Catecholamine-independent transient expression of tyrosine hydroxylase in primary auditory neurons is coincident with the onset of hearing in the rat cochlea
Abstract
During the last stages of neuronal maturation, tyrosine hydroxylase is transiently expressed in the absence of the other catecholamine-synthesizing enzymes. We show here that it is expressed in rat spiral ganglion neurons between postnatal days 8 and 20, with a peak of expression at postnatal day 12. These tyrosine hydroxylase-immunoreactive neurons did not display aromatic amino acid decarboxylase- or dopamine-β-hydroxylase-immunoreactivities, ruling out the possibilities of dopamine or noradrenaline synthesis. They also did not display peripherin- or intense neurofilament 200-kDa-immunoreactivities, two indicators of type II primary auditory neurons. Tyrosine hydroxylase-immunoreactive dendrites were seen in synaptic