Tyrosinase gene expression is not detected in mouse brain outside the retinal pigment epithelium cells
Abstract
Tyrosinase is the rate-limiting enzyme for melanin synthesis. Its gene is expressed in two cell types: melanocytes, derived from migrating neural crest cells, and, in the CNS, retinal pigment epithelium cells, derived from the optic cup. Its absence from the eye results in profound pathway selection errors of optic fibres at the chiasm and, hence, it has been implicated as a developmental regulator of CNS pathway selection. Recently, it has been proposed that tyrosinase can also be expressed in the developing and adult brain, although the methods used were indirect. Its presence in the brain could be very significant in terms of a potentially wider role in pathway finding. Here, we have evaluated the presence of tyrosinase expression in mouse developing, perinatal and adult brain by in situ hybridization in whole-mount embryos and histological sections and by real-time reverse transcription–polymerase chain reaction. We find no evidence for tyrosinase gene expression in the CNS outside the retinal pigment epithelium cells.
Introduction
Tyrosinase, the rate-limiting enzyme for melanin biosynthesis, is encoded in the mouse by a gene located in chromosome seven which is expressed in two cellular types: melanocytes, derived from migrating neural crest cells, that colonize iris, cochlea, skin and choroid and retinal pigment epithelium (RPE) cells, derived from the optic cup. During mouse embryogenesis, tyrosinase gene can first be detected from +10.5 days postcoitum (d.p.c.) onwards in the RPE cells and from +16.5 d.p.c. in skin melanocytes (Beermann et al., 1992; Steel et al., 1992). These results were established by in situ hybridization on mouse embryos. No direct systematic assessment was reported on tyrosinase in mouse developing brain in these earlier studies.
In contrast to the above, a series of studies using transgenic tyrosinase reporter mice has been published documenting lacZ expression in developing and adult mouse brain, under the control of mouse tyrosinase gene promoter and 5′ upstream regulatory sequences (Tief et al., 1996a,b, 1998; Camacho-Hubner & Beermann, 2001). These data had been taken as an indirect indication for endogenous tyrosinase expression in the mouse developing brain.
However, to date, endogenous tyrosinase expression had not been specifically analysed in developing or adult mouse brain by direct means, namely in situ hybridization or real-time reverse transcription–polymerase chain reaction (RT–PCR) techniques. It is important that the original observations using indirect methods are confirmed, as tyrosinase plays a key role in axon guidance in the visual system (Jeffery et al., 1994; Jeffery, 1997) and may have a wider role regulating such mechanisms in the brain.
Materials and methods
Animals
Outbred albino NMRI mice (Harlan Iberica, Barcelona, Spain) were time mated. The day on which the vaginal plug was found was defined as 0.5 d.p.c. Developing animals were killed between 10.00 and 12.00 a.m. for +8.5, +9.5, +10.5, +11.5, +12.5, +13.5, +14.5, +16.5 and +18.5 d.p.c. stages and between 10.00 and 12.00 p.m. for +9, +10, +11, +12, +13 and +14 d.p.c. stages. The age of embryos was confirmed by morphological traits (Kaufman, 1995). Adult mice were killed by cervical dislocation. Neonates, fetuses at term and embryos were killed by decapitation, after short exposure to cool. All experiments complied with local and European legislation concerning vivisection and the experimentation and use of animals for research purposes.
In situ hybridization
In situ hybridization of RNA probes to tissue sections (cryostat, 20–25 µm thick) and whole-mount embryos was done as described by Wilkinson & Nieto (1993). Tyrosinase antisense (experimental) and sense (control) RNA probes were generated from plasmid pmcTyr1 (Ruppert et al., 1988), that carries a full-length mouse tyrosinase cDNA, using the DIG RNA labelling kit (Roche). Five mouse embryos were used for whole-mount in situ hybridization for each developmental day and probe. Four mouse embryos were used for in situ hybridization to tissue sections for each developmental day and probe. Photographs were taken on a digital camera (DC100; Leica) using QWin (Leica) software.
