the multiplication of M. tuberculosis in the lungs of mice infected via the intravenous route, 3 weeks after infection (Camacho et al., 1999; Cox et al., 1999). To better characterize the role of DIMs in a more natural model of tuberculosis infection and to assess the role of these molecules in the persistence of M. tuberculosis within the host, we infected BALB/c mice intranasally with the wild-type M. tuberculosis strain, Mt103, or its DIM-deficient mutant, 1A29, and analysed bacterial loads in the lungs and spleen of infected animals over a 4 month period.
1A29 replicated less extensively than Mt103 in the lungs and spleen (Fig. 1). During the acute phase of infection (from day 1 to 21), the apparent doubling times of the wild-type and mutant strains were 49.4 ± 1.2 and 67.7 ± 2.2 h, respectively, in the lungs and 43.4 ± 2.4 and 82.8 ± 9.6 h, respectively, in the spleen. Colony-forming unit (cfu) counts for both strains then decreased in the lungs during the chronic phase of infection, but this decrease was more marked for 1A29 than for Mt103 (Fig. 1A). In the spleen, both strains persisted equally well, suggesting that DIMs are required for multiplication but not for persistence in this organ (Fig. 1B).
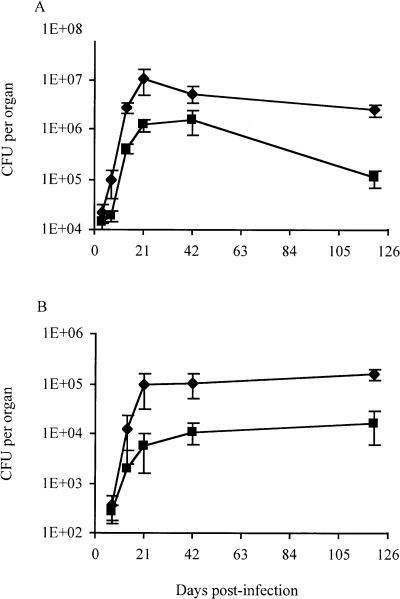
Multiplication and persistence of M. tuberculosis Mt103 wild-type and 1A29 mutant strains in the lungs and spleen of mice. Multiplication and persistence of Mt103 (diamonds) and 1A29 (squares) in the lungs (A) and in the spleen (B) of BALB/c mice infected intranasally. Values represent the means ± standard deviations (error bars) of cfu counts for four or five infected mice.
Analysis of the granulomatous response elicited by Mt103 and 1A29 in the lungs of mice revealed that the organized granuloma-like structures (Gonzalez-Juarrero et al., 2001) typically found in the lungs of Mt103-infected animals 42 days after infection only appeared in the lungs of 1A29-infected mice about 4 months later (data not shown). At this late time point, granuloma-like structures in the lungs of 1A29-infected mice occupied about 22% of histological sections, a value that is comparable to that found in lung sections of Mt103-infected mice on day 42 (25%). This delay in granuloma formation is probably related, at least partly, to the differences in bacterial loads between the two strains during the acute phase of infection.
DIM production is not required for the growth of M. tuberculosis within resting macrophages nor for the inhibition of phagosome–lysosome fusion within these cells
The attenuation of the DIM-deficient mutant in mice during the acute phase of infection suggested that the ability of this mutant to multiply or persist within macrophages was affected. To test this hypothesis, we first compared the growth kinetics of 1A29 and Mt103 in 7H9 broth (data not shown) and within resting mouse bone marrow macrophages. Under these two culture conditions, 1A29 replicated as well as Mt103 over a 7 day period (Fig. 2A), indicating that DIM deficiency did not affect the ability of M. tuberculosis to replicate under axenic conditions or inside macrophages. The calculated intracellular apparent doubling times of Mt103 and 1A29 during the 7 days of infection were 42.0 ± 2.6 and 35.3 ± 1.9 h, respectively, and wild-type and mutant cfu counts were not statistically different throughout the kinetics (Student's t-test, P < 0.05).
