Developmental elimination of ectopic projection sites for the transgenic OR gene that has lost zone specificity in the olfactory epithelium
Abstract
In rodents, olfactory receptor (OR) genes are expressed in one of four zones in the olfactory epithelium (OE), and olfactory sensory neurons (OSNs) expressing the same OR project their axons to a specific set of glomeruli on the olfactory bulb (OB). Using the yeast artificial chromosome (YAC) transgenic system, we have analysed the expression of the murine OR gene MOR29A of the MOR28 cluster located on chromosome 14. Although expression of the endogenous MOR29A was restricted to the most dorsomedial zone, the transgenic MOR29A (Tg MOR29A) was expressed in all four zones of the OE. When the OB of the transgenic mouse was analysed, the axons of the OSNs expressing Tg MOR29A were found to project not only to the dorsal side but also to the ventral side of the OB as well. The ectopic projection sites on the ventral side gradually disappear during postnatal development. Naris occlusion prevents this elimination, suggesting that odorant stimulation is involved in eliminating the ectopic projection sites.
Introduction
A multigene family encoding olfactory receptors (ORs) was first identified in rats, which consists of about 1000 OR genes (Buck & Axel, 1991). In situ hybridization with OR gene probes demonstrated that the OE can be divided into four distinct zones, and that each OR gene is expressed in one of four particular zones (Ressler et al., 1993; Vassar et al., 1993). Furthermore, in each olfactory sensory neuron (OSN), only one member of an OR gene family is expressed in a mono-allelic manner (Chess et al., 1994; Malnic et al., 1999; Ishii et al., 2001).
Although OSNs expressing a given OR gene are scattered within a zone, they converge their axons to a specific set of glomeruli on the olfactory bulb (OB) (Ressler et al., 1994; Vassar et al., 1994; Mombaerts et al., 1996). Thus, odorant stimuli received in the olfactory epithelium (OE) appear to be converted to a topographic map of activated glomeruli on the OB (Rubin & Katz, 1999; Uchida et al., 2000; Fried et al., 2002). Mutations and base substitutions in the OR coding regions appear to change the projection sites on the OB (Wang et al., 1998; Ishii et al., 2001). Swapping of coding sequences has resulted in projection to a set of glomeruli different from that of the wild type (Wang et al., 1998). These results indicate an instructive role of OR molecules in selecting the target sites on the OB. In targeting the axons of OSNs, odorant-evoked activity has been implicated to have a critical role. Zheng et al. (2000) reported that absence of OCNC1 perturbed the targeting of M72-expressing OSNs, although Lin et al. (2000) obtained an opposite result with a different OR gene, P2. In heterozygous female mice, OCNC1-deficient neurons were depleted during development, and this depletion was prevented by naris occlusion (Zhao & Reed, 2001).
Using yeast artificial chromosome (YAC) transgenic mice (Serizawa et al., 2000; Ishii et al., 2001), we have been studying the expression of the murine MOR28 gene cluster, containing seven OR genes (Tsuboi et al., 1999; Nagawa et al., 2002). In situ hybridization of mouse OE sections revealed that the MOR28, 10 and 83 genes in the cluster are expressed in the most ventrolateral zone (zone 4 in the nomenclature of Ressler et al., 1993), whereas the MOR29A, 29B, 30A and 30B genes are expressed in the most dorsolateral zone (zone 1). During the process of analysing our YAC transgenic mice, we noticed that the zone-specific expression of Tg MOR29A is disregulated, as has been reported for the transgenic minigenes (Vassalli et al., 2002). Expression of the transgenic OR genes is not restricted to one particular zone of the OE. Furthermore, OSNs expressing such transgenes targeted their axons to multiple sites on the ventral side of the OB in addition to the dorsal side (zone 1).
In the present study, we have found that ectopic projection sites on the ventral side disappear as the mice aged. It was also found that naris occlusion blocked the developmental elimination of ectopic projection sites. Such observations may indicate an activity-dependent elimination of ectopic glomeruli during development of the olfactory system.
