Cerebellar contribution to spatial event processing: do spatial procedures contribute to formation of spatial declarative knowledge?
Abstract
Spatial knowledge of an environment involves two distinct competencies: declarative spatial knowledge, linked to where environmental cues are and where the subject is with respect to the cues, and, at the same time, procedural spatial knowledge, linked to how to move into the environment. It has been previously demonstrated that hemicerebellectomized (HCbed) rats are impaired in developing efficient exploration strategies, but not in building spatial maps or in utilizing localizing cues. The aim of the present study was to analyse the relationships between spatial procedural and declarative knowledge by using the open field test. HCbed rats have been tested in two different protocols of the open field task. The results indicate that HCbed animals succeeded in moving inside the arena, in contacting the objects and in habituating to the new environment. However, HCbed animals did not react to environmental changes, when their impaired explorative pattern was inappropriate to the environment, suggesting that they were not able to represent a new environment because they were not able to explore it appropriately. Nevertheless, when their altered procedures were favoured by object arrangement, they detected environmental changes as efficiently as did normal rats. This finding suggests that no declarative spatial learning is possible without appropriate procedural spatial learning.
Introduction
Most recent research on cerebellar physiology has focused on the cognitive role of this subcortical region. Considering the wide reciprocal neuroanatomical projections between cortex and cerebellum, it has been advanced that cerebellar circuits may play a key role in processing information relayed from and to the cortex (Schmahmann, 1996). In particular, one of the most intriguing cognitive cerebellar functions is the capacity to cope with spatial demands (Gandhi et al., 2000; Joyal et al., 2001). It requires close integration between environmental (sensory) information and explorative (motor) acts, thus mimicking the sensorimotor role classically attributed to cerebellar circuits. Recently, it was demonstrated that the cerebellar function in spatial event processing is primarily linked to the acquisition of procedural elements (Petrosini et al., 1996; Leggio et al., 1999; Mandolesi et al., 2001; Rondi-Reig et al., 2002). By using the Morris water maze, we demonstrated that hemicerebellectomized (HCbed) rats display a severe impairment in exploration strategies that are maintained even in the presence of a cerebellar lesion, if they are preoperatively learned. Impaired performances are also displayed by HCbed animals in the radial maze task, demonstrating that cerebellar damage induces an inflexible use of procedures (if indeed any procedure can be acquired in the presence of a cerebellar lesion) and a severe impairment in working memory processes (Mandolesi et al., 2001). Data obtained in mutant mice support the cerebellar involvement in spatial learning (Lalonde, 1987; Caston et al., 1998; Belzung et al., 2001; Lalonde & Strazielle, 2003). A number of studies in mice strains with various types of cerebellar degeneration have shown navigational impairment and deficits in spontaneous alternation and exploration (Lalonde & Botez, 1985; Lalonde et al., 1988, 1989).
The purpose of the present study was to analyse the relationships between spatial procedural and declarative knowledge. The open field was chosen to study this facet of the spatial function. In this task, no explicit reward is present and no stimulus–response association is required. It consists of placing rats in a wide arena containing some objects and, after habituation, examining their reactivity to object displacement (spatial change) or change (object novelty). A response to change indicates that the animal is reacting to the mismatch between the current new situation and the representation of the initial one, built up during previous exploration (Poucet, 1989, 1993; Thinus-Blanc et al., 1992). Thus, a renewal of exploration in response to spatial or object change is a powerful indicator of the ability of animals to form a spatial place competence (Denenberg, 1969; Save et al., 1992). At the same time, the analysis of explorative behaviour provides important insights about progressively developed procedural competence.
General materials and methods
Subjects and experimental groups
The animals used in the present research were adult male Wistar rats (250–300 g). They were housed two animals to a cage and maintained on a standardized dark/light schedule (10 : 14 h), following the guidelines for ethical conduct developed by the American Psychological Association's Committee on Animal Research and Ethics (CARE). Each animal was randomly assigned to one of two experimental groups: Control group (n = 27), comprising intact naïve animals; and HCbed group (n = 29), comprising hemicerebellectomized animals, behaviourally tested when cerebellar symptomatology stabilized. Before testing, both groups of animals were extensively handled and manipulated to reduce possible stress from testing procedure.