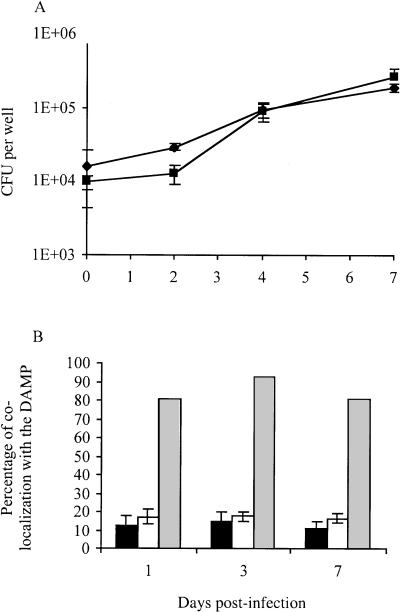
Multiplication and trafficking of M. tuberculosis Mt103 wild-type and 1A29 mutant strains inside resting macrophages. A. Multiplication of Mt103 (diamonds) and 1A29 (squares) in resting mouse bone marrow macrophages. Values represent the means ± standard deviations (error bars) of cfu counts obtained from three independent wells in one typical experiment. B. Co-localization of Mt103 (black bars) and 1A29 (white bars) with DAMP in resting BALB/c murine bone marrow macrophages. Mt103 killed with 4% paraformaldehyde (grey bars) serves as a positive control. Data represent the means ± standard deviations (error bars) of co-localization counts from three coverslips per sample, with at least 100 phagosomes analysed per coverslip, in one typical experiment.
As prevention of phagolysosome fusion is one of the mechanisms by which M. tuberculosis survives within macrophages (Armstrong and Hart, 1971; Clemens and Horwitz, 1995; for a review, see Russell, 2001), we sought to determine whether DIM deficiency affected the intracellular fate of M. tuberculosis within resting macrophages. For this, we compared the trafficking pattern of 1A29 and Mt103 inside resting mouse bone marrow macrophages. Macrophages were infected with Mt103 and 1A29 expressing the gfp gene, and the co-localization of phagosomes with a marker of phagosome maturation was analysed at different times after infection. DAMP, a weak base that accumulates in acidic compartments, was used as the marker (Anderson et al., 1984). Consistent with the fact that M. tuberculosis is able to prevent phagolysosome fusion, <16% of the green fluorescent protein (GFP)-labelled Mt103 co-localized with DAMP during the first 7 days after phagocytosis (Fig. 2B). The mutant did not behave differently from the wild-type strain (Fig. 2B). In comparison, > 80% of the PFA-killed Mt103 co-localized with DAMP during the same period (Fig. 2B). Thus, DIMs do not contribute significantly to the inhibition of phagolysosome fusion by M. tuberculosis within resting macrophages.
The presence of DIMs in the cell envelope contributes to the protection of M. tuberculosis against the bactericidal activity of RNIs released by activated macrophages
As the role of DIMs in the permeability of the M. tuberculosis cell envelope (Camacho et al., 2001) may contribute to the resistance of this bacterium to the bactericidal mechanisms that accompany macrophage activation, we next compared the growth kinetics of 1A29 and Mt103 inside activated mouse bone marrow macrophages.
1A29 replicated less efficiently than Mt103 inside activated macrophages (Fig. 3A). Over a 3 day period, the apparent doubling times of Mt103 and 1A29 were 35.0 ± 4.0 and 92.6 ± 27.4, respectively, and the wild-type and mutant cfu counts were significantly different on days 3 and 8 (Student's t-test, P < 0.05). Interestingly, this difference in growth between wild-type and mutant strains was totally abolished when macrophages were treated with L-NAME, an analogue of l-arginine that inhibits the production of RNIs by the inducible nitric oxide synthase, NOS2 (Fig. 3B). Over a 3 day period, the apparent doubling times of Mt103 and 1A29 in L-NAME-treated macrophages were 39.4 ± 5.1 and 41.7 ± 7.8, respectively, with no statistical difference in cfu counts between the wild-type and mutant strains throughout the kinetics. Therefore, DIM production increases the resistance of M. tuberculosis to the bactericidal activity of NO radicals and related RNIs produced by macrophages.