Real-time reverse transcription–polymerase chain reaction
Total RNA was extracted from the indicated tissues (brain, liver, skin and eyes) of three individuals of each age using the RNeasy kit (Qiagen). RNase-free DNaseI (Roche)-treated total RNA (1 µg) was retrotranscribed with SuperScript RNase H− reverse transcriptase (Invitrogen) and random primer oligonucleotides (hexamers) (Invitrogen) and the quality of cDNA products verified on a standard 1.5% agarose gel. For real-time PCR reactions, an aliquot of cDNA, corresponding to 1 or 10 ng total RNA, was amplified in a 25-µL final volume with AmpliTaq Gold (Roche), SYBR Green (PE Applied) and 0.1 µm specifically designed tyrosinase primers (forward, 1031–1049, within exon 2, and reverse, 1160–1140, within exon 3; nucleotide positions are referred to GenBank X12782) on an ABI-Prism 7000 (Applied Biosystems). Amplification parameters for the 130-bp cDNA-derived PCR product were 10 min at 95 °C and 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The endogenous β-actin gene expression was used as an internal control (primers: forward, 320–339; reverse, 445–428; GenBank X03765). A series of diluted gel-purified tyrosinase and β-actin cDNA PCR products, quantified using PicoGreen (Molecular Probes) and a fluorometer TD360 (Turner Biosystems) and corresponding to 5.6 × 105−5.6 × 100 and 2.6 × 105−2.6 × 101 range of tyrosinase or β-actin mRNA molecules/µL, respectively, was included as standard references to transform experimental real-time PCR raw data. For normalization purposes, real-time PCR data were expressed using the comparative CT method (Applied Biosystems) as the number of mRNA molecules of tyrosinase/ng of total RNA and the number of mRNA molecules of tyrosinase/mRNA molecule of β-actin, as recommended (Applied Biosystems; Bustin, 2002).
Results and discussion
We have used in situ hybridization and real-time RT–PCR techniques to examine the expression of the mouse tyrosinase gene. Outbred albino NMRI mice were used for all the analyses. The tyrosinase protein made by the albino allele (Tyrc; Jackson & Bennett, 1990) is not functional and, hence, pigment is not accumulated, avoiding interference with in situ hybridization signals. However, transcription is not affected and tyrosinase mRNA can be detected at wild-type levels (Beermann et al., 1992; Schedl et al., 1993).
First, using a tyrosinase-specific antisense RNA probe, we systematically hybridized whole-mount mouse embryos from early to mid-embryogenesis (+8.5 to +14.5 d.p.c.). We could only detect tyrosinase-positive signals from day +10.5 d.p.c. in the RPE cells (Fig. 1), as reported previously (Beermann et al., 1992). None of these embryos showed any sign of tyrosinase expression in developing brain outside the RPE cells. In particular, no sign of tyrosinase expression was observed in telencephalic vesicles from +10.5 d.p.c. (Fig. 1a), +11.5 d.p.c. (Fig. 1b), +12 d.p.c. (Fig. 1c) or +13.5 d.p.c. (Fig. 1f) where strong lacZ staining had been reported (Tief et al., 1996a; Camacho-Hubner & Beermann, 2001). Hybridization with tyrosinase sense control probes did not show any specific signal (Fig. 1d).
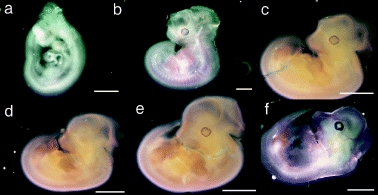
In situ hybridization of whole-mount mouse embryos with tyrosinase RNA probes. Representative whole-mount mouse embryos from (a) +10.5 days postcoitum (d. p. c. ), (b) +11.5 d.p.c., (c) +12 d.p.c., (d and e) +12.5 d.p.c. and (f) +13.5 d.p.c. hybridized with (a–c, e and f) antisense tyrosinase RNA probe or (d) sense control tyrosinase RNA probe. Specific tyrosinase expression (stain) of developing retinal pigment epithelium can be seen in (a–c, e and f) but is absent in (d). Mouse embryo in (d) is shown at a slightly lower magnification compared with age-matched mouse embryo in (e). Scale bars, (a and b), 1 mm; (c–f), 2 mm.
Second, we carried out in situ hybridization of tyrosinase RNA probes on histological sections. The expected tyrosinase-specific labelling of RPE cells (Fig. 2a and c) was found in mouse developing eyes at +13.5 and +14.5 d.p.c. whereas no tyrosinase-specific expression was detected in the telencephalon of +12.5 d.p.c. (Fig. 2e), +13.5 d.p.c. (Fig. 2f) and +14.5 d.p.c. (Fig. 2g) embryos. No specific signals were observed with the control probe (Fig. 2b, d, h–j). Tyrosinase-specific expression was also not detected in the telencephalon of +16.5 d.p.c. mouse embryos (not shown).
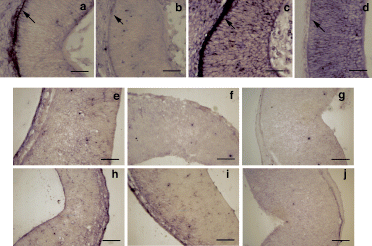
In situ hybridization of developing mouse embryo histological sections with tyrosinase RNA probes. Cryostat sections from (a–d) mouse developing retinae and brain transverse (horizontal) sections showing representative segments of neural tissue corresponding to the (e–j) telencephalic vesicles (future lateral ventricles) from (e and h) +12.5 days postcoitum (d. p. c. ), (a, b, f and i) +13.5 d.p.c. and (c, d, g and j) +14.5 d.p.c. embryos hybridized with (a, c, e, f and g) tyrosinase antisense RNA probe and (b, d, h, i and j) tyrosinase sense control RNA probe. Retinal pigment epithelium cells are indicated with arrows in (a–d). Specific tyrosinase expression (stain) can only be detected in developing retinal pigment epithelium cells (a and c) and is absent in all the other panels. Scale bars, (a–d), 50 µm; (e–j), 150 µm.
Third, we evaluated tyrosinase gene expression using real-time RT–PCR. The presence of tyrosinase transcripts in ectopic body locations had been addressed previously by standard PCR methods (Battyani et al., 1993; Xu et al., 1997; Tief et al., 1996b). However, those approaches were, at best, semiquantitative. In contrast, we decided to run real-time RT–PCR experimental assays using three experimental approaches. First, we quantified the relative tyrosinase gene expression in relation to an endogenous gene (β-actin), using the comparative CT method (Applied Biosystems). Second, we calculated the tyrosinase gene expression applying a reference made up of different amounts of tyrosinase mRNA molecules and third, we considered the number of tyrosinase mRNA molecules in relation to the number of β-actin mRNA molecules. Specific tyrosinase gene expression was measured in RNAs obtained from skin and eyes of +18.5 d.p.c. fetuses, postnatal day 3 pups and adults, whereas very low or undetectable values, similar to those obtained in blank nontemplate control, were measured in ectopic (brain and liver) samples (Fig. 3).
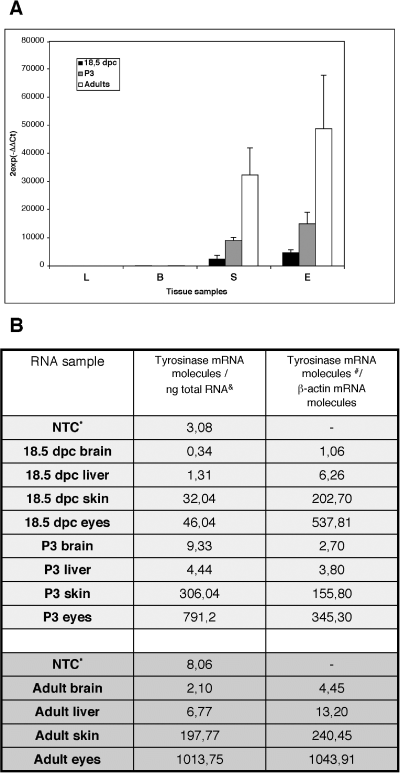
Quantification of tyrosinase gene expression by real-time reverse transcription–polymerase chain reaction in RNA samples from 18.5 days postcoitum (d.p.c.) fetuses, postnatal day 3 (P3) pups and adults. (A) Measurement of tyrosinase gene expression using the comparative CT method () (Applied Biosystems). Bars represent the mean (± SD) from triplicate experiments of relative tyrosinase gene expression values, normalized to a β-actin endogenous reference and relative to a calibrator, in 18.5 d.p.c. fetuses (▪), P3 pups (grey bars) and adults (□) in the following tissue samples: L, liver; B, brain; S, dorsal skin and E, eyes. Tyrosinase gene expression in ectopic tissues (liver or brain) is very low or undetectable. (B) Evaluation of tyrosinase gene expression using the number of tyrosinase mRNA molecules. The table shows three columns corresponding to the RNA samples, the number of tyrosinase mRNA molecules/ng of total RNA and the number of tyrosinase mRNA molecules relative to the number of β-actin mRNA molecules. *NTC, Nontemplate control; &, mean values from duplicate amplification experiments; #, mean values from duplicate amplification experiments and transformed by an arbitrary value (× 105). Observed variation of values was low (∼5–20%) in brain and liver RNA samples and higher (∼10–40%) in skin and eye RNA samples, similar to values obtained in (A). Tyrosinase gene expression in ectopic tissues (such as liver or brain) was very low or undetectable, within the range of the NTC values.
The strong ectopic brain-specific expression of tyrosinase-lacZ transgenes, as previously reported, did not appear in all transgenic mouse lines analysed carrying different tyrosinase promoter fragments (reviewed in Camacho-Hubner & Beermann, 2001). In contrast, the addition of different regulatory sequences derived from the locus control region of the mouse tyrosinase gene (Montoliu et al., 1996) resulted in the loss of lacZ staining in the brain in all lines analysed (Camacho-Hubner & Beermann, 2001). These results prompted the authors to suggest the presence of a repressor sequence within the locus control region that could be responsible for the loss of brain-specific expression of tyrosinase-lacZ transgenes.
Alternatively, it might be argued that the addition of endogenous regulatory sequences, such as the locus control region, restores rather than alters the endogenous-specific tyrosinase gene expression pattern, overcoming the common position effects observed with standard transgenesis (Gimenez et al., 2001; Giraldo & Montoliu, 2001, 2002). Indeed, the use of lacZ reporter transgenes in mice has been often associated with ectopic expression (MacKenzie et al., 1997; Zhao & Overbeek, 1999), variegation (Montoliu et al., 2000; Ramirez et al., 2001), interference or silencing (Cohen-Tannoudji et al., 2000) in adults and during development, due to the prokaryotic nature of lacZ sequences.
The putative existence of tyrosinase-like activity in some areas of the brain has been controversial, considering catecholamine synthesis in tyrosine hydroxylase-deficient mice (Rios et al., 1999) or explaining the presence of neuromelanin (Xu et al., 1997; Ikemoto et al., 1998; Tief et al., 1998; Matsunaga et al., 1999). However, it has not been possible to document tyrosinase-specific activity in brain protein extracts (Tief et al., 1996b).
In summary, we could not find detectable levels of tyrosinase gene expression in developing, perinatal or adult mouse brain, other than RPE cells or skin melanocytes, after evaluation by in situ hybridization and real-time RT–PCR techniques. The lack of endogenous regulatory sequences plus the intrinsic nature of lacZ sequences might have triggered the brain-specific expression of previously reported tyrosinase-lacZ constructs. This unexpected pattern disappeared on the addition of the endogenous tyrosinase locus control region sequences, thereby counteracting the position effects of unprotected transgenes and restoring what appears to be the normal endogenous expression pattern of the mouse tyrosinase gene, as we have documented here.
Acknowledgements
This work was supported by funds from the Spanish Ministry of Science and Technology Bio97-0628, Bio2000-1653, FEDER 2FD1997-2059 and Laboratorios Dr Esteve S.A. to L.M. The authors are grateful to F. Solano, J.C. García-Borrón, M. Torres and G. Jeffery for useful comments and N. Mercader, M. Cantero and P. Cozar for technical assistance.
Abbreviations
-
- d.p.c.
-
- days postcoitum
-
- RPE
-
- retinal pigment epithelium
-
- RT–PCR
-
- reverse transcription–polymerase chain reaction.