Surgery and motor assessment
The rats were anaesthetized with a solution of ketamine and xylazine (ketamine, 90 mg/kg; xylazine, 15 mg/kg), administered intraperitoneally. A craniotomy was performed over the right hemicerebellum. The dura was excised and the right cerebellar hemisphere and hemivermis were ablated by suction; care was taken not to lesion extracerebellar structures. The cavity was filled with sterile gel foam, the wound edges were sutured, and the animals were allowed to recover from anaesthesia and surgical stress. Testing was performed 2 weeks after the hemicerebellectomy (HCb), when no change in cerebellar symptomatology was observed. Animals were behaviourally tested only if they exhibited stable motor symptomatology consistent with a cerebellar lesion. The following postural and motor aspects were taken into account: head and body tilts, position of either hindlimb in relation to trunk, presence of ataxia, tremor, rearing behaviour, falls to lesion side, wide-based locomotion, collapsing on the belly, pivoting, vestibular drop reactions, and the ability to traverse a narrow path and be suspended on a wire. Details and time course of the cerebellar symptoms in the rats have been described elsewhere (Molinari et al., 1990). It is noteworthy that previous reports demonstrated that the akinetic symptoms of HCbed animals are not so severe that they affect their performances.
Apparatus
The apparatus consisted of a circular wooden box (diameter 140 cm) delimited by a wall 30 cm high. The floor was painted pale grey and divided into sectors by black lines. Three concentric 20-cm annuli surrounded a central circle that was 20 cm in diameter. The arena was located in a dimly lit and soundproof cubicle with one door and was bordered by some extramaze cues held in constant spatial relationships throughout the experiment. Some intramaze cues were also provided on the internal wall delimiting the open field (conspicuous stripes, 5 cm wide and 25 cm high). Five objects were simultaneously present in the open field: (A) a metallic bar with a conic red base (10 cm base diameter, 25 cm bar height); (B) a plunger (10 cm base diameter, 10 cm height); (C) a high steel rod (3 cm base diameter, 8 cm height); (D) a yellow rubber plug (5 cm base diameter, 5 cm height); (E) a black cylinder with a plastic glass turned upside down on the top (5 cm base diameter, 10 cm height). Object arrangement changed according to the paradigm. A sixth object was used to examine reactivity to object change. It consisted of a green hemisphere (15 cm base, 10 cm height). At the end of each session, the animal was removed, defaecation boluses were counted, and both the arena and the objects were cleaned with alcohol.
Behavioural parameters
The parameters used to assess the animals' performances allowed us to distinguish different task aspects more closely, even if they were not exclusively linked to emotional, motor, spatial or procedural components.
- 1
Emotional parameters: number of defecation boluses; motionless time.
- 2
Motor parameters: total distance (in m) travelled in the arena (TD); percentage of TD engaged in exploring peripheral sectors (PD); number of rearing responses.
- 3
Spatial parameters: total time spent in contacting objects (a contact was defined as a subject's snout actually touching an object, when the animal was sniffing the object for at least 1 s).
Procedural parameters
In addition to the parameters classically used in open field testing, such as locomotor activities and time spent in contact with the objects, locomotor trajectories were analysed in detail to categorize the exploration strategies put into action. Thus, by considering spatial and temporal distribution of trajectories, path length, percentage of time spent in inner or outer annuli and position of contacted objects, exploration behaviour was classified into five main categories: circling, peripheral locomotor activity without contacting objects; peripheral exploration of two objects, locomotor activity mainly performed in the peripheral annulus contacting 1–2 objects; peripheral exploration of five objects, locomotor activity mainly performed in the peripheral annulus contacting 3–5 objects; central exploration of two objects, locomotor activity performed also in the central area of the arena contacting 1–2 objects; and central exploration of five objects, locomotor activity performed also in the central area of the arena contacting 3–5 objects. The explorative strategies put into action during S2–S9 sessions were then classified according to this categorization and the frequency of single categories was calculated as follows:
[(F × 100)/(N × 8)], where F is the frequency of a single strategy, N the number of groups of animals, 8, the number of sessions with objects.
Histological controls
When the behavioural testing was finished, the HCbed animals were deeply anaesthetized and perfused with saline followed by 10% buffered formalin. The extent of the cerebellar lesion was determined from Nissl-stained 60-µm frozen sections. Animals were included in the present study if they had received a complete right HCb with total ablation of deep nuclei (Fig. 1). In all cases reported here, the left side of the cerebellum and all extracerebellar structures were completely spared, except for the dorsal cap of the right Deiters' nucleus, which was slightly affected in some cases. Variability in the extent of the floccular and vermian lesions was considered not to be of influence because in all cases these structures were functionally disconnected owing to the ablation of the cerebellar peduncles and deep nuclei of the right side.
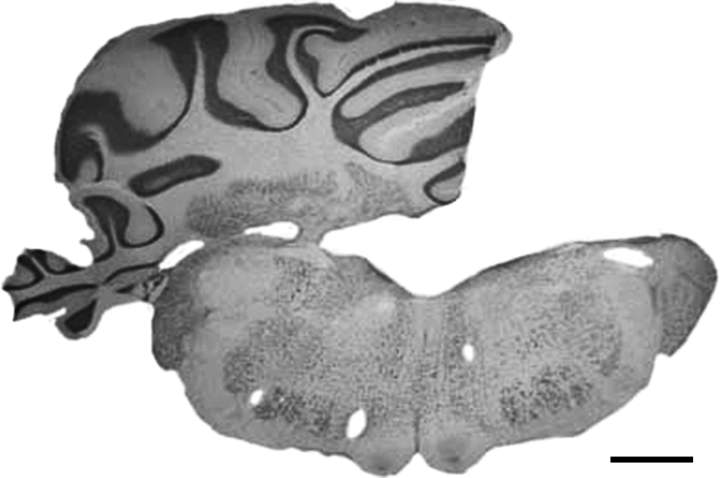
Nissl-stained coronal section through cerebellum and brain stem in a hemicerebellectomized rat. Note the complete absence of the hemisphere and deep nuclei of the right hemicerebellum with complete sparing of surrounding structures. Calibration bar, 1 mm.
Statistical analysis
Metric unit results of animals belonging to the different experimental groups were first tested for homoscedasticity of variance and then compared using analyses of variance (anovas) with repeated measures, followed by multiple comparisons using Duncan's test. P-values <0.05 were considered significant.
Experiment 1
To analyse whether a cerebellar lesion causes a deficit in detecting environmental (spatial or object) changes, in the first experiment the object arrangement classically used in this test was employed. It consists of a square configuration with a central object. No object was placed in an overtly peripheral position. The differences from the classical protocol were shortened session duration (4 min vs. 6 min) and extended intersession intervals (8 min vs. 3 min), counterbalanced by an increased number of sessions (nine vs. seven sessions). These changes were made based on the results of a pilot study indicating that HCbed rats were very fatigued after a 6-min session because of their ataxic and asthenic symptoms (L. Mandolesi, unpublished observation). To prevent these motor impairments from masking possible cognitive defects, the schedule was modified as previously described.
After the habituation phase, experiment 1 was then subdivided into two protocols (1a and 1b). Each protocol took into account which objects were preferentially contacted by each animal in a different way.
Methods (Experiment 1)
Subjects
Useful data were collected from 17 HCbed animals, when cerebellar symptomatology stabilized, and from 18 control animals. Experiment 1 included two different protocols that differed in the objects changed in the spatial change or in the object novelty phases. Nine HCbed animals and eight controls were tested according to protocol 1a, and eight HCbed animals and ten controls were tested according to protocol 1b.
Behavioural testing
In both protocols, the first five sessions were identical. To familiarize the animals with the apparatus, during session 1 (S1) each rat was allowed to move within the empty open field, and the baseline level of activity was measured. During S2–S5, the five objects were placed in a square arrangement with a central object (Fig. 2A). Four objects were placed in the middle annulus of the arena and one was placed in the central area. Habituation in object exploration was recorded. Before S6 (spatial change), the spatial configuration was changed by moving two objects.
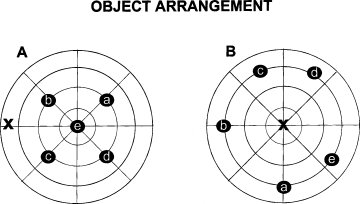
Schematic representations of the apparatus and object configurations in the two experiments. X indicates the starting point. (A) Object arrangement of Experiment 1 in sessions S2–S5; (B) object arrangement of Experiment 2 in sessions S2–S5.
Protocol 1a Object E replaced object B, which was in turn put between objects A and E, so that the initial square arrangement was changed to a polygon-shaped configuration, without any central object. In S7, the object configuration was unchanged to let the rats habituate to the new object placement. In S8 (object novelty), a new object F replaced one of the nondisplaced objects (D) at the same location. In S9, the same object arrangement was maintained.
Protocol 1b The spatial change concerned the two most explored objects. The new spatial arrangement was maintained in S7. In S8 (object novelty), the new object F replaced the third most explored object at the same location. In S9, the same object arrangement was maintained. Obviously, in this protocol the objects that were displaced or substituted were different for each animal. Protocol 1b rewarded biased explorations, allowing us to analyse whether a failure in detecting environmental changes could be due to an explorative defect.