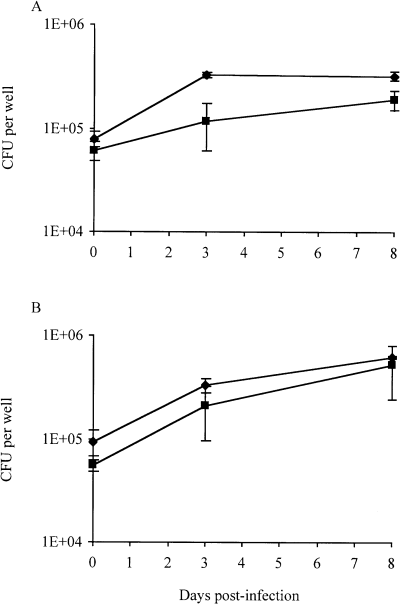
Growth of M. tuberculosis Mt103 wild-type and 1A29 mutant strains inside activated macrophages. Survival of Mt103 (diamonds) and 1A29 (squares) in untreated activated mouse bone marrow macrophages (A) and activated macrophages treated with the inhibitor of RNI production, L-NAME (B). Values represent the means ± standard deviations (error bars) of cfu counts obtained from three independent wells in one typical experiment.
Cytokine secretion by antigen-presenting cells infected in vitro with the wild-type Mt103 strain or with Mt103 mutants deficient in the production or transport of DIM
The apparent replication of the tubercle bacilli in vivo not only depends on their intrinsic ability to multiply inside host cells but also reflects the capacity of the host's immune system to limit their growth. Thus, we examined whether Mt103 and 1A29 activate immune cells differently. Antigen-presenting cells (APCs), such as macrophages and dendritic cells (DCs), play an essential role in initiating the immune response against pathogens. For this reason, we determined the amounts of the proinflammatory cytokines tumour necrosis factor (TNF)-α, interleukin (IL)-6 and IL-12p40 released by macrophages and DCs upon in vitro infection with each of the two strains. All these cytokines are required for proinflammatory response, granuloma formation and development of a type 1 response during tuberculosis (for a review, see Orme, 1995; Flynn and Chan, 2001; Kaufmann, 2001). The mmpL7 mutant of M. tuberculosis Mt103 (strain 8B152), which synthesizes wild-type amounts of DIMs but fails to transport them from the cytoplasm to the cell envelope, was included in this study (Camacho et al., 2001). This mutant displays a similar attenuation level to 1A29 in BALB/c mice (Camacho et al., 1999) and grows similarly in resting mouse bone marrow macrophages (data not shown). Quantification of the inocula and of intracellular cfu during the course of infection confirmed that the number of bacteria used to infect the cells and replication inside macrophages and DCs were similar for the three strains (data not shown).
The cytokine secretion profiles of APCs infected with the wild-type strain or with the two mutants were markedly different. 1A29 and 8B152 induced the secretion of significantly higher amounts of TNF-α by macrophages and DCs than Mt103 (Fig. 4A). This difference was statistically significant on days 1, 2 and 3 (Student's t-test, P < 0.05). 1A29- and 8B152-infected macrophages and DCs also secreted more IL-6 than Mt103-infected cells (Fig. 4B). This difference between wild-type and mutant strains was statistically significant 48 h and 72 h after infection in macrophages, and at all time points in DCs. No significant differences were found between the three strains with regard to the secretion of IL-12p40 by macrophages and DCs (Fig. 4C). The fact that 1A29 and 8B152 induced similar cytokine responses indicates that the location of DIMs is critical for the type of immune response induced by M. tuberculosis.
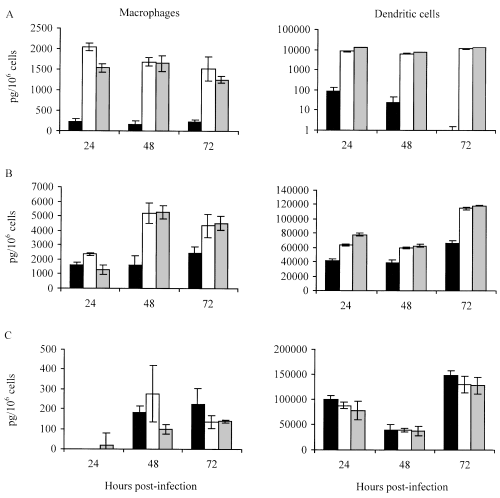
Cytokine secretion by macrophages (left) and dendritic cells (right) in response to infection with Mt103 (black bars), 1A29 (white bars) or 8B152 (grey bars). Supernatants of infected macrophages and dendritic cells were assayed for the production of TNF-α (A), IL-6 (B) and IL-12p40 (C). Values represent the means ± standard deviations (error bars) of cytokine production measured in three independent wells in one typical experiment.
Purified DIMs do not have any effect on the activation of antigen-presenting cells in vitro
As Mt103 and its isogenic mutants 1A29 and 8B152 differ in their cell envelope DIM content, we next tested whether DIMs alone have a direct effect on APCs in vitro. For this, we examined the activation levels of mouse bone marrow-derived DCs and macrophages after incubation with various concentrations of purified DIM molecules. This treatment caused macrophages and dendritic cells to swell, indicating that DIMs were taken up efficiently by these cells (data not shown). The acquisition of the IAd major histocompatibility complex (MHC) class II marker and of the B7.2 maturation marker by DCs was analysed by flow cytometry. No significant difference was observed between unstimulated DCs and DCs incubated for 48 h with 1 or 100 µg of purified DIMs (Table 1). In comparison, DCs stimulated for 48 h with 10 µg of lipopolysaccharide (LPS) expressed more MHC class II and B7.2 molecules on their surface (Table 1). This increase in LPS-stimulated cells was 1.5- to twofold in the case of the mean fluorescence intensity for IAd and at least sevenfold in the case of the percentage of B7.2-positive cells (Table 1). When purified DIMs were added before or after the treatment of DCs with LPS, they had no inhibitory effect on cell activation (Table 1). Moreover, purified DIMs (10 µg) did not modify TNF-α secretion by DCs in response to LPS stimulation (data not shown). Similarly, no significant difference in activation levels was found between unstimulated macrophages and macrophages incubated for 24 h with 1–50 µg of purified DIMs (Fig. 5). Furthermore, DIMs did not inhibit the activation of macrophages by interferon (IFN)-γ and TNF-α (Fig. 5).
| Treatment | Maturation marker | |||
|---|---|---|---|---|
| IAd | B7.2 | |||
| % positive cells | MFI | % positive cells | MFI | |
| LPS 10 µg | ||||
| No DIMs added | 1.43 | 1.84 | 7.50 | 0.88 |
| +DIMs 1 µg | 1.53 | 1.60 | 7.00 | 0.87 |
| +DIMs 100 µg | 1.45 | 1.64 | 7.50 | 0.90 |
| DIMs 1 µg | ||||
| No LPS added | 1.35 | 1.26 | 1.10 | 0.90 |
| +LPS 10 µg | 1.48 | 1.57 | 10.00 | 0.72 |
| DIMs 100 µg | ||||
| No LPS added | 1.33 | 1.29 | 1.10 | 0.97 |
| +LPS 10 µg | 1.45 | 1.89 | 9.00 | 0.63 |
- Bone marrow-derived DCs were either unstimulated or treated with LPS (10 µg per 106 cells) or DIMs (1–100 µg per 106 cells) for 48 h. Effect of purified DIMs on LPS-induced maturation of DCs was also tested by adding DIMs 24 h before or after the treatment with LPS. Acquisition of maturation markers was analysed by flow cytometry using anti-IAd (MHC class II) antibodies and anti-B7.2 antibodies. Both the percentage of positive cells and the mean fluorescence intensity (MFI) were monitored. Results are expressed as a fold increase relative to unstimulated cells. The table shows the results of a representative fluorescence-activated cell sorting (FACS) experiment.
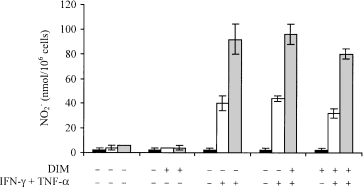
Effect of purified DIM molecules on the activation of macrophages in vitro. Murine bone marrow macrophage activation was assessed by measuring the concentration of NO 2 – in the culture medium using the Griess test. ‘+’ indicates that either DIMs or (IFNγ + TNFα) were added to the culture medium 24 h before NO 2 – release was measured. Measurement was performed on three consecutive days: day 1 (black bars); day 2 (white bars); day 3 (grey bars). Values represent the means ± standard deviations (error bars) of NO 2 – release in three independent wells in one typical experiment.
Thus, purified DIMs alone do not seem to have any positive or negative effect on the activation of macrophages and dendritic cells in vitro.
Discussion
The recent demonstration that the virulence of isogenic DIM-deficient mutants of M. tuberculosis is attenuated in the lungs of mice 3 weeks after intravenous infection clearly proved that DIMs are required for the full virulence of the tubercle bacillus in vivo (Camacho et al., 1999; Cox et al., 1999). However, the precise role of these complex lipids in the course of infection remained to be determined.
To determine more precisely at what stage of the infection DIMs are required, we analysed the replication and persistence of a fadD26 mutant of M. tuberculosis Mt103, which is completely devoid of DIMs (Camacho et al., 1999; Cox et al., 1999), in intranasally infected BALB/c mice over a 4 month period. The intranasal route of infection was chosen because it resembles more closely the natural route by which humans acquire infection. Unexpectedly, the DIM-deficient mutant replicated less extensively than the wild-type parental strain in both lungs and spleen during the acute phase of infection. Thus, DIMs are clearly required for the multiplication of M. tuberculosis Mt103 in both organs. These findings are not consistent with the observations made earlier on the virulence of DIM-deficient mutants of M. tuberculosis Erdman, which showed that DIMs are required for growth in the lungs but not in the spleen of C57BL/6 mice infected intravenously (Cox et al., 1999). As the intranasal infection of C57BL/6 mice with 1A29 and Mt103 yielded similar results to those found in BALB/c mice (data not shown), it is unlikely that this discrepancy between the results of Cox and collaborators and ours is due to the type of mice used. Rather, the ability of DIM-deficient mutants to replicate in the spleen might depend on the inoculation route used or on the M. tuberculosis strain. The role of DIMs during the persistence phase of infection was not as obvious as during the acute phase, as the fate of the DIM-deficient mutant at later stages of infection depended on the organ studied (Fig. 1).
As the attenuation of the 1A29 strain during the acute phase of infection may have been caused by reduced abilities to multiply and/or to persist inside host phagocytic cells, we examined the growth characteristics and trafficking of the DIM-deficient mutant in resting and activated macrophages. Interestingly, 1A29 did not display any major growth or trafficking defects within resting macrophages but was impaired for growth within activated macrophages. This attenuation of the mutant appeared to be directly attributable to its higher sensitivity to RNIs, as blocking the synthesis of these compounds with an inhibitor of the nitric oxide synthase NOS2, L-NAME, allowed 1A29 to grow as efficiently as the wild-type Mt103 in activated macrophages. Thus, the production of DIMs is not essential for the arrest of phagosome maturation but contributes to the resistance of M. tuberculosis to the most effective antimicrobial agent produced by murine macrophages against virulent mycobacteria (Chan and Flynn, 1999): RNIs. As mice with mutations in the macrophage-inducible NOS2 are more susceptible to M. tuberculosis infection (Scanga et al., 2001) and a correlation exists between the resistance to RNIs among various M. tuberculosis strains and their virulence in vivo (O’Brien et al., 1994), it can inferred that the limited ability of the DIM-deficient mutant to replicate in mice is, at least partly, due to its increased sensitivity to RNIs.
The ability of M. tuberculosis strains to multiply in vivo also greatly depends on the ability of the host immune response to contain the infection. For instance, differences in the way 1A29 and Mt103 activate early immunological responses in the host could also contribute to their different apparent growth rates during the acute phase of infection. As DIM deficiency alters the permeability of the cell envelope of M. tuberculosis (Camacho et al., 2001), it is likely that 1A29 secretes qualitatively and quantitatively different compounds from the wild-type parental strain or exposes different molecules at its surface. Such differences in the cell envelope composition of the two strains, including their DIM content, could dramatically affect the early immune response that they induce.
To investigate whether Mt103 and 1A29 stimulated the host immune response differently, we compared the ability of both strains to activate resting macrophages and dendritic cells in vitro. These two types of antigen-presenting cell are crucial for the initiation of the host immune response in the course of infection. In both cell types, the DIM-deficient mutant, 1A29, induced the production of more TNF-α and IL-6 than the wild-type parental strain, Mt103. This difference in cytokine secretion was not the result of differences in intracellular bacterial loads as both bacterial strains replicated and survived equally well in these cell types. Both TNF-α and IL-6 play important roles in the host immune response to M. tuberculosis. TNF-α participates in cell recruitment, granuloma formation (Bean et al., 1999; Roach et al., 2002) and enhances the release of reactive nitrogen intermediates from macrophages (Ding et al., 1988; Bose et al., 1999). IL-6 is required for the early production of IFN-γ which helps to limit bacterial growth (Saunders et al., 2000). TNF-α- or IL-6-deficient mice infected with M. tuberculosis display increased bacterial loads and decreased survival time, compared with control mice (Ladel et al., 1997; Bean et al., 1999; Saunders et al., 2000; Roach et al., 2002). Thus, it is likely that the greater amounts of TNF-α and IL-6 secreted by APCs at the site of 1A29 infection than at the site of Mt103 infection overstimulate the recruitment and activation of immune cells, processes that are important for the initial containment of mycobacterial growth. Consequently, macrophages may rapidly become less permissive to bacterial growth in the case of 1A29, a fact that is probably exacerbated by the increased sensitivity of the mutant to RNIs. In addition, other immune cells could participate in the limitation of the growth of 1A29 in vivo. It is for instance the case with neutrophils that have been shown to be activated by IL-6 (Borish et al., 1989) and to become more mycobactericidal in the presence of TNF-α (Kisich et al., 2002). Thus, reducing TNF-α and IL-6 production could be an escape mechanism of DIM-producing M. tuberculosis strains that limits the activation of immune cells during the very early steps of infection, thereby favouring bacterial growth.
Taken together, our findings suggest that the bacilli of the M. tuberculosis complex use DIM production as a means of lessening the effectiveness of the bactericidal activity of macrophages and controlling the release and exposure of immunologically active components on their cell envelope in order to modulate the responses of APCs. The existence of putative regulatory elements in the promoter regions of mas and fadD28, two genes involved in the biosynthesis of DIMs (Sirakova et al., 2002), the upregulation of these two genes after phagocytosis of M. bovis BCG by macrophage-like THP-1 cells (Li et al., 2001) and the fact that M. bovis produces large amounts of DIMs during mice infection (Kanai et al., 1970; Kondo and Kanai, 1972) further support this concept.
What our findings also clearly show is that the proper location of DIM molecules in the cell envelope is critical to their biological effects. Indeed, the 8B152 mutant, which synthesizes wild-type amounts of DIMs but fails to export them to the cell envelope (Camacho et al., 2001), behaved like 1A29 in terms of both induction of cytokines in vitro and virulence in vivo (Camacho et al., 1999).
The fact that purified DIM molecules had no stimulatory or inhibitory effect on the activation of macrophages and dendritic cells in vitro could suggest that DIMs by themselves are not responsible for the modulation of cytokine secretion by M. tuberculosis-infected APCs. Although this assumption is consistent with the idea that DIM production controls the secretion or exposure of immunogenic compounds to some extent, one cannot exclude the possibility that DIM molecules carried by whole tubercle bacilli, the presentation of which as cell-associated molecules and trafficking inside APCs are probably different from those of purified DIM molecules, also have a direct immunomodulatory role. Likewise, the effect of DIM production on the resistance of M. tuberculosis to RNIs is most likely the result of a passive role of DIMs in the cell wall permeability barrier, but could also be attributable to scavenger properties of these molecules on NO radicals. In this regard, it is interesting to note that the structurally related phenolic glycolipid produced by Mycobacterium leprae, PGL-1, was found to be a potent oxygen radical scavenger (Chan et al., 1989). However, the scavenging properties of PGL-1 were shown to be mainly associated with the glycosylated phenol moiety of the molecule of which M. tuberculosis DIMs are devoid. Thus, DIMs are unlikely to exhibit a similar scavenging activity. Clearly, more experiments are required to clarify these points.
In conclusion, our results suggest that the major roles of DIM production in virulence are in the modulation of the host immune response in the very early steps of infection and in the protection of M. tuberculosis against the NO-dependent killing of macrophages.
Experimental procedures
Bacterial strains and growth conditions
Mycobacterium tuberculosis Mt103, the wild-type strain used in this study, was isolated from an immunocompetent tuberculosis patient ( Jackson et al., 1999 ). Strains 1A29 and 8B152 were isolated using the STM procedure, as described previously ( Camacho et al., 1999 ). 1A29 and 8B152 harbour IS 1096::km insertions within the fadD26 and mmpL7 genes respectively. The biochemical phenotypes of Mt103, 1A29 and 8B152 were described by Camacho et al. (2001 ). In the wild-type strain, Mt103, DIMs are mostly found associated with the cell wall. 8B152 produces wild-type amounts of DIMs that primarily remain associated with the cytosol plus plasma membrane fraction. 1A29 does not produce detectable amounts of DIM molecules.
Mycobacterium tuberculosis Mt103, its fadD26 transposon mutant, 1A29, and its mmpL7 transposon mutant, 8B152, were grown at 37°C in Middlebrook 7H9 broth (Difco) supplemented with 10% ADC enrichment (5% bovine serum albumin fraction V, 2% dextrose, 0.003% beef catalase) and 0.05% Tween 80, or on agar Middlebrook 7H11 medium (Difco) supplemented with OADC (0.05% oleic acid, 5% bovine serum albumin fraction V, 2% dextrose, 0.004% beef catalase, 0.85% NaCl). Kanamycin (20 µg ml −1 ) was added to the culture medium of the mutant strains. Mt103 and 1A29 expressing the green fluorescent protein (GFP-Mt103 and GFP-1A29) were obtained by transformation with the GFP-encoding plasmid, pEGFP, kindly provided by G. Stewart (Imperial College, London, UK) and cultured in the presence of hygromycin B (50 µg ml −1 ).
Growth and persistence of M. tuberculosis in mice
Female BALB/c mice (6–8 weeks old) purchased from CERJanvier were infected intranasally with M. tuberculosis Mt103 and the fadD26 mutant strains. Glycerol stocks of both strains were washed in PBS (pH 7.4) containing 0.05% Tween 80 and centrifuged at 20 g for 10 min to remove bacterial clumps. Bacterial suspensions consisting of ≈ 2 × 105 cfu ml−1 were then prepared, and 50 µl drops corresponding to 104 cfu were deposited at the entry of the nostril until complete inhalation. Four to five mice were used per experimental point and per strain. On days 1, 7, 14, 21, 42 and 120 after infection, mice were killed. Their lungs and spleen were removed aseptically and homogenized in Sauton medium diluted 1:4 and supplemented with Middlebrook OADC. The viable bacteria in the organs of infected animals were counted by plating serial dilutions of the organ homogenates onto solid 7H11 or 7H11-Km medium.
Histopathology and immunohistochemistry
For histopathological studies, four additional mice were infected in each experimental group. Lungs from killed mice were removed aseptically. The superior, median and inferior lobes of the right lung were fixed in Zn fixative and embedded in paraffin, as described previously (Steedman, 1957; Ferrero et al., 2000). Serial 4 µm sections were cut and stained with haematoxylin and eosin or used for immunostaining. The surface occupied by granulomas and infiltrates was estimated on 6–12 histological lung sections from five to six mice infected with 1A29 or Mt103 in two independent experiments, with the aid of a 2.5× magnification objective. The surface of granulomas was calculated using the Leica qwin program (Leica Microsystems Imaging Solutions).
Preparation and infection of murine bone marrow macrophages and dendritic cells
Murine bone marrow macrophages were prepared as described previously (Rousseau et al., 2003). Macrophages were seeded in 24-well plates (Tpp) 105 cells per well for the infection experiment, 106 cells per well for cytokine measurement and allowed to differentiate for 7 days. For experiments with activated macrophages, 100 U ml−1 IFN-γ (R and D Systems) and 10 ng ml−1 TNF-α (R and D Systems) were added to the medium 24 h before infection and maintained throughout the infection process. Activation of macrophages was checked just before infection using the Griess reagent kit (Molecular Probes). Dendritic cells were prepared as described previously (Méderléet al., 2002). On day 10, loosely adherent cells were harvested, adjusted to 2 × 105 cells ml−1 in fresh culture medium and seeded in six-well plates. Infection assays were carried out as follows: Mt103 and 1A29 bacterial cells from fresh cultures were washed in PBS (pH 7.4) containing 0.05% Tween 80, and bacterial concentrations were adjusted in cell culture medium. An aliquot of the bacterial suspensions used to infect the cells was plated on 7H11 plates to establish the exact number of bacteria in the inoculum. Macrophages and dendritic cells were infected at 37°C in a 5% CO2 atmosphere for 4 h at mutiplicities of infection (MOI) of 1:2 bacteria per cell and 5:1 bacteria per cell. Infections were terminated by removing the overlying medium and washing each well three times with Dulbecco's modified Eagle medium (DMEM) before adding fresh culture medium to the wells. On days 0 (4 h), 3, 5 and 7 after infection (resting macrophages) or on days 0 (4 h), 3 and 8 after infection (activated macrophages), the infected cell monolayers (three wells per strain) were lysed in 200 µl of cell culture lysis reagent (Promega), and the number of viable intracellular cfu was evaluated by plating the lysis solution on 7H11 or 7H11-Km. To inhibit RNI production, 200 µM Nω-nitro-l-arginine methyl ester hydrochloride (L-NAME; Sigma) was added to the culture medium of activated macrophages 24 h before induction and maintained throughout the infection process. The effectiveness of macrophage treatment with L-NAME was checked using the Griess reagent kit (Molecular Probes).
Immunofluorescence and confocal experiments
Bone marrow macrophages from BALB/c mice were cultured on glass coverslips in 24-well plates (106 cells per well in a volume of 1 ml). Macrophages were infected with GFP-Mt103 and GFP-1A29 at an MOI of 5:1 bacteria per cell (for the 24 h time point) or 1:2 bacteria per cell (for 3 and 7 day time points). After 4 h at 37°C, the medium was removed, and the wells were washed three times with DMEM. At various time points after infection, the coverslips were removed from wells (three coverslips per strain) and fixed with 4% paraformaldehyde in PBS. For the staining of acidic vacuoles, cells were incubated with 30 µM N-(3-((2,4-dinitrophenyl)amino)propyl)-N-(3-aminopropyl)methylamine, dihydrochloride (DAMP) (Molecular Probes) for 30 min and then washed three times with DMEM before fixation. Coverslips were then incubated for 20 min in PBS with 1% BSA, 0.1% fetal bovine serum and 0.05% saponin to permeabilize cells. Anti-DNP antibodies (Molecular Probes) were used to detect DAMP. Cy3-conjugated secondary antibodies were purchased from Amersham. After labelling, coverslips were set in Fluoromount G (Southern Biotechnology Associates) on microscope slides. Slides were observed by confocal microscopy using a LSM510 laser Ar/He-Ne Zeiss microscope.
Effect of purified DIMs on the activation of macrophages and dendritic cells in vitro
Dendritic cells were activated by 10 µg of LPS (Sigma) per 106 cells. Macrophages were activated as described above, using 100 U ml−1 IFN-γ (R and D Systems) and 10 ng ml−1 TNF-α (R and D Systems).
An aqueous emulsion of purified DIMs, kindly provided by P. E. Kolattukudy (University of Central Florida, Orlando, FL, USA), was prepared in 0.1% polyvinylpyrrolidone (Sigma) at a final concentration of 1 µg µl−1. The effect of DIMs on dendritic cells and macrophages was tested by adding 1–100 µg of purified DIMs per 106 dendritic cells and 1–50 µg of purified DIMs per 105 macrophages. The stabilizer, polyvinylpyrrolidone, alone had no effect on the cells.
Flow cytometry analysis of cell surface markers
Dendritic cells were prepared and activated as described above. Fc receptors were blocked for 20 min at 4°C with anti-mouse CD16-CD32 (mouse Fc block, clone 2.4G2; Pharmingen). Approximately 2 × 105 cells were placed into microplates and stained for surface markers for 30 min at 4°C using anti-CD11c antibodies (phycoerythrin-conjugated anti-mouse CD11c clone HL3; Pharmingen), anti-MHC class II antibodies (biotinylated anti-mouse IAd–IEd clone 2G9; Pharmingen) and anti-B7.2 antibodies (biotinylated anti-mouse B7.2 clone GL1; Pharmingen) diluted in PBS containing 1% heat-inactivated fetal calf serum, 1% mouse antibodies and 0.05% sodium azide. Biotinylated antibodies were labelled with streptavidin-PerCP (Pharmingen) for 30 min at 4°C. Cells were fixed in 4% paraformaldehyde for 20 min at 4°C and analysed by flow cytometry (FACStar; Becton Dickinson) using the cellquest software (Becton Dickinson Immunocytometry System).
Measurement of cytokine production
Supernatants from control and infected macrophages and dendritic cells were harvested at different time points after infection, filtered on 0.22 µm pore-size polyvinylidene difluoride (PVDF) filters (Millipore) and stored at −80°C. TNF-α, IL-6 and IL-12p40 were detected by enzyme-linked immunosorbent assay (ELISA; OptEIA, Pharmingen), according to the supplier's instructions. Recombinant cytokines (Pharmingen) were used to generate standard curves. For each condition tested, cytokine production was measured in three independent wells and in three independent experiments using different cell preparations.
Acknowledgements
This work was supported by the Institut Pasteur and the European Commission contract QLK2-CT-1999-01093, ‘A cluster for tuberculosis vaccine development’. C.R. is a recipient of a Ministère de la Recherche fellowship.