Materials and methods
Targeting vectors
A plasmid vector p29IG, containing the MOR29A coding and 3′-untranslated region tagged with IRES-gap-EGFP, was derived from p28DU (Serizawa et al., 2000). For the tagging, an SacII–NotI fragment containing IRES-gap-EGFP was isolated from pGKI (Serizawa et al., 2000) and inserted into the SacII and NotI sites of p28DU, generating pIG28D. The C57Bl/6 mouse genomic sequence of the MOR29A coding region was amplified with a pair of primers, 29a-SacI (GAGCTCTGCTGTGGTGACAGATTTCC) and 29a-SacII (CCGCGGCCACTGGCTACTTCTCTCC), and inserted into the SacI and SacII sites of pIG28D, generating p29IG28D. The 3′-untranslated region of MOR29A was amplified with another set of primers, 29a3U-NotI (GCGGCCGCACACATCATCGTCATCACC) and 29a3U-EcoRI (GAATTCTAGGATCATAGGCCTGCACC), and inserted into the NotI and EcoRI sites of p29aIG28D, generating p29IG.
To make the targeting vector, pKOV-KanF-SacB, the flanking regions of the SacB gene were amplified from the bacterial artificial chromosome (BAC) cloning vector pBACe3.6 with two sets of primers: 5SacB-F (GATCCTTCTATAGTGTCACC) and 5SacB-R (GGGCGCGCCAGCTGTCCCACACATCAAGTC), and 3SacB-F (GGGCGCGCCATGATCGCGTAGTCGATAG) and 3SacB-R (GATGCAAGTGTGTCGCTGTC). The amplified fragments were inserted into the SalI site of pKOV-KanF (Lalioti & Heath, 2001), generating pKOV-KanF-SacB. To produce pKOV-KanF-28Z, a 3.6-kb MunI–MunI genomic fragment containing the MOR28 coding region and 3′UTR was inserted into pKOV-KanF, generating pKOV-KanF-28. The IRES-tau-lacZ fragment was then inserted into the SpeI site downstream of the MOR28 coding region in pKOV-KanF-28, generating pKOV-KanF-28Z. To produce pKOV-KanF-29G, an EcoRI–BamHI fragment containing MOR29A-IRES-gap-EGFP was isolated from the YAC-290G construct and inserted into pKOV-KanF.
Transgenic constructs
The YAC-290 construct was generated by truncating the YAC-460 with a fragmentation vector pU-V22 (Serizawa et al., 2000). The YAC-290G construct, in which MOR28 and MOR29A are tagged with IRES-tau-lacZ and IRES-gap-EGFP, respectively, was generated from the YAC-290 and targeting vector p29IG by yeast homologous recombination (Bungert et al., 1995).
The BAC-200G construct, in which MOR28 and MOR29A are tagged with IRES-tau-lacZ and IRES-gap-EGFP, respectively, was generated from the BAC clone of the C57Bl/6 mouse, RPCI-23 339 P24, by targeting the plasmid vectors, pKOV-KanF-SacB, pKOV-KanF-28Z, and pKOV-KanF-29G (Lalioti et al., 2001).
Transgenic mice
YAC DNA was separated in Seakem Gold agarose by preparative pulse-field gel electrophoresis (FMC BioProducts, Rockland, MD, USA). The DNA was isolated and concentrated by electrophoresis in 4% Nusieve GTG (FMC BioProducts). An agarose piece containing the YAC DNA was incubated overnight in YAC buffer [10 mm Tris Cl (pH 8.0), 0.1 mm EDTA, 100 mm NaCl, 30 µm spermine, and 70 µm spermidine] to replace the electrophoresis buffer (0.5 × TBE). Agarose was digested with β-agarase (New England Biolabs, Beverly, MA, USA) and DNA was injected into fertilized mouse eggs. BAC DNA was linearized by AscI digestion and isolated with preparative pulse-field gel electrophoresis.
Olfactory tissues
All experiments were carried out in accordance with the guidelines at The University of Tokyo. All efforts were made to minimize the number of animals used. Mice were anaesthetized with sodium pentobarbital (2.5 mg/animal) and perfused intracardially with 4% paraformaldehyde in phosphate-buffered saline (PBS). OE and OB were dissected and fixed overnight with 4% paraformaldehyde in PBS. The OE but not the OB was decalcified in 0.5 m EDTA at 4 °C for 1–2 days. Tissues were then placed in 30% sucrose, and embedded briefly in O.C.T. compound (Tissue-Tek, Torrance, CA, USA) in dry acetone ice. Serial coronal sections (16 µm for the OE, 18 µm for the OB) were prepared with JUNG CM3000 cryostat (Leica, Nussloch, Germany) and collected onto glass slides coated with 3-aminopropyl-triethoxysilane.
In situ hybridization
The 3′-untranslated region of MOR29A in pBluescript II (Stratagene, La Jolla, CA, USA), and the coding region of the mouse OCAM in pCRII (a generous gift from Dr Y. Yoshihara at RIKEN) were used as templates to make probes for the MOR29A and OCAM genes, respectively. Digoxigenin-labelled probes were prepared with a DIG RNA labelling kit (Hoffmann-La Roche, Basel, Switzerland).
To detect the MOR29A transcripts, coronal sections of the OE (16 µm each) from the 3-week-old mice were hybridized with the probe as described (Hirota et al., 1992) to detect the MOR29A transcripts. For detection of the OCAM RNA, the Tyramide signal amplification kit (PerkinElmer, Wellesley, MA, USA) was used. After treatment with anti-DIG polyclonal antibodies (Hoffmann-La Roche), sections were incubated with biotin-conjugated anti-sheep IgG (Chemicon International, Temecula, CA, USA) 1 : 200 in PBS for 1 h at room temperature. Samples were washed three times (5 min each) with Wash buffer [100 mm Maleic acid (pH 7.5), 150 mm NaCl, and 0.3% Tween20], reacted with horseradish peroxidase (HRP)-conjugated streptavidin (PerkinElmer) 1 : 100 in PBS for 30 min, and washed again three times. Signals were visualized by incubating the samples with TSA-Flu for 10 min.
Immunohistochemistry
Coronal sections of the OE (16 µm each) were incubated overnight with rabbit antibodies against β-galactosidase (ICN Biochemicals, Aurora, OH, USA) or with antibodies against enhanced green fluorescent protein (EGFP; Clontech, Palo Alto, CA, USA) 1 : 1000. Samples were treated with the Cy3-conjugated antibody against rabbit IgG (Chemicon International, Temecula, CA) 1 : 500 for 3 h at room temperature. Fluorescence photographs were taken with a cooled CCD camera, model C4742-95–12ERG (Hamamatsu Photonics K.K., Shizuoka, Japan) coupled to a fluorescence microscope, model IX-70 (Olympus, Tokyo, Japan).
For the OB studies, coronal sections (18 µm each) were incubated overnight with rabbit antibodies against OCAM (a generous gift from Drs H. Nagao and K. Mori at the University of Tokyo) (1 : 500). Samples were then treated with the Cy3-conjugated antibody against rabbit IgG (Chemicon International) 1 : 200 for 2 h at room temperature. Whole nuclei were counterstained with DAPI (Hoffmann-La Roche). Fluorescent images of EGFP, Cy3 and DAPI were photographed as described above.
Naris occlusion
Five-day-old mice were placed on ice to anaesthetize them. One of the two nostrils was lightly cauterized with a fine-tip surgical cautery, SURE SX-10C (Ishizaki, Japan). Scar formation leads to naris closure that can be confirmed by stereoscopic visual examination at time of tissue harvest.
Results
The Tg MOR29A is expressed in all four zones of the OE
We have studied the murine MOR28 gene cluster that contains seven OR genes on mouse chromosome 14 (Serizawa et al., 2000; Ishii et al., 2001; Nagawa et al., 2002). In our YAC constructs, the first four OR genes, MOR28, 10, 83 and 29A, are tandemly linked; however, the three other genes, MOR29B, 30A and 30B, are missing owing to a deletion of 60 kb in the 100-kb downstream region of MOR29A (Fig. 1a).
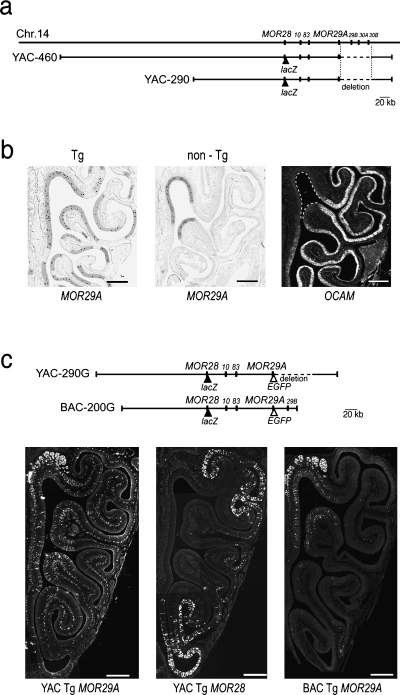
Disregulated zonal expression of the transgenic MOR29A in the YAC-290 construct. (a) Structure of the YAC-290 and YAC-460 constructs containing four mouse OR genes. On the mouse chromosome 14, seven OR genes, MOR28, 10, 83, 29 A, 29B, 30A and 30B, are tandemly linked. Both the YAC-290 and YAC-460 constructs contain a 60-kb deletion downstream of the Tg MOR29A, deleting three OR genes, MOR29B, 30A and 30B. (b) Detection of MOR29A transcripts by in situ hybridization. Coronal sections of the OE from the YAC-290 transgenic mouse (Tg), or from the non-transgenic mouse (non-Tg) were hybridized with a Dig-labelled antisense probe. Because the 3′-UTR was used as a probe, it is specific to MOR29A and not cross hybridizable to MOR29B. Hybridized areas are highlighted. Hybridization signals (dots) are restricted to zone 1 in the non-Tg mouse, but not in the Tg mouse. To define zone 1, OCAM was used as a zonal marker. A coronal section of the OE was hybridized with a Dig-labelled antisense OCAM probe. The OCAM-negative region (zone 1) is indicated by broken lines. Scale bars, 0.5 mm. (c) Expression of the Tg MOR29A and Tg MOR28 in the YAC-290G transgenic mouse. In the YAC-290G construct, Tg MOR28 and MOR29A are differentially tagged with IRES-tau-lacZ and IRES-gap-EGFP, respectively. Consecutive OE sections from the 3-week-old mouse (line G3) were immunostained with anti-β-galactosidase and anti-EGFP antibodies to detect the expression of the Tg MOR28 and Tg MOR29A, respectively. Expression of the Tg MOR28 is restricted to zone 4, but Tg MOR29A is expressed in all four zones. As a control, the BAC-200G transgenic mouse was analysed for Tg MOR29A expression. A coronal section of the OE from the 3-week-old BAC-200G transgenic mouse (line b) was immunostained with anti-EGFP antibodies. The BAC Tg MOR29A is expressed mainly in zone 1. Scale bars, 0.5 mm.
In situ hybridization of OE sections indicated that the zone-specificity of the Tg MOR29A is likely to be disregulated in three transgenic lines out of seven (Fig. 1b, Table 1). When the non-transgenic (non-Tg) mouse was analysed as a control, transcripts of the endogenous MOR29A were detected largely in zone 1 of the OE (Fig. 1b), defined as an OCAM-negative region (Yoshihara et al., 1997). When the transgenic mouse (Tg), YAC-290, was analysed, hybridization signals for the MOR29A transcripts were not restricted to zone 1 (Fig. 1b).
| Transgenic construct | Line | Copy number | Expression zone(s) |
|---|---|---|---|
| YAC-290 | 72 | 10 | Zone 1 |
| 75 | 7 | Zones 1–4 | |
| YAC-460 | a | 1 | Zone 1 |
| b | 2 | Zones 1–4 | |
| YAC-290G | G1 | 1 | Zone 1 |
| G2 | 3 | Zone 1 | |
| G3 | 5 | Zones 1–4 | |
| BAC-200G | 1 | 14 | Zone 1 |
| 2 | 3 | Zone 1 |
- Copy numbers of the transgenic constructs were quantified by Southern hybidization. Expressions of MOR29A in each transgeneic mouse were either confined to zone 1 (zone 1) or found from all four zones (zones 1–4).
Because the transcripts of Tg MOR29A cannot selectively be detected from those of endogenous MOR29A, we generated a new transgenic mouse line carrying EGFP-tagged Tg MOR29A. In this animal (YAC-290G), Tg MOR29A and Tg MOR28 are differentially tagged with IRES-gap-EGFP and IRES-tau-lacZ, respectively (Fig. 1c). Coronal sections of the OE from a 3-week-old transgenic mouse were examined. In YAC-290G line G3 mice, the EGFP-fluoresced OSNs expressing Tg MOR29A were found not only in zone 1 but in all three other zones, whereas lacZ-positive OSNs expressing Tg MOR28 (immunostained with anti-β-galactosidase antibodies) were restricted to zone 4 (Fig. 1c). In YAC-290G line G3 mice, the zone-specificity is disregulated for Tg MOR29A, whereas Tg MOR28 is expressed in a zone-specific manner.
Note that Tg MOR29A in the BAC-200G construct, which does not have the deletion between the Tg MOR29A and 29B genes, was expressed largely in zone 1 for two lines (Fig. 1c). Copy numbers and expression zone of MOR29A in each transgenic mouse line are given in Table 1.
Detection of ectopic projection sites for Tg MOR29A
For the projection of OSNs, a zone-to-zone correlation has been proposed between the spatial topography in the OE and the glomerular sheet on the OB (Mori et al., 1999). Because of disregulation of zonal expression of Tg MOR29A in the OE of YAC-290G mice, we analysed the axonal projection of the transgene-expressing OSNs. Projection sites for Tg MOR29A were detected with EGFP fluorescent light on whole-mount OBs of 3-week-old heterozygous mice (Fig. 2a). In BAC-200G transgenic mice, in which zonal regulation of Tg MOR29A appears to be normal (Fig. 1c), the transgene-expressing OSNs projected their axons to the dorsal side of the OB. Two symmetrical pairs of projection sites were detected in the dorsolateral and the dorsomedial areas of the OB, although the dorsolateral projection sites were sometimes split. In YAC-290G mice, projection sites for Tg MOR29A were detected not only on the dorsal side but also on the ventral side of the OB (Fig. 2a). In two-thirds of the samples, the ventral side of the OBs showed two symmetrical pairs of projection sites (one on the medial and the other on the lateral side). In the remaining samples, only one projection site was formed in the middle of each medial and lateral side. The projection pattern on the dorsal side was basically the same as that for the BAC-200G mouse.
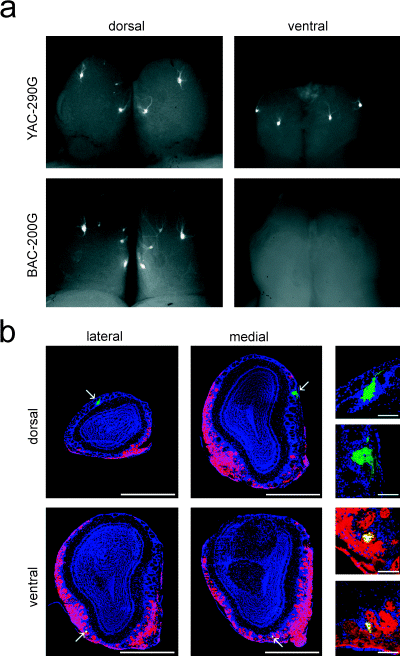
Projection of OSNs expressing the Tg MOR29A. (a) Projection sites for the Tg MOR29A were detected with EGFP fluorescent light on whole mount OBs. Dorsal and ventral views are shown for 3-week-old mice heterozygous for the transgenic construct, YAC-290G or BAC-200G. (b) Coronal sections of the OB were analysed for the Tg MOR29A projection sites (arrows). Sections were also immunostained with anti-OCAM and Cy3-conjugated secondary antibodies (red), and counterstained with DAPI (blue). The transgene-expressing OSNs projecting to the OCAM-negative area (zone 1) were stained green, whereas those projecting to the OCAM-positive regions were stained yellow. Scale bars, 1 mm. Higher magnifications of the projection sites are shown to the right (scale bars, 0.1 mm).
Coronal sections of the OB from YAC-290G mice were examined for the zonal distribution of projection sites for Tg MOR29A (Fig. 2b). We also used the OCAM protein as a zonal marker in the OB. Because OCAM is transported from the OE through the axons to the OB, glomeruli projected by the OSNs in zones, 2, 3 and 4 can be stained with anti-OCAM antibodies (Yoshihara et al., 1997). In the OCAM-negative (dorsal) area of the OB, two major projection sites were detected, one on the lateral and the other on the medial side (Fig. 2b). In situ hybridization indicated that these loci were identical or very close to those for the endogenous MOR29A, whose expression was confined to zone 1 (data not shown). This observation indicates that the transgene-expressing axons targeting to the OCAM-negative projection sites on the OB originate from the OCAM-negative area in the OE, i.e. zone 1. Transgenic projection sites in the OCAM-negative area of the OB are not co-innervated by the OCAM-positive (red) and Tg MOR29A-expressing (green) OSNs, which should appear yellow. By contrast, the transgenic projection sites in the OCAM-positive area of the OB are stained yellow (Fig. 2b).
Ectopic projection sites disappear during development
To study the fate of the ectopic projection sites during development, we tracked the developmental changes of the projection sites for Tg MOR29A in heterozygous YAC-290G mice of 1.5–7 weeks of age. As shown in Fig. 3, projection sites on the ventral side of the OB disappeared as the mice aged, whereas those on the dorsal side remained unchanged. The projection sites on the ventral side are visible in 1.5- and 3-week-old mice, but not always in 4- and 5-week-old animals. No signals are found on the ventral side at 6 and 7 weeks after birth. The time course of disappearance is variable for the ectopic glomeruli. The same results were obtained for mice over 7 weeks old up to 17 months of age (data not shown). After elimination of ectopic glomeruli, ectopic axons were diffusely innervating on the OB (data not shown).
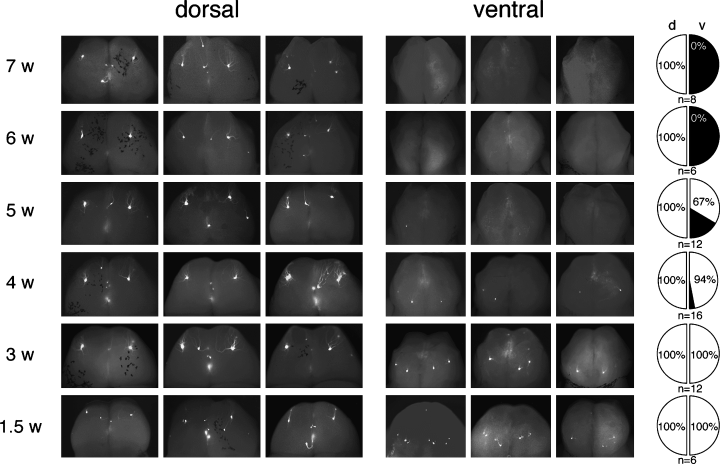
Elimination of ectopic projection sites during development. Whole mount views are shown for OBs from YAC-290G transgenic mice (heterozygous line G3). Projection sites for the Tg MOR29A were detected with EGFP fluorescence. Transgenic animals of various ages (w: weeks after birth) were analysed for the transgenic projection sites on the dorsal and ventral sides of the OB. Pie-charts on the right-hand side show ratios (%) of the number of bulbs with the transgenic projection sites (white) to those without the projection site (black). The dorsal (d) and ventral (v) sides were analysed separately.
Elimination of ectopic glomeruli is blocked under naris occlusion
Because developmental elimination of ectopic glomeruli appeared to be induced by odorant-evoked activity, we performed unilateral naris occlusion on YAC-290G mice shortly after birth (postnatal day 5). The OE and OB were examined 6, 10 and 19 weeks after birth (Fig. 4). The non-occluded contralateral naris served as a positive control in each animal. A dramatic difference was found on the ventral side between the naris-occluded and non-occluded OB. The transgenic projection sites that disappeared on the non-occluded side persisted in the occluded side of 19-week-old mice (Fig. 4a).
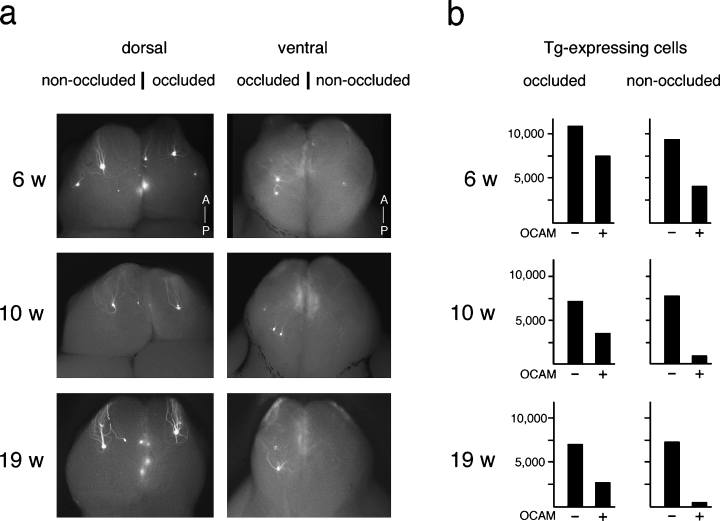
Naris occlusion of YAC-290G transgenic mice. (a) Transgenic projection sites on whole mount OBs. Unilateral naris occlusion was performed on YAC-290G mice on postnatal day 5. OBs were isolated from 6-, 10- and 19-week-old mice. Projection sites for the Tg MOR29A were detected with EGFP fluorescence. Naris-occluded and non-occluded sides of OBs were compared. Both dorsal and ventral views are shown. (b) Numbers of OSNs expressing the Tg MOR29A in individual animals. Coronal sections (16 µm) of the OE were prepared from the non-occluded and occluded nares and the numbers of EGFP-positive OSNs were counted every 14 sections. The OCAM-negative (–) and positive (+) regions were analysed separately.
We also analysed coronal sections of the OE for the number of OSNs expressing Tg MOR29A in the OCAM-negative region (zone 1) vs. positive regions (Fig. 4b). In non-occluded samples, the number of cells expressing the transgene decreased in the OCAM-positive regions as the mice aged, but remained unchanged in zone 1. However, in occluded samples, the transgene-expressing cells in the OCAM-positive regions did not disappear as rapidly as in the non-occluded samples.
Discussion
Disregulation of the zone specificity for the Tg MOR29A gene
In the present study, we have demonstrated that the zonal specificity of the transgenic (Tg) MOR29A gene is disregulated in three lines out of seven YAC transgenic mice: Tg MOR29A is expressed in all four zones of the OE, whereas wild-type MOR29A is expressed largely in zone 1. A similar observation has been reported by Vassalli et al. (2002) with the transgenic minigenes: The Tg M71 minigene of 9.2 kb showed disregulated zonal expression in one out of four lines, presumably owing to the influence of transcriptional activity near the integration site on the chromosome. They also found that the zonal specificity was disregulated for the Tg MOR23 minigene when large DNA deletions were introduced into the intron.
In our YAC Tg mice, the zonal specificity of the Tg MOR29A was disregulated in three lines out of seven (Table 1). Sequence analysis revealed that the YAC-290G construct does not contain any mutations or deletions in the intron or in the 9-kb upstream region of the Tg MOR29A. However, the YAC-290G construct was found to carry a 60-kb deletion downstream of the Tg MOR29A. It is possible that the transcriptional activity near the integration site influenced the zonal specificity of the Tg MOR29A because the 60-kb deletion has brought the transgene closer to the 3′-end of the YAC construct.
Formation and elimination of ectopic projection sites
OSNs expressing the Tg MOR29A in the YAC-290G construct projected their axons not only to the dorsal area (zone 1) of the OB but also to ectopic projection sites on the ventral side. We have found that the dorsal projection sites for the Tg MOR29A are targeted by the OSNs residing in zone 1 of the OE (OCAM-negative), whereas the ectopic projection sites on the ventral side are mainly targeted by those in zones 2, 3 and 4 (OCAM-positive). A similar observation, supporting the idea of a zone-to-zone correlation between the OE and OB, was made by Vassalli et al. (2002) with transgenic minigenes M71 and MOR23.
In our transgenic animals, the ectopic projection sites on the ventral side disappeared as the mice aged. Because transgenic MOR29A-expressing neurons existed in all zones of the OE as the ventral projection sites disappear (data not shown), we suspected that these cells were generated and then quickly disappear. As naris occlusion prevented the elimination of these ectopic sites, an activity-dependent process may be responsible for such changes. It is interesting that the fate of ectopic OSNs expressing the Tg MOR29A is similar to that of the OCNC1-deficient neurons in heterozygous female mice (Zhao et al., 2001). The OCNC1-deficient OSNs, in which activity is not evoked by odorants, become depleted in the heterozygous animal. This depletion does not occur when the mouse is naris occluded shortly after birth. It is possible that the dysfunctional OSNs are selectively eliminated, when they are situated in a competitive environment with functional OSNs. We assume that the ectopic projection sites for the Tg MOR29A cannot function properly, possibly because they cannot be activated by the odour signals in concert with the surrounding glomeruli. Failure of connections with the external tufted cells (Lodovichi et al., 2003) may be responsible at least in part for the elimination of the ectopic glomeruli. The ventral glomeruli may be sensitive to a threshold number of axons innervating them, below which the glomerular structure and connectivity cannot be maintained (Ebrahimi & Chess, 2000). Because decreased airflow and odorant stimulation is known to increase the half-life of OSNs in the occluded naris (Farbman et al., 1988), the number of neurons innervating the ventral glomeruli might have stayed above the threshold number necessary to maintain a glomerulus. Regenerational dynamics of OSNs may affect the elimination of ectopic glomeruli.
Ectopic glomeruli often appear also for endogenous OR genes tagged with lacZ or EGFP (Royal & Key, 1999; Gogos et al., 2000; Key & St John, 2002; Lodovichi et al., 2003). Although one can argue that the formation of the ectopic projection site is as a result of tagging, such ectopic glomeruli may be generated during the process of odour-map formation. Elimination of ectopic glomeruli we have described may play an important role in tailoring the odour map during development of the olfactory system.
Acknowledgements
This work was supported by the CREST programme of Japan Science and Technology (JST) Corporation, and grants from the Ministry of Education, Culture and Science, Mitsubishi Foundation and Nissan Science Foundation. H.N. was supported by Hayashi Memorial Foundation. S.S. is an investigator of the PRESTO programme of JST. T.I. is a predoctoral fellow of the Japan Society for Promotion of Science. We are grateful to Yoshihiro Yoshihara for plasmid, and Hiroshi Nagao and Kensaku Mori for antibody samples. We also thank Misao Suzuki for making transgenic mice, and Hirofumi Nishizumi and Hitomi Sakano for helpful comments regarding the manuscript.
Abbreviations
-
- BAC
-
- bacterial artificial chromosome
-
- EGFP
-
- enhanced green fluorescent protein
-
- OB
-
- olfactory bulb
-
- OE
-
- olfactory epithelium
-
- OR
-
- olfactory receptor
-
- OSN
-
- olfactory sensory neuron
-
- Tg
-
- transgenic
-
- YAC
-
- yeast artificial chromosome.