Results (Experiment 1)
In this section, because S1 and the subsequent four habituation sessions were identical in both protocols, the related results will be pooled and described together.
In S1, control and HCbed animals exhibited a comparable level of anxiety. In fact, defaecation boluses did not show significant differences between groups (F1,33 = 0.05). The other open-field parameter indicative of a high-stress state is the ‘freezing’ or motionless periods. Also with regard to this parameter no significant difference between groups was found (F1,33 = 0.14). Of course, high statistical significance was displayed in motor parameters. The HCbed group exhibited a significantly lower number of rears (F1,33 = 64.70, P < 0.0001) (Fig. 3A). With regard to the distance travelled within the arena, HCbed animals exhibited a significantly lower value. A two-way anova revealed significant effects of group (F1,33 = 13.19, P < 0.0001) and session (F8,264 = 24.32, P < 0.0001). Interaction was not significant (F8,264 = 1.2). It is interesting to note that HCbed animals spent a significantly higher percentage of their path in the peripheral annulus (Fig. 3A). A two-way anova revealed significant effects of group (F1,33 = 31.66, P < 0.0001) and session (F8,264 = 15.01, P < 0.0001). Interaction was also significant (F8,264 = 1.92; P < 0.05).
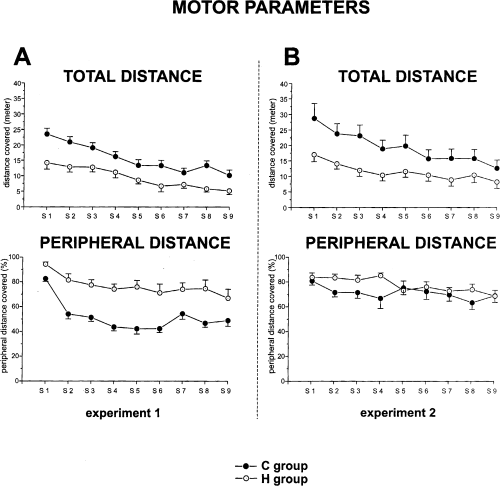
Total distance (calculated in metres) and peripheral distance (calculated as the percentage of total distance engaged in exploring peripheral sectors of the arena) travelled by control and HCbed rats in Experiments 1 and 2. In this and in the following figures vertical bars indicate SEM.
Figure 4 shows the mean time of contacts with objects in S2–S5 in the two experimental groups. Both groups of animals showed habituation (one-way anovas – controls: F3,51 = 8.73, P < 0.0001; HCbed animals: F3,48 = 5.58, P < 0.01). A two-way anova (group × session) revealed significant effects of group (F1,33 = 28.11, P < 0.0001) and session (F3,99 = 11.03, P < 0.0001). Interaction was also significant (F3,99 = 4.81, P < 0.01).
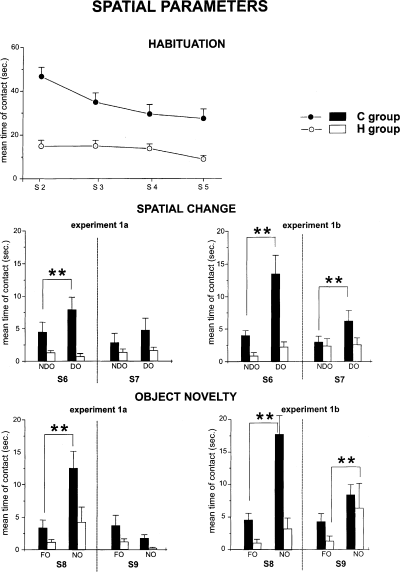
Habituation (upper), reactivity to spatial change (middle) and reactivity to object novelty (lower) in the two protocols (1a and 1b) of Experiment 1. In the upper part of the figure, the mean duration of contact with the five objects during the four habituation sessions is represented. The histograms represent the mean time spent in contacting the displaced (DO) or the nondisplaced (NDO) objects in S6 and S7, or the mean time spent contacting the new (NO) or the familiar (FO) objects in S8 and S9. **P < 0.005 DO vs. NDO and NO vs. FO.
Protocol 1a
In S6, control rats clearly explored displaced objects more than nondisplaced objects. This difference in time of contact with the two categories of objects was reduced in S7. It is interesting to note that HCbed animals behaved as if no spatial displacement had occurred. In S6 and S7, they showed no significant difference between object categories. A three-way anova (group × session × object) revealed significant effects of group (F1,15 = 7.07, P < 0.05) and category of object (F1,15 = 4.81, P < 0.05). Session effect was not significant (F1,15 = 4.37). Interaction among factors was not significant (F1,15 = 1.75). Post-hoc analysis revealed a significant difference between displaced and nondisplaced objects only in control animals.
As shown in Fig. 4, a similar explorative pattern was also observed in object novelty detection. In S8, control animals reacted markedly to object substitution, contacting the novel object significantly more. Conversely, although the HCbed animals reacted to the novel object, their reaction did not reach significance. A three-way anova (group × session × object) revealed significant effects of group (F1,15 = 5.39, P < 0.05), session (F1,15 = 21.74, P < 0.001), category of object (F1,15 = 21.40, P < 0.001). Interaction among factors was not significant (F1,15 = 3.93). Post-hoc analysis revealed a significant difference between new and familiar objects in control but not in HCbed rats.
Protocol 1b
In S6, control rats contacted displaced objects much more than nondisplaced objects (Fig. 4). This difference in time spent in contacting the two categories of objects was reduced, but maintained, in S7. Again, HCbed animals behaved as if no object displacement had occurred. In S6 and S7, they showed no significant difference between object categories. A three-way anova (group × session × object) revealed significant effects of group (F1,16 = 7.87, P < 0.05), session (F1,16 = 6.69, P < 0.05) and category of object (F1,16 = 13.98, P < 0.01). A post-hoc analysis revealed a significant difference between displaced vs. nondisplaced objects only in control animals.
In S8, control animals reacted markedly to object substitution, contacting the novel object significantly more (Fig. 4). It is interesting to note that HCbed animals did not reach the significance level in contacting the new object in S8, but explored the novel object significantly more than the familiar ones in S9. A three-way anova (group × session × object) revealed significant effects of group (F1,16 = 7.51, P < 0.05) and category of object (F1,16 = 30.98, P < 0.0001). Session effect was not significant (F1,16 = 2.55). Interaction among factors was significant (F1,16 = 8.80, P < 0.01). A post-hoc analysis revealed a significant difference between new vs. familiar objects in both control and HCbed rats.
Pooling data obtained with both protocols, we analysed the procedural parameters of the two experimental groups (Fig. 5). The control animals mainly put into action an explorative strategy that traversed more than once the centre of the arena, to contact all objects (CE 5 Ob), regardless of their spatial localization. Conversely, HCbed animals mainly performed strategies linked to the exploration of the peripheral portions of the arena (C, PE 2 Ob, PE 5 Ob), and the central object was often completely neglected.
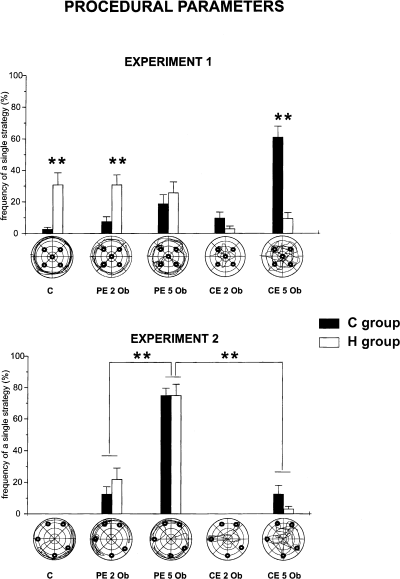
Mean percentages of the five spatial strategies by the experimental groups throughout the S2–S9 sessions of the open field test in Experiments 1 and 2. Under the abscissas the typical explorative patterns of the five categories are represented. C, circling, peripheral locomotor activity without contacting objects; PE 2 Ob, peripheral exploration and contact of two objects; PE 5 Ob, peripheral exploration and contact of five objects; CE 2 Ob, central exploration and contact of two objects; CE 5 Ob, central exploration and contact of five objects.
Discussion (Experiment 1)
In the presence of a cerebellar lesion, many postural and motor deficits are inescapably present. In spite of this, the HCbed animals succeeded in moving inside the arena and in contacting the objects. They habituated to the new environment as with the control animals. This observation is in agreement with data reported by Dahhaoui et al. (1992) in cerebellectomized rats, and by Caston et al. (1998) in intact or cerebellectomized Lurcher mutant mice, suggesting that the cerebellum is not involved in the habituation of exploration behaviour. The explorative pattern displayed by HCbed animals was markedly different from that of the controls. The HCbed rats persisted in exploring the outer annulus, even in the last session of the habituation phase, very rarely visiting the inner areas (Fig. 3A). They made few contacts, especially with the object in the centre of the arena. In sessions 6 and 8, cerebellar animals exhibited significantly decreased ability to react to a new arrangement (Fig. 4), whereas the control rats reacted to changes and spent more time contacting the displaced or new objects. This reduced amount of activity could be an indication of increased level of anxiety of HCbed animals. To test this hypothesis, defaecation and freezing were used. Defaecation, the parameter for which the open field was designed, is one, if not the most widely used, measure together with the prime index of emotionality, the validity of its role having been confirmed by factor analytic studies (Whimbey & Denenberg, 1967). The other parameter usually taken as indicative of a high-stress state is the absence of movement or freezing, a behavioural response occurring in the face of perceived danger. Elements such as spatial dislocation or abrupt change are reported to be particularly potent eliciting factors (Walsh & Cummins, 1976). However, both indexes failed to reveal any difference between HCbed and control animals. It is interesting to note also that nervous mutant mice that display a loss of Purkinje cells do not demonstrate freezing responses (Lalonde & Botez, 1985). Thus, the emotional reactivity seems to be not markedly altered by the cerebellar lesion, at least under some experimental conditions, in which, for example, before testing cerebellar as well as control animals were repeatedly handled to reduce fear reactions and timidity.
When an animal explored only some objects and neglected the others, it is reasonable to expect a reaction to changes in the former objects and not in the latter ones. To overcome the eventuality of bias in object exploration, we planned protocol 1b, in which only the most contacted objects were changed. In this protocol, the cerebellar animals never recognized spatial changes in S6 and S7 and succeeded in detecting object novelty only in S9. Behaviours characterized by prevalently peripheral exploration and by reduced (if any) reaction to environmental changes can be provoked by different factors. Theoretically, this poorly reactive spatial behaviour can result from a high level of anxiety, a low motivation to explore, or from an impairment in building the spatial place competence of the environment, perhaps linked to a procedural impairment in putting efficient explorative strategies into action. As mentioned above, in the open field task HCbed animals did not appear very anxious, considering either specific parameters, such as defaecation and freezing, or their general behaviour in the arena. The second factor inevitably affecting exploration behaviour is a motivational factor. Recently, it has been reported that Lurcher mutant mice, whose cerebellar cortex is lacking almost all Purkinje and granule cells, exhibited a low level of exploration in a hole-board matrix (Caston et al., 1998). Furthermore, nervous mutants exhibit deficits in spontaneous alternation rates (Lalonde & Botez, 1985). These deficits were interpreted as due both to a decreased motivation to explore a new environment and to spatial deficits. In fact, in addition to its role in cognitive functions (Schmahmann, 1997), such as executive function, memory, visuospatial analysis and language, it has been suggested that the cerebellum is also implicated in motivational states determining the motivational disorders associated with neurodevelopmental pathologies, such as autism (Pierce & Courchesne, 2001). Although it cannot be ruled out that some motivational factors contributed to affect the exploration of HCbed animals in the open field task, some consideration of the features of protocol 1b strengthen the hypothesis of a spatial deficit in the presence of a cerebellar lesion. In fact, protocol 1b, in which just the most contacted objects were changed, emphasizes even low levels of motivation, allowing reducing the motivational load in the explorative function. If a subject contacts only two objects, it is certainly possible that its reduced attitude to explore is provoked by low levels of motivation. Regardless, however low it may be, a motivational drive high enough to explore those particular objects is present. The same motivation should operate when just these objects are changed.
The third factor theoretically involved in the difficulty displayed by HCbed animals in reacting to environmental changes is the impairment in forming the declarative knowledge of the environment, suggesting that cerebellar networks could be involved in processes of forming and updating spatial relationships among environmental stimuli. However, before attributing this role to cerebellar circuits, the role played by procedural competencies in acquiring the spatial declarative competence must be analysed, because of the already demonstrated cerebellar function in gaining procedural competence. As repeatedly observed (Petrosini et al., 1996, 1998; Leggio et al., 1999), HCbed animals are severely impaired in acquiring the appropriate spatial explorative procedures (Fig. 5). Indeed, it is possible that the subject cannot acquire a declarative competence in the presence of a cerebellar lesion, because the cerebellar networks are not only involved in procedural learning but also in the representational phase of a new environment (neither declarative nor procedural learning). Conversely, it is possible that a subject that is not able to explore a new environment appropriately, in the presence of a cerebellar lesion, will not be able to represent it either (no declarative learning as a result of no procedural learning). To discriminate between hypotheses, the environment was modified according to the impaired spatial procedures of cerebellar animals.
Experiment 2
The change introduced in this experiment was object localization. All five objects were placed on the periphery. Particular care was taken to choose a large enough distance that the animals thigmotaxically linked to or leaning against the arena walls would not accidentally bump into objects and, by contrast, that it was sufficiently far from arena walls to require active exploration. However, this distance did not demand any exploration of the inner areas of the arena, thus fostering the biased explorative strategies of cerebellar animals. If the new arrangement allowed animals to detect the environmental changes, spatial procedures are evidently capable of influencing the representational phase linked to building of the declarative knowledge of the environment. By contrast, if cerebellar animals failed again to detect environmental changes, this shows that not only are the procedural components of spatial learning impaired by a cerebellar lesion but so also are the place representations.
Methods (Experiment 2)
Subjects
Data were collected from 12 HCbed animals, when cerebellar symptomatology stabilized, and from nine control animals.
Behavioural testing
Rats were tested with the same apparatus and with the same procedures described in Experiment 1. The protocol was modified by locating all objects 15 cm from arena walls (Fig. 2B)
Results (Experiment 2)
As in experiment 1, in S1, control and HCbed animals exhibited a comparable level of anxiety, as indicated by no significant difference in defaecation boluses (F1,19 = 1.22) or in motionless periods (F1,19 = 0.14). Of course, high statistical significance was obtained for motor parameters. Groups of animals significantly differed in rearing behaviours (F1,19 = 38.5, P < 0.0001), because HCbed animals exhibited fewer rears than controls. HCbed animals displayed a lower value for distance travelled in the arena (Fig. 3B). A two-way anova revealed a significant effect of group (F1,19 = 5.91, P < 0.05) and session (F8,152 = 17.5, P < 0.0001). Interaction was also significant (F8,152 = 2.05, P < 0.05). It is interesting to note that with the new spatial arrangement both experimental groups spent the highest percentage of their path in the peripheral annulus. A two-way anova revealed a significant effect only for session (F8,152 = 2.57, P < 0.05). The effects of group (F1,19 = 2.00) and interaction (F8,152 = 1.45) were not significant, demonstrating that the exploration of the cerebellar animals in the arena was similar to that of controls. As shown in Fig. 6, which depicts the mean time of contacts in S2–S5 with all five objects, both groups of animals showed habituation (one-way anovas – control group: F3,24 = 19.02, P < 0.0001; HCbed animals: F3,33 = 27.66, P < 0.0001). A two-way anova revealed no significant group effect (F1,19 = 3.25), whereas session effect (F3,57 = 43.60, P < 0.0001) and interaction (F3,57 = 3.82, P < 0.05) were significant. In S6, both control and HCbed rats contacted displaced objects significantly more than nondisplaced ones. This difference in contacting the two object categories was maintained in S7, although it was reduced. A three-way anova (group × session × object) revealed no significant effect of group (F1,19 = 4.37) and session (F1,19 = 0.02). Category of object displayed a significant effect (F1,19 = 22.48, P < 0.0001). Interactions between and among factors were not significant. Post-hoc analysis revealed significant differences between displaced vs. nondisplaced objects in control and HCbed animals
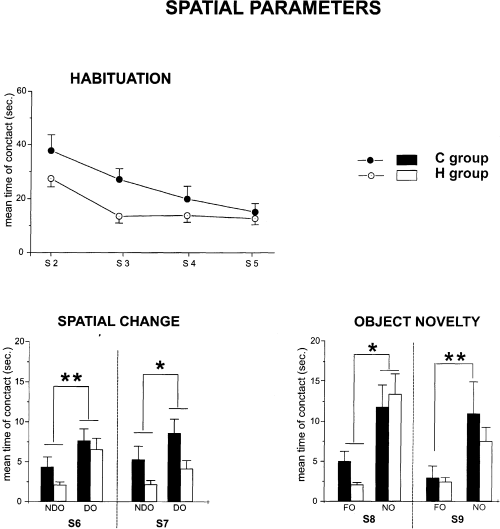
Habituation (upper), reactivity to spatial change (middle) and reactivity to object novelty (lower) in Experiment 2. In the upper part of the figure, the mean duration of contact with the five objects during the four habituation sessions is represented. The histograms represent the mean time spent in contacting the displaced (DO) or the nondisplaced (NDO) objects in S6 and S7, or the mean time spent contacting the new (NO) or the familiar (FO) objects in S8 and S9. *P < 0.05 and **P < 0.001 DO vs. NDO and NO vs. FO.
A similar pattern was also observed in object novelty detection (Fig. 6). In S8, both control and HCbed animals reacted to object substitution, contacting the novel object significantly more often. This pattern was maintained in S9. A three-way anova (group × session × object) revealed no significant effect of group (F1,19 = 0.48) and session (F1,19 = 3.7). Category of object displayed a highly significant effect (F1,19 = 31.7, P < 0.0001). Interactions between and among factors were not significant. Post-hoc analysis revealed significant differences between new vs. familiar objects in control and HCbed rats.
When procedural parameters were analysed, a marked analogy in the explorative patterns of the two experimental groups was noted (Fig. 5). Both control and HCbed animals mainly chose the explorative strategy that contacted all objects through peripheral trajectories, and avoiding ‘expensive’ and unmotivated passing through the centre of the arena.
Discussion (Experiment 2)
The findings of Experiment 2 indicate that HCbed animals were able to detect environmental changes once their altered procedures were favoured by object arrangement. Cerebellar animals reacted to both spatial and object changes, just as did controls. This finding strongly suggests that motivational factors, even if present, are not the main cause of the impaired exploration displayed in the Experiment 1. It is interesting to note that in this new spatial arrangement even their explorative strategies were identical. This indicates that a subject is not able to represent a new environment when it is not able to explore it appropriately, as in the case of a cerebellar lesion. This suggests that no declarative learning is possible without the appropriate procedural learning.
These data extend earlier findings on the involvement of cerebellar circuits in procedural spatial learning (Petrosini et al., 1996, 1998; Leggio et al., 1999; Mandolesi et al., 2001), showing that the spatial procedural impairment resulting from a cerebellar lesion conditions the processes of forming and updating spatial relationships among environmental stimuli. Altered explorative strategies make the acquisition of the declarative knowledge of the environment extremely demanding. This impairment is so marked that no detection of environmental changes was evidenced in Experiment 1, in which no attempt was made to foster the procedural explorative behaviour of HCbed rats. Conversely, when the environment was shaped according to the explorative abilities of the cerebellar animals, and thus their procedural deficits were bypassed, as in Experiment 2, cerebellar animals were able to react to environmental changes. Once they were allowed to explore a new environment keeping to their own strategies, no significant difference between experimental groups was found regarding reaction to change or procedural components put into action.
This finding indicates that the acquisition of the declarative competence of an environment does not require cerebellar involvement per se. Cerebellar mediation is fundamental in acquiring the procedural competence that appears to be a prerequisite of any localizatory knowledge. In fact, to understand an environment fully, in addition to learning to locate specific cues (localizatory learning), it is necessary to learn how to move in that environment (procedural learning). Procedural components include behaviours such as suppression of fear in an open space, leaving peripheral areas, development of efficient explorative strategies, and acquisition and utilization of snapshots of the target view and of the representation-forming procedures (Fenton & Bures, 1993). The localizatory and procedural aspects of spatial learning are widely inter-related, even if multiple and differentiated brain areas contribute to them. The most influential theory on spatial learning suggests that hippocampal areas have a selective role in high-order components, such as place learning (O'Keefe & Nadel, 1978; Jarrard, 1993; O'Keefe, 1993; Moser et al., 1995; Stupien et al., 2003) or processes underlying the establishment of relational representations (Sutherland & Rudy, 1989; Eichenbaum et al., 1994). By contrast, hippocampal-independent mechanisms, suggested to be involved in those forms of learning related to procedural components, bring brain structures into play that are primarily involved in sensorimotor functions, such as the cerebellum (Thompson & Kim, 1996; Guillou et al., 1999). These competencies represent the environment in a different manner. Through localizatory knowledge, the environment is described independently from action (for example, cue X is near cue Y) (Schenk & Morris, 1985). Conversely, through procedural knowledge, spatial competence is built into movements performed within the environment. In this case, the building of the spatial map may be defined within a distinct reference frame, defined by self-motion (for example, cue X is three steps forward from here). Thus, the contribution of spatial procedures to the mental representation of an environment is not location-based, but route-based. According to the present results, procedural aspects are necessary appropriately to organize spatial information and to represent an environment. It is usually held that mammals organize complex spatial information into coherent reference frames that are used to guide behaviour efficiently.
The present data indicate that efficient behaviour is used to guide the building of the internal representation of the environment.
Acknowledgements
This research was supported by MIUR grants to L.P.
Abbreviation
-
- HCbed
-
- hemicerebellectomized.