Helicobacter pyloriγ-glutamyltranspeptidase upregulates COX-2 and EGF-related peptide expression in human gastric cells
Summary
Gastric mucosa responds to Helicobacter pylori-induced cell damage by increasing the expression of COX-2 and EGF-related peptides. We sought to investigate the bacterial virulence factor/s and the host cellular pathways involved in the upregulation of COX-2, HB-EGF and amphiregulin in MKN 28 and AGS gastric mucosal cells. H. pylori strain CCUG 17874 was grown in Brucella broth supplemented with 0.2% (2,6-dimethyl)-β-cyclodextrins. The soluble proteins released in the culture medium by the bacterium were fractionated by exclusion size and anion exchange chromatography. A single peak retaining the ability to upregulate COX-2 and HB-EGF mRNA and protein expression was obtained. SDS-PAGE analysis of the peak showed two peptides with an apparent molecular weight of 38 and 22 kDa, which were identified by automated Edman degradation analysis as the N-terminal and C-terminal peptides of H. pyloriγ-glutamyltranspeptidase respectively. Acivicin, a selective γ-glutamyltranspeptidase inhibitor, counteracted H. pylori-induced upregulation of COX-2 and EGF-related peptide mRNA expression. An H. pylori isogenic mutant γ-glutamyltranspeptidase-deficient strain did not exert any effect on COX-2, HB-EGF and amphiregulin mRNA expression. Blockade of phosphatidylinositol-3 kinase and p38 kinase, but not MAP kinase kinase, inhibited H. pyloriγ-glutamyltranspeptidase-induced upregulation of COX-2 and EGF-related peptide mRNA expression.
Introduction
Helicobacter pylori is the major causative agent of chronic superficial gastritis and peptic ulcer disease and plays an important role in the development of adenocarcinoma of the distal stomach in humans ( Zarrilli et al., 1999a ; Peek and Blaser, 2002 ; Ricci et al., 2002 ).
Helicobacter pylori -induced gastroduodenal disease depends on the inflammatory response of the host and on the production of a number of virulence factors, such as urease, responsible for ammonia generation ( Ricci et al., 1997 ), a vacuolating cytotoxin (VacA) ( Cover and Blaser, 1992 ) and a cytotoxin-associated protein (CagA), one of the products of a 40 kb cag pathogenicity island ( cag PAI) ( Censini et al., 1996 ). Colonization with cag + strains induces a more intense tissue response, as reflected by enhanced mucosal inflammation, cell proliferation and cell death ( Peek et al., 1999 ). The cag PAI encodes a type IV secretion system, by which H. pylori delivers CagA into gastric epithelial cells ( Censini et al., 1996 ). The intracellular CagA activates the Ras/MAPKK/MAPK1 pathway and a growth factor-like cell response that results in abnormal proliferation and movement of gastric epithelial cells ( Higashi et al., 2002 ; Mimuro et al., 2002 ). However, additional effector factors different from CagA seem to be involved in the epithelial cytokine/chemokine response to H. pylori infection ( Fischer et al., 2001 ).
The mechanism whereby H. pylori contributes to gastric carcinogenesis is still unknown. Infection in humans is associated with increased proliferation of gastric epithelial cells (Jones et al., 1997; Peek et al., 1997) that may be sustained by hypergastrinaemia (Wang et al., 2000), increased expression of epidermal growth factor (EGF)-related peptides (Romano et al., 1998a; Keates et al., 2001; Tuccillo et al., 2002; Wallasch et al., 2002) and activation of the EGF receptor (EGFr) signal transduction pathway in gastric epithelial cells (Keates et al., 2001; Wallasch et al., 2002). In addition, it has been demonstrated that H. pylori upregulates the expression of cyclooxygenase-2 (COX-2), the inducible isoform of the enzyme responsible for prostaglandin production, in human gastric epithelial cells in vitro (Romano et al., 1998b) as well as in human gastric mucosa in vivo (Fu et al., 1999; Zarrilli et al., 1999b). The upregulation of growth factors and COX-2 might increase the mitogenic activity of H. pylori-infected gastric mucosa in view of the absence of a corresponding increase in apoptosis (Jones et al., 1997; Peek et al., 1997), which may therefore be regarded as early events in gastric carcinogenesis associated with H. pylori infection (Zarrilli et al., 1999a; Peek and Blaser, 2002). Upregulation of COX-2 and inducible nitric oxide synthase (iNOS) expression (Fu et al., 1999; Zarrilli et al., 1999b) may also contribute to the high oxidative DNA damage observed during H. pylori infection (Baik et al., 1996), and this may increase the mutation rate in infected hyperproliferative gastric mucosa.
We have shown previously that either H. pylori whole bacteria or broth culture filtrates (BCFs) upregulated the expression of EGF-related growth factors and COX-2 in MKN 28 human gastric epithelial cells in vitro (Romano et al., 1998a,b), and that these phenomena were independent of the expression of known bacterial virulence factors, such as VacA, CagA or urease (Romano et al., 1998a,b). This study was designed to identify the bacterial virulence factor/s and the host cellular pathways involved in the upregulation of COX-2 and EGF-related peptides in gastric mucosal cells. We show that H. pylori-induced upregulation of COX-2 and EGF-related peptide expression in MKN 28 and AGS human gastric epithelial cells depends on the bacterial production of γ-glutamyltranspeptidase (GGT). A common signal transduction pathway that relies on the activation of a phosphatidylinositol-3 kinase (PI-3 kinase) and p38 kinase, but not MAP kinase kinase, is responsible for H. pylori GGT-induced upregulation of COX-2 and EGF-related peptide expression in MKN 28 gastric mucosal cells.
Results
Biochemical fractionation of COX-2 and HB-EGF upregulating activities in MKN 28 gastric cells from H. pylori BCFs
Our previous data demonstrated that bacterial suspensions or BCFs from H. pylori were able to upregulate the expression of HB-EGF and amphiregulin (AR) growth factors (members of the EGF receptor family of ligands) and COX-2 in MKN 28 gastric mucosal cells. These effects were specifically related to H. pylori because they were not observed with Escherichia coli and were independent of known H. pylori virulence factors such as VacA, CagA or urease (Romano et al., 1998a,b). Also, increased expression of both EGF-related growth factors and COX-2 was mediated by a soluble factor (of apparent molecular mass >12 kDa) released by H. pylori in the culture medium (Romano et al., 1998a; M. Romano and R. Zarrilli, unpublished data).
To identify the virulence factor(s) responsible for these phenomena, we performed a biochemical fractionation of soluble bacterial products released in BCFs and analysed these fractions for their COX-2 and EGF-related growth factor upregulating activities in MKN 28 gastric mucosal cells. H. pylori strain CCUG 17874 was grown in Brucella broth supplemented with 0.2% (2,6-dimethyl)-β-cyclodextrins (CD) instead of 5% fetal calf serum (FCS) to obtain the BCFs. Then, H. pylori soluble bacterial products were fractionated through a four-step biochemical procedure.
First, bacterial BCFs were fractionated through a G-50 gel filtration chromatography to exclude CD and low-molecular-mass components of Brucella broth medium. The fractions in either the exclusion or the inclusion volume of the column were pooled and analysed for their protein composition and COX-2 and EGF-related growth factor upregulating activities. As shown in Fig. 1A, pools of fractions from the exclusion volume of the column loaded with H. pylori broth filtrates, but not with control uBCFs, showed a complex protein pattern and retained COX-2 and HB-EGF upregulating activities. No biological activities were present in pools of fractions from the inclusion volume of columns loaded with either H. pylori BCFs or uninoculated broth culture filtrates (uBCFs; data not shown).
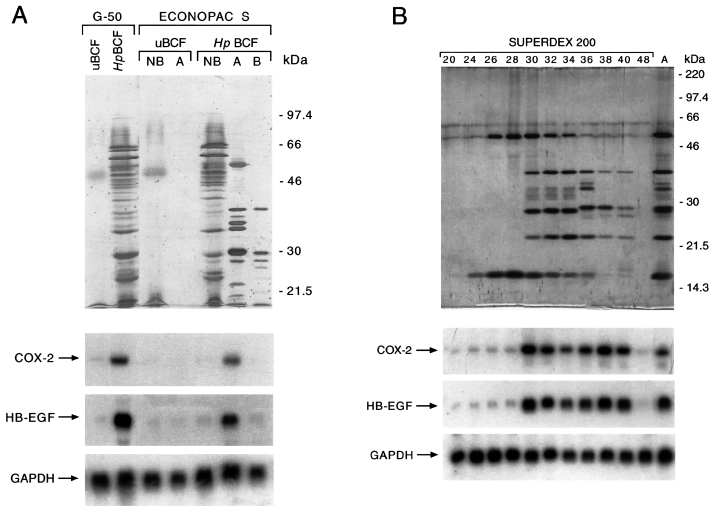
Biochemical fractionation of COX-2 and HB-EGF upregulating activities from H. pylori broth culture filtrates.Top. A. Silver nitrate staining of 10% SDS-PAGE of uninoculated broth culture filtrate (uBCF) and H. pylori broth culture filtrate ( Hp BCF) sequentially fractionated onto a Sephadex G-50 and an Econopac S FPLC. Pools of the exclusion volume fractions from Sephadex G-50 (G-50) and aliquots of either not bound samples (NB) or samples eluted in a first (A) or a second (B) peak from Econopac S FPLC are shown.B. Silver nitrate staining of 12% SDS-PAGE of H. pylori proteins eluted from peak A of the Econopac S FPLC column before (lane A) and after Superdex 200 FPLC fractionation. Fraction numbers of the Superdex 200 FPLC are indicated. Migration of molecular weight markers is indicated on the right.Bottom. Northern blot analysis of COX-2, HB-EGF and GAPDH mRNA expression in MKN 28 cells incubated for 18 h with H. pylori fractionated proteins shown in the top photos.
In the second fractionation step, the proteins in the exclusion volume of the G-50 column were loaded onto a cation exchange chromatography column and eluted with a 0.1–1 M NaCl linear gradient at pH 7.4. Two peaks (A and B) were eluted from H. pylori filtrates, showing patterns of 10 and five major proteins in the SDS-PAGE analysis respectively (Fig. 1A). COX-2 and HB-EGF upregulating activities were retained in the H. pylori proteins eluted in peak A, but not in peak B or in those not bound to the affinity column (Fig. 1A). Also, no COX-2 and HB-EGF upregulating activities were found in the not bound flowthrough (NB) or in the fractions eluted at the same ionic strength of peak A (A) from control uBCF (Fig. 1A). The affinity chromatography purification step enriched COX-2 and HB-EGF upregulating activities by approximately 15-fold compared with H. pylori BCFs (Table 1).
| Purification step | COX-2/GAPDH (mRNA levels µg−1 proteina) | HB-EGF/GAPDH (mRNA levels µg−1 proteina) |
|---|---|---|
| None | 1 | 1 |
| Sephadex G-50 | 2.5 ± 0.3 | 3.3 ± 0.4 |
| Econo-Pac S FPLC | 18.7 ± 1.2 | 15.8 ± 1.4 |
| Superdex 200 FPLC | 22.6 ± 1.8 | 19.6 ± 1.6 |
| Econo-Pac S FPLC pH 8.3 | 44.4 ± 2.5 | 38.7 ± 3.1 |
- a . COX-2/GAPDH and HB-EGF/GAPDH mRNA ratios from MKN 28 cells incubated with H. pylori soluble virulence factors at the different purification steps are shown. Values are expressed as arbitrary densitometric units normalized on the amount of H. pylori protein samples and are the mean ± SD of at least three independent experiments. None, H. pylori BCF. mRNA levels of MKN 28 cells incubated with crude H. pylori BCF are arbitrarily taken as 1. BCF, broth culture filtrate; FPLC, fast performance liquid chromatography.
Next, H. pylori proteins in the peak of activity were separated through fast performance liquid chromatography (FPLC) exclusion size chromatography (Superdex 200 FPLC column), and the fractions were analysed by SDS-PAGE. As shown in Fig. 1B, fractions 24–28 contained two proteins with an apparent molecular mass of 60 kDa and 16 kDa, fractions 30–34 contained the same two proteins and three additional proteins with an apparent molecular mass of 38 kDa, 29 kDa and 22 kDa, fractions 36–40 contained three different proteins with an apparent molecular mass of 35 kDa, 34 kDa and 29.5 kDa and low amounts of the proteins eluted in fractions 30–34. Northern blot analysis of MKN 28 cells incubated with the eluted fractions from the column showed that COX-2 and HB-EGF upregulating activities were retained in fractions 30–40, but not in fractions 24–28 of the column (Fig. 1B). The calculated molecular mass of proteins eluted in fractions 30–40 from the FPLC exclusion size chromatography were estimated as being between 70 kDa and 50 kDa (data not shown).
Purification and identification of H. pylori COX-2 and HB-EGF upregulating activities in MKN 28 gastric mucosal cells
Based on the different protein composition of the fractions showing biological activity after exclusion size chromatography (Fig. 1B), the active eluted fractions were divided into two independent pools (pool I and pool II, from fractions 30–34 and fractions 36–40 respectively), loaded onto a cation exchange column and eluted with a 0.1–1 M NaCl linear gradient at pH 8.3. In fact, it has been shown that the proteins in H. pylori contained twice as many of the basic amino acids arginine and lysine as proteins in other organisms and that several surface and soluble proteins from H. pylori possessed basic isoelectric points (Tomb et al., 1997; Alm et al., 1999). At the end of the purification procedure, a single peak (A*) retaining both COX-2 and HB-EGF upregulating activities was obtained from both pools of fractions, whereas no activity was observed in the proteins that did not bind to the column (Fig. 2A). Moreover, COX-2 and HB-EGF upregulating activities at this purification step increased by ≈ 40-fold compared with those in H. pylori BCFs (Table 1). SDS-PAGE analysis of the peak A* obtained from pool I showed two peptides with an apparent molecular mass of 38 kDa and 22 kDa (Fig. 2A), whereas the same two peptides of 38 kDa and 22 kDa plus an additional one with an apparent molecular mass of 30 kDa were observed in the peak obtained from pool II (data not shown).
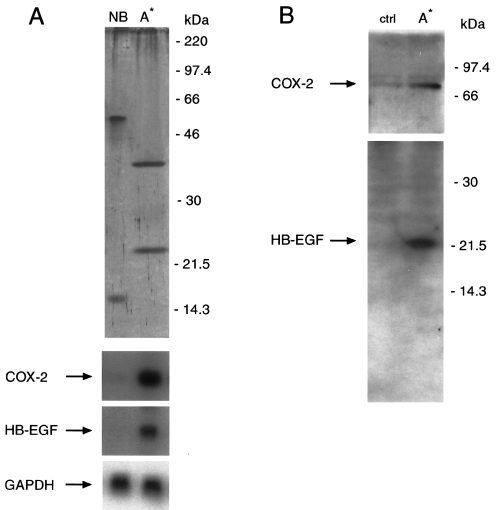
pH 8.3 Econopac S FPLC fractionation of COX-2 and HB-EGF upregulating activities from H. pylori soluble proteins. A. Top. Silver nitrate staining of 12% SDS-PAGE of H. pylori protein pool from fraction 30–34 of the Superdex 200 column after elution with Econopac S FPLC column run at pH 8.3. Aliquots of either not bound samples (NB) or samples eluted in the single A* peak from Econopac S FPLC are shown. Migration of molecular weight markers is indicated on the right. Bottom. Northern blot analysis of COX-2, HB-EGF and GAPDH mRNA expression in MKN 28 cells incubated for 18 h with H. pylori fractionated proteins shown at the top.B. Western blot analysis of COX-2 and HB-EGF protein expression in MKN 28 cells after treatment with H. pylori soluble proteins eluted in the single A* peak from Econopac S FPLC column run at pH 8.3. COX-2 and HB-EGF immunoreactive bands are indicated on the left. Migration of molecular weight markers is shown on the right.
To determine whether COX-2 and HB-EGF protein synthesis correlated with mRNA expression, cellular extracts from peak A* soluble protein-treated or untreated (control) MKN 28 cells were analysed by Western blot analysis using antisera specific for COX-2 and HB-EGF respectively (Fig. 2B). A COX-2 immunoreactive peptide of ≈ 70 kDa was detected in untreated (control) cells that increased by eightfold in peak A*-treated cells (Fig. 2B, top). On the other hand, an HB-EGF immunoreactive peptide of ≈ 22 kDa was detected in peak A*-treated, but not in untreated (control), MKN 28 cells (Fig. 2B, bottom). In contrast, H. pylori soluble proteins eluted in peak A* did not affect the expression of COX-1 mRNA levels in MKN 28 cells, as assessed by reverse transcription polymerase chain reaction (RT-PCR) analysis (data not shown). We also studied whether induction of COX-2 mRNA expression was associated with an increased release of PGE2 in the conditioned medium by MKN 28 cells. Incubation for 18 h with H. pylori soluble proteins eluted in peak A* caused an approximately fourfold increase in PGE2 release by MKN 28 cells compared with control cells (493 ± 134 pg mg−1 protein versus 118 ± 37 pg mg−1 protein respectively; P < 0.05).
Automated Edman degradation analysis was performed on the two proteins eluted in the peak A* of activity, and obtained peptide sequences were aligned with those deduced from the two complete sequences of H. pylori available (Tomb et al., 1997; Alm et al., 1999). As shown in Fig. 3, N-terminal sequences from 38 kDa and 22 kDa peptides aligned with residues 27–35 and 380–387 of H. pylori GGT, respectively, which corresponded to the N-terminal sequences of large and small subunits generated by H. pylori pro-GGT through cleavage of the N-terminal signal sequence and processing of the protein by autocatalytic activity at the amino acidic residue 379 (Chevalier et al., 1999; Bumann et al., 2002). Also, the calculated molecular mass of the large and small GGT subunit of 38 226 Da and 20 378 Da, respectively, as deduced from the published sequence of H. pylori 26695 (Tomb et al., 1997), was in agreement with the apparent molecular mass of 38 kDa and 22 kDa of the purified proteins on which sequence analysis had been performed.

Amino acid alignment of N-terminal sequences from 38 kDa (A) and 22 kDa (B) H. pylori soluble proteins eluted in the single A* peak from Econopac S FPLC column run at pH 8.3 with H. pylori 26695 GGT. Amino acid residues of H. pylori 26695 GGT are indicated. Multiple sequence alignment has been performed by searching the PyloriGene™ database at Institut Pasteur ( http://pim.hybrigenics.com ) with the program fasta ( Pearson and Lipman, 1988 ).
Effect of γ-glutamyltranspeptidase inhibitor acivicin on COX-2, HB-EGF and AR upregulating activities of purified γ-glutamyltranspeptidase from H. pylori filtrates in MKN 28 gastric cells
To demonstrate further that the induction COX-2 and HB-EGF expression was dependent on the bacterial production of GGT, we studied the effect of acivicin, a specific non-competitive inhibitor of GGT enzyme, on the ability of purified GGT from H. pylori BCFs to upregulate COX-2, HB-EGF and AR expression. As shown in Fig. 4, treatment of MKN 28 cells with acivicin at doses of 100 µM and 200 µM inhibited by more than 90% the upregulation of COX-2, HB-EGF and AR expression induced by either the H. pylori proteins eluted in the exclusion volume of the G-50 column during the first purification step or the purified H. pylori GGT in the peak A* of activity from the fourth purification step. To support further a role for GGT in H. pylori-induced upregulation of COX-2 and EGF-related peptide expression, acivicin also counteracted COX-2, HB-EGF and AR overexpression induced by crude BCF obtained from wild-type H. pylori strain CCUG 17874 (data not shown). Acivicin treatment at the doses used did not affect COX-2 and EGF-related growth factor expression in MKN 28 cells (Fig. 4), nor did it alter cell viability (data not shown).
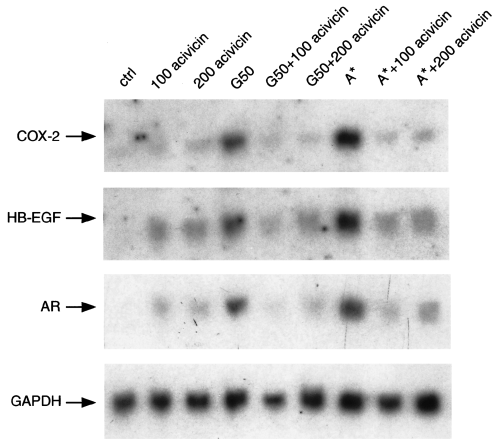
Effect of acivicin, a selective GGT inhibitor, on COX-2, HB-EGF and AR upregulating activities of purified GGT from H. pylori filtrates in MKN 28 gastric mucosal cells. Northern blot analysis of COX-2, HB-EGF, AR and GAPDH mRNA expression in MKN 28 cells incubated for 18 h with H. pylori proteins eluted in the exclusion volume of the G-50 column or in the single A* peak from Econopac S FPLC column run at pH 8.3 in the presence of acivicin is shown. 100 acivicin and 200 acivicin, acivicin at a concentration of 100 µM and 200 µM respectively.
Effect of purified γ-glutamyltranspeptidase from H. pylori filtrates on COX-2, HB-EGF and AR mRNA expression in AGS gastric cells
In order to rule out the possibility that GGT-dependent upregulation of COX-2 and EGF-related peptides was a cell line-specific phenomenon, we investigated the effect of the purified H. pylori GGT in the peak A* of activity from the fourth purification step in the AGS gastric cell line. As shown in Fig. 5, H. pylori GGT induced the expression of COX-2, HB-EGF and AR mRNA, and this effect was inhibited by acivicin treatment. In contrast, purified H. pylori GGT did not affect the expression of COX-1 mRNA levels in AGS cells, as assessed by RT-PCR analysis (data not shown).
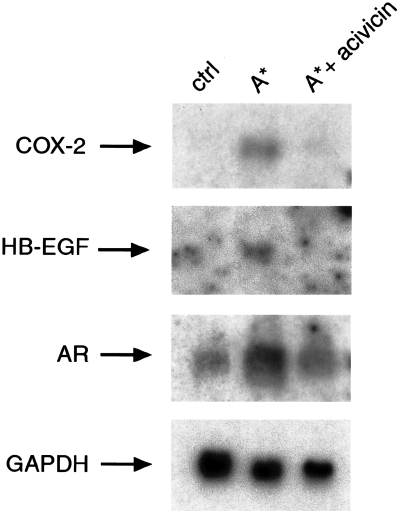
Effect of acivicin, a selective GGT inhibitor, on COX-2, HB-EGF and AR upregulating activities of purified GGT from H. pylori filtrates in AGS gastric mucosal cells. Northern blot analysis of COX-2, HB-EGF, AR and GAPDH mRNA expression in AGS cells incubated for 18 h with H. pylori proteins eluted in the single A* peak from Econopac S FPLC column run at pH 8.3 in the absence and presence of acivicin GGT inhibitor at 100 µM is shown.
COX-2, HB-EGF and AR upregulating activities of an H. pylori isogenic mutant γ-glutamyltranspeptidase-defective strain in MKN 28 gastric mucosal cells
To elucidate further the role of GGT in the upregulation of COX-2 and EGF-related peptides, we evaluated whether an H. pylori GGT null mutant strain retained these activities compared with its parental strain. As shown in Fig. 6, bacterial suspensions from the H. pylori wild-type strain M5 increased COX-2, HB-EGF and AR mRNA expression by 10-fold, whereas those from its isogenic GGT null mutant M5 ggt::aph did not. Accordingly, BCFs from wild-type strain M5, but not from its isogenic mutant lacking ggt, increased COX-2, HB-EGF and AR mRNA expression by threefold. On the other hand, bacterial suspension and BCFs from the H. pylori strain CCUG increased COX-2 and EGF-related peptide mRNA expression by 10- and five fold, respectively (Fig. 6).
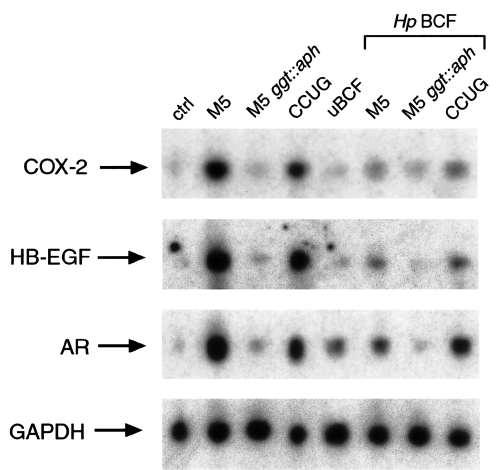
COX-2, HB-EGF and AR upregulating activities of HpM5 parental strain and its γ-glutamyltranspeptidase-defective isogenic mutant strain. Northern blot analysis of COX-2, HB-EGF, AR and GAPDH mRNA expression in MKN 28 cells incubated for 18 h with DMEM (control), uninoculated broth culture filtrate (uBCF), a bacterial suspension (5 × 10 7 cfu ml −1 ), broth culture filtrate ( Hp BCF) from H. pylori M5 strain or its isogenic ggt mutant (M5 ggt::aph ) or CCUG 17874 strain is shown.
Effect of PI-3 kinase, MAP kinase kinase or p38 kinase inhibitors on COX-2, HB-EGF and AR upregulating activities of purified γ-glutamyltranspeptidase from H. pylori filtrates
All the above data indicate that a single bacterial virulence factor was able to upregulate the expression of at least two different functions, COX-2 and EGF-related growth factors in MKN 28 cells. Because EGF-related growth factors are known to upregulate COX-2 expression through the activation of EGFr in colon cancer cells (Coffey et al., 1997), we asked whether H. pylori GGT-dependent upregulation of COX-2 expression in gastric mucosal cells might be consequent on the induction of EGF-related growth factors. To address this issue, we performed a time course analysis of H. pylori GGT effect on COX-2, HB-EGF and AR expression. As shown in Fig. 7A, purified H. pylori GGT induced COX-2, HB-EGF and AR mRNA expression at similar time points (i.e. 12 and 18 h), thus making it unlikely that COX-2 upregulation is contributed to by EGF-related growth factors. It has been shown previously that different signal transduction pathways, either MAP kinase or PI-3 kinase, or stress kinase regulated the expression of COX-2 and EGF-related growth factors in inflammatory and epithelial eukaryotic cells (Barnard et al., 1995; Barry et al., 1999; Di Popolo et al., 2000; Sheng et al., 2001). Also, the above signal transduction pathways have been demonstrated to be activated in gastric mucosal cells during H. pylori infection (Naumann et al., 1999; Higashi et al., 2002; Mimuro et al., 2002; Tuccillo et al., 2002). To identify the signal transduction pathway/s responsible for GGT-dependent upregulation of COX-2 and EGF-related growth factor expression in MKN 28 gastric mucosal cells, we analysed whether this was affected by PI-3 kinase, MAP kinase kinase or p38 kinase inhibition. Treatment of MKN 28 cells with the PI-3 kinase inhibitor LY294002 or the p38 kinase inhibitor SB203580 reduced GGT-dependent upregulation of COX-2 and EGF-related growth factor expression by 80% and 70% respectively (Fig. 7B). On the other hand, treatment with the MAP kinase kinase inhibitor PD98059 did not affect GGT-dependent upregulation of COX-2 and EGF-related growth factor expression (Fig. 7B). All the pharmacological inhibitors at the doses used did not affect COX-2 and EGF-related peptide expression (Fig. 7B) or viability in MKN 28 cells (data not shown). Altogether, these data suggested that PI-3 kinase- and p38 kinase-dependent common signal transduction pathways mediated the effect of H. pylori GGT on COX-2 and EGF-related growth factor expression in MKN 28 gastric mucosal cells.
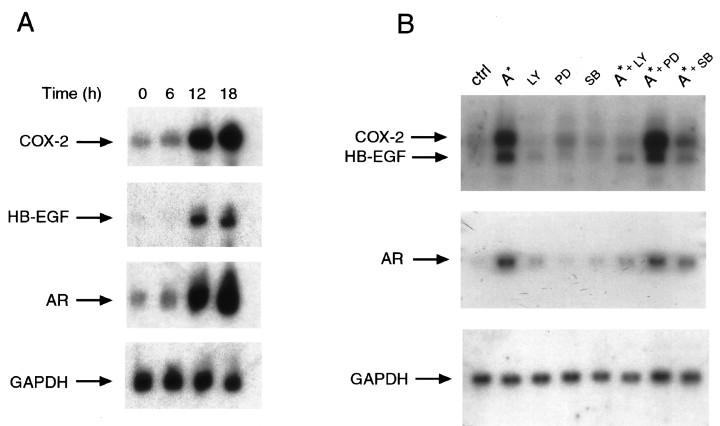
Signal transduction pathways involved in H. pylori γ-glutamyltranspeptidase-dependent upregulation of COX-2 and EGF-related peptides. A. Time course of COX-2, HB-EGF and AR expression in MKN 28 cells incubated with purified GGT from H. pylori filtrates. Northern blot analysis of COX-2, HB-EGF, AR and GAPDH mRNA expression in MKN 28 cells incubated with H. pylori proteins eluted in the single A* peak from Econopac S FPLC column run at pH 8.3 for up to 18 h is shown.B. Effect of PI-3 kinase, MAP kinase kinase or p38 kinase inhibitors on COX-2, HB-EGF and AR upregulating activities of purified GGT from H. pylori filtrates in MKN 28 cells. Northern blot analysis of COX-2, HB-EGF, AR and GAPDH mRNA expression in MKN 28 cells incubated for 18 h with H. pylori proteins eluted in the single A* peak from Econopac S FPLC column run at pH 8.3 and inhibitors is shown. LY, PI-3 kinase inhibitor LY294002 at a concentration of 50 µM; PD, MAP kinase kinase inhibitor PD98059 at a concentration of 40 µM; SB, p38 kinase inhibitor SB203580 at a concentration of 5 µM.
Discussion
The present study was designed to identify the bacterial virulence factor(s) and the host cellular pathway(s) responsible for H. pylori-dependent upregulation of the expression of HB-EGF and AR growth factors (members of the EGFr family of ligands) and of COX-2 expression in MKN 28 human gastric mucosal cells. Our previous data demonstrated that these effects were specifically related to H. pylori because they were not observed with E. coli and were independent of known H. pylori virulence factors, such as VacA, CagA or urease (Romano et al., 1998a,b). Also, increased expression of both EGF-related growth factors and COX-2 was mediated by a soluble H. pylori factor > 12 kDa in size (Romano et al., 1998a; M. Romano and R. Zarrilli, unpublished data).
Here, we demonstrate that GGT is the bacterial product responsible for H. pylori-dependent upregulation of COX-2 and EGF-related peptide expression in MKN 28 and AGS human gastric mucosal cells. Moreover, we demonstrate that H. pylori GGT stimulates the release of PGE2 in MKN 28 cells. A biochemical fractionation procedure of the soluble proteins released in the culture medium by the bacterium lead us to isolate two peptides with an apparent molecular mass of 38 kDa and 22 kDa, which retain the ability to upregulate EGF-related growth factors and COX-2 in gastric mucosal cells. The above two peptides were identified by automated Edman degradation analysis followed by alignment with amino acid sequences deduced from the two complete sequences of H. pylori available (Tomb et al., 1997; Alm et al., 1999) as the N-terminal and C-terminal peptides of H. pylori GGT respectively. Also, the apparent molecular mass of 38 kDa and 22 kDa of the purified proteins is in agreement with the calculated molecular mass of the large and small GGT subunit of 38 226 Da and 20 378 Da, respectively, as deduced from the published sequence of H. pylori 26695 (Tomb et al., 1997). In keeping with our data, it has been shown that two peptides of 38 kDa and 23 kDa are generated by H. pylori pro-GGT through cleavage of the N-terminal signal sequence and processing of the protein by autocatalytic activity at the amino acid residue 379 respectively (Chevalier et al., 1999). Our data are also in agreement with a recent report showing that two GGT fragments of 38 kDa and 21 kDa are two major components of the proteins secreted by H. pylori (Bumann et al., 2002). This finding, together with the presence of a signal peptide sequences for sec-dependent transport across the bacterial plasma membrane (Tomb et al., 1997; Alm et al., 1999; Chevalier et al., 1999), is consistent with our previous studies showing that increased expression of both EGF-related growth factors and COX-2 was mediated by a soluble H. pylori factor > 12 kDa in size (Romano et al., 1998a; M. Romano and R. Zarrilli, unpublished data). We have shown previously that broth culture filtrates and suspensions from E. coli did not have any effect on EGF-related peptide and COX-2 mRNA expression in MKN 28 gastric mucosal cells (Romano et al., 1998a,b). The enzyme GGT is expressed by several bacterial species, including E. coli (Suzuki et al., 1986; reviewed and discussed in Chevalier et al., 1999). One possible explanation for this discrepancy might be the different biochemical properties between E. coli GGT and H. pylori GGT, E. coli GGT activity being greatly affected by growth temperature and absent at 37°C (Suzuki et al., 1986), whereas H. pylori GGT is not (Chevalier et al., 1999). Also, the possibility exists that H. pylori secretes higher levels of GGT compared with other organisms. However, no quantitative data are available on GGT production from H. pylori in infected gastric mucosa. We also find that the ability of either purified H. pylori GGT or crude H. pylori BCF to upregulate EGF-related peptides and COX-2 expression in MKN 28 and AGS gastric epithelial cells is counteracted by acivicin, a selective non-competitive GGT inhibitor. Moreover, we demonstrate that an H. pylori isogenic mutant GGT-defective strain does not exert any effect on COX-2, HB-EGF and amphiregulin mRNA expression, whereas both M5 and CCUG wild-type strains are able to upregulate COX-2 and EGF-related peptide expression. The fact that BCFs from CCUG are more effective in the induction of COX-2 and EGF-related peptide mRNA than those from M5 suggests that differences in secretion/shed of GGT in these two strains may exist. However, all the above data reinforce the concept that COX-2 and EGF-related peptide upregulation by H. pylori depends on the bacterial production of GGT.
GGT is an enzyme that plays a major role in glutathione metabolism (Tate and Meister, 1981). In mammals, GGT is a heterodimeric plasma membrane ectoenzyme, composed of two glycosylated subunits (heavy subunit of 55–60 kDa and light subunit of 21–30 kDa), which catalyses the transfer of a γ-glutamyl residue from glutathione (GSH) to amino acids or peptides, thus forming glutamate and cysteinylglycine. These components are then transported into cells for resynthesis of GSH (Tate and Meister, 1981). Because the maintenance of intracellular levels of glutathione is critical in response to oxidative stress injury, GGT has been interpreted as being an antioxidant enzyme. However, recent studies indicate that the extracellular cleavage of GSH by GGT leads to reactive oxygen species (ROS) production (del Bello et al., 1999) and that purified GGT generates ROS in the presence of GSH and transferrin, as a source of iron (Drozdz et al., 1998), thus suggesting a pro-oxidant role for GGT. The fact that bacterial GGT may have a pro-oxidant effect during H. pylori infection is further supported by the finding that antral glutathione concentration and glutathione S-transferase activity are reduced during H. pylori infection (Verhulst et al., 2000; Shirin et al., 2001) and that incubation of cultured gastric mucosal cells with H. pylori depletes intracellular GSH stores (Shirin et al., 2001). In keeping with this, it has been shown recently that the extracellular cleavage of GSH by GGT mediates nuclear factor-κB activation in normal and transformed eukaryotic cells through ROS production (Accaoui et al., 2000) or lipid peroxidation (Djavaheri et al., 2002). In the above two experimental systems, the GGT pro-oxidant effect is based on the generation of cysteinylglycine (Accaoui et al., 2000; Djavaheri et al., 2002). We postulate that H. pylori GGT induces an oxidative response in gastric mucosal cells that results in upregulation of COX-2 and EGF-related growth factor expression. In partial support of this hypothesis, treatment of MKN 28 gastric mucosal cells (50 µM) with the iron-chelator desferrioxamine, which inhibits the formation of ROS generated by cysteinylglycine in the presence of transition metals (Djavaheri et al., 2002), inhibited by 40% purified H. pylori GGT-dependent upregulation of COX-2 and EGF-related peptide mRNA expression (data not shown).
Some experimental evidence indicates that GGT is an important virulence factor for H. pylori. A whole-genome microarray identified the presence of the gene in all H. pylori clinical isolates analysed (Salama et al., 2000). Also, the synthesis of a catalytically active GGT has been demonstrated in H. pylori (Chevalier et al., 1999). GGT seems to play an important role during the establishment of H. pylori infection, H. pylori GGT-defective isogenic strains being unable to colonize (Chevalier et al., 1999) or impaired (McGovern et al., 2001) in the colonization of the gastric mucosa of mice or piglets. Dissecting out the response of the host to H. pylori infection and identifying the responsible bacterial virulence factors may be relevant to the understanding of the mechanism(s) whereby H. pylori alters gastric homeostasis and renders gastric mucosa more susceptible to disease. In particular, we identified a novel physiological role for H. pylori GGT, i.e. the activation of specific host signalling pathways that lead to the induction of cell cycle mediators such as EGF-related growth factors and COX-2. This contributes to unravelling the role of specific bacterial products in the pathogenesis of H. pylori-related gastric carcinogenesis and might provide a clue for targeted therapeutic intervention to overcome H. pylori-related gastric cancer. However, our data must be interpreted with caution, because the cells used in this study are from an adenocarcinoma, and the effects observed could potentially reflect the biology of a tumour cell as opposed to that of normal, non-transformed cells. On the other hand, MKN 28 and AGS cell lines have been proved to be appropriate models for the study of the response of gastric epithelial cells to H. pylori (Ricci et al., 1997; Romano et al., 1998a,b; Naumann et al., 1999; Peek et al., 1999; Pomorski et al., 2001). Moreover, we have previously compared MKN 28 cells with human gastric epithelial cells in primary culture and found comparable results with regard to their response to damaging agents in vitro (Romano et al., 1988).
Upregulation of COX-2 expression and activity has been considered as early events in H. pylori-associated gastric carcinogenesis, and its effect has been explained at least in part by the inhibition of cellular apoptosis (Zarrilli et al., 1999a; Peek and Blaser, 2002). The fact that H. pylori GGT upregulates the expression of COX-2 therefore supports a role for GGT in H. pylori-related gastric carcinogenesis. However, this finding is apparently in contrast to a recent report demonstrating that GGT plays a significant role in H. pylori-mediated apoptosis of AGS gastric epithelial cells (Shibayama et al., 2003). This discrepancy may depend on different experimental systems and/or different experimental conditions. In partial support of this hypothesis, our data show that H. pylori GGT-dependent induction of COX-2 mRNA levels is higher in MKN 28 cells compared with AGS cells. In further support of this hypothesis, we found that H. pylori is able to induce apoptosis in AGS cells but not in MKN 28 cells (R. Zarrilli, unpublished observation).
Several signal transduction pathways are activated during the inflammatory and proliferative response of gastric mucosal cells to H. pylori infection (Zarrilli et al., 1999a; Peek and Blaser, 2002; Ricci et al., 2002). The expression of genes within the cag island activates nuclear factor-κB, mitogen-activated protein kinase- and stress kinase-dependent signal transduction pathways, thus ultimately leading to chemokine secretion in gastric mucosal cells (Naumann et al., 1999; Peek et al., 1999). Also, translocation of CagA in gastric epithelial cells induces a growth factor like-response through Grb2 and the Ras/MAPKK/MAPK pathway (Higashi et al., 2002; Mimuro et al., 2002). On the other hand, H. pylori infection activates EGFr signalling and expression of EGF-related growth factors in gastric mucosal cells, and these effects are not dependent on genes in the H. pylori cag island (Romano et al., 1998a; Tuccillo et al., 2002).
Our data demonstrate that the blockade of the PI-3 kinase or the p38 kinase, but not the MAP kinase, pathway inhibits H. pylori GGT-dependent upregulation of COX-2 and EGF-related growth factor expression in MKN 28 gastric mucosal cells. We also show that purified H. pylori GGT induced COX-2, HB-EGF and AR mRNA expression at similar time points, thus making it unlikely that COX-2 upregulation is contributed to by EGF-related growth factors. In keeping with our finding, it has been demonstrated that H. pylori induces the release of prostaglandin E2 from gastric epithelial cells by cytosolic phospholipase A2 activation via the p38 kinase pathway (Pomorski et al., 2001). Our data are also in agreement with those obtained in different experimental systems showing that COX-2 upregulation in platelets is mediated through PI-3 kinase, MAP kinase and p38 kinase (Barry et al., 1999) and that COX-2 overexpression in colon cancer cells is regulated through a PI-3 kinase-dependent pathway (Di Popolo et al., 2000; Sheng et al., 2001).
In conclusion, we demonstrate that H. pylori-related upregulation of COX-2 and EGF-related peptide expression depends on the production by the bacterium of GGT and the subsequent activation of PI-3 kinase- and p38 kinase-dependent signal transduction pathways in human gastric mucosal cells. Because EGFr activation and COX-2 increased expression play an important role in the host response to H. pylori infection and during gastrointestinal carcinogenesis (Zarrilli et al., 1999a; Peek and Blaser, 2002), based on our findings, we postulate that development of gastric adenocarcinoma associated with H. pylori infection may also depend on the activation of GGT-related events.
Experimental procedures
Bacterial strains and growth conditions
We used the wild-type (VacA+/cag+/urease+) CCUG 17874 strain (from the culture collection of the University of Goteborg, Goteborg, Sweden). We also used the mouse-adapted H. pylori strain M5 and its GGT-defective isogenic mutant M5 ggt::aph, in which the ggt gene was disrupted by insertional mutagenesis (McGovern et al., 2001). Bacteria were grown routinely on Columbia agar supplemented with 1% Vitox and 10% defibrinated sheep blood (Oxoid) at 37°C under microaerobic conditions. Bacteria were grown in Brucella broth (Difco) supplemented with 1% Vitox (Oxoid) and 5% fetal calf serum (FCS) to prepare standard crude BCFs (Romano et al., 1998a,b) or 0.2% (2,6-dimethyl)-β-cyclodextrins (CD; from Sigma-Aldrich) (Marchini et al., 1995) to purify soluble bacterial factors for 36–48 h at 37°C in a thermostatic shaker (150 oscillations min−1) under microaerobic conditions. When bacterial suspensions reached 1.2 optical density units at 450 nm (corresponding to a bacterial concentration of 5 × 108 cfu ml−1), bacteria were removed by centrifugation (12 000 g for 15 min), and the supernatants were sterilized by passage through a 0.22-µm pore size cellulose acetate filter (Nalgene) to obtain the BCFs. Proteins in the BCFs were processed further for biochemical analysis. In the experiments with crude H. pylori BCFs, BCFs were used at dilution of 1:3, and uninoculated BCF (uBCF) served as a control.
Biochemical fractionation of soluble bacterial proteins
Step 1. BCFs were 20-fold concentrated by a Heidolph Roto-vapor apparatus at 37°C and loaded onto a Sephadex G-50 column (Amersham Pharmacia Biotech, size 2.5 × 60 cm), which was equilibrated and eluted with 25 mM Hepes, 100 mM NaCl, pH 7.4, at a flow rate of 1.5 ml min−1 and 4°C.
Step 2. The fractions eluted in the exclusion volume were pooled and loaded onto a cation exchanger column (Bio-Rad, Econo-Pac S-Cartridge, 5 ml) connected to an FPLC system (Amersham Pharmacia Biotech), equilibrated with 25 mM Hepes, 100 mM NaCl, pH 7.4, and eluted with a linear gradient of 0.1–1 M NaCl at a flow rate of 1 ml min−1 and room temperature.
Step 3. The eluted fractions retaining the biological activity were pooled, concentrated by Roto-vapor and loaded onto a Superdex 200 High Load column (Amersham Pharmacia Biotech, 1 × 30 cm) connected to an FPLC system (Amersham Pharmacia Biotech) and eluted with 25 mM Hepes, 100 mM NaCl, pH 7.4, at a flow rate of 0.3 ml min−1 and room temperature. Gel filtration low- and high-molecular-weight calibration kits (Amersham Pharmacia Biotech) were used to calibrate the column and to estimate the molecular weight of the eluted samples.
Step 4. The eluted fractions retaining the biological activity were pooled, dialysed against 25 mM phosphate buffer, 100 mM NaCl, pH 8.3, and loaded onto a cation exchanger column (Bio-Rad Laboratories, Econo-Pac S-Cartridge, 5 ml) connected to an FPLC system (Amersham Pharmacia Biotech), equilibrated with the same buffer and eluted with a linear gradient of 0.1–1 M NaCl at a flow rate of 1 ml min−1 and room temperature.
SDS-PAGE analysis
Protein concentration of samples was determined by the dye-binding assay of Bradford (using protein assay reagent from Bio-Rad Laboratories). SDS-PAGE analysis was carried out under reducing conditions according to the method of Laemmli (1970); after the run, the proteins were stained by Coomassie brilliant blue R250 or silver nitrate as described previously (Rabilloud et al., 1994).
Automated Edman degradation analysis
For amino acid sequence analysis, protein samples were transferred from 12% SDS-PAGE gels onto polyvinylidene difluoride (PVDF) membranes (ImmobilonP; Millipore) by electroblotting in 100 mM CAPS (3-cyclohexylamino-1-propanesulphonic acid), 20% methanol, pH 11.0. After Coomassie blue staining, the bands of interest were excised, and N-terminal sequence analysis was performed with a Perkin-Elmer–Applied Biosystems 477A pulsed-liquid protein sequencer equipped with a Perkin-Elmer–Applied Biosystems 120A high-performance liquid chromatography (HPLC) apparatus for phenylthiohydantoin (PTH)-amino acid identification as reported previously (Zappacosta et al., 1997). The obtained peptide sequences were used to identify proteins by searching the PyloriGene™ database of Institut Pasteur (http://pim.hybrigenics.com) with the program fasta (Pearson and Lipman, 1988).
Human gastric epithelial cells and culture conditions
We used two different human gastric cell lines, the MKN 28 cell line, which derives from a well-differentiated human gastric tubular adenocarcinoma and shows gastric-type differentiation (Romano et al., 1988), and the human gastric adenocarcinoma cell line AGS (ATCC, CRL-1739). Both cell lines were grown as monolayers in DMEM supplemented with 10% FCS (Gibco BRL) at 37°C in a humidified atmosphere of 5% CO2. To evaluate COX-2 and EGF-related growth factor upregulating activities, MKN 28 cells were incubated for 18 h with H. pylori soluble bacterial factors in standard culture medium (DMEM supplemented with 10% FCS). In the experiments with bacterial suspensions, cells were incubated with bacterial preparations at a concentration of 5 × 107 cfu ml−1 in DMEM supplemented with 10% FCS. In the experiments with the pharmacological inhibitors, MKN 28 cells were treated with one or other of (i) acivicin, a specific non-competitive inhibitor, at a concentration of 100 µM and 200 µM (Maellaro et al., 2000); (ii) PD98059, an inhibitor of the activation of MAP kinase kinase, at a concentration of 40 µM (Alessi et al., 1995); (iii) SB203580, a specific inhibitor of p38 kinase, at a concentration of 5 µM (Cuenda et al., 1995); (iii) LY294002, a specific inhibitor of PI-3 kinase, at a concentration of 50 µM (Vlahos et al., 1994).
RNA isolation, Northern analysis and RT-PCR analysis
Total RNA was isolated from MKN 28 cells by the guanidinium thiocyanate acid–phenol procedure and subjected to Northern analysis as described previously (Romano et al., 1998a,b). In brief, 10 µg of total RNA per lane was separated by electrophoresis in 1% agarose–formaldehyde gels. RNA was transferred to Hybond-N+ (Amersham Italia), cross-linked (UV Stratalinker-1800, Stratagene) and hybridized to 32P-labelled cDNA probes. 32P-labelled isotopes were from Amersham Italia. The human COX-2 probe was a 0.276 kb EcoRI cDNA, the human heparin-binding EGF-like growth factor (HB-EGF) probe was a 1.102 kb EcoRI cDNA, the human amphiregulin (AR) probe was a 0.87 kb EcoRI–HindIII cDNA, and the human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe was a 1.5 kb BamHI cDNA. COX-2, HB-EGF and AR mRNA levels were normalized to mRNA levels of the constitutively expressed GAPDH gene (Romano et al., 1998a,b). mRNA levels were quantified by densitometric scanning of the autoradiograph using a Howtek Scanmaster-3 densitometer with rfl print software (Amersham Pharmacia Biotech). RT-PCR analysis of COX-1 mRNA expression was performed as described previously (Zarrilli et al., 1999b).
Preparation of cell extracts and Western blot analysis
At the end of the incubation period, cells were washed twice with PBS, scraped in lysis buffer containing 20 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM sodium orthovanadate, 1 mM phenylmethylsulphonyl fluoride (PMSF; Sigma Chemical), 1% NP40 in the presence of a protease inhibitor cocktail (CompleteTM tablets; Roche Diagnostics) and then incubated for 30 min at 4°C with shaking. Total lysates were cleared by microfuging at 14 000 r.p.m. for 30 min at 4°C; 80 µg of proteins was separated by 12% SDS-PAGE and blotted to BA 85 0.45 µm protran nitrocellulose membrane (Schleicher and Schuell). After blocking with 10% BSA in TTBS (10 mM Tris, pH 7.5, 150 mM NaCl, 0.1% Tween 20), filters were incubated with specific antibodies (sc-7951 rabbit polyclonal anti-human COX-2 from Santa Cruz Biotechnology; 2998 rabbit polyclonal anti-human HB-EGF, kindly provided by Dr M. Klagsbrun, Boston, MA, USA), and proteins were visualized with anti-rabbit IgG peroxidase-linked antibody (Amersham Pharmacia Biotech) using the ECLplus system (Amersham Pharmacia Biotech).
PGE2 assay
PGE2 concentration in the conditioned media of MKN 28 cells was measured with a 125I radioimmunoassay as described previously (Romano et al., 1998b).
Acknowledgements
We thank Dr Maria Monti from CEINGE, Napoli, Italy, for technical support in protein sequencing, Drs Angela Maria Acquaviva and Fabrizio Gentile for scientific advice, Mario Berardone for excellent artwork, and Rita Cerillo for technical assistance. This work was supported in part by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC) and Ministero dell’Università e della Ricerca Scientifica e Tecnologica (COFIN 2002 to V.R. and R.Z.), Italy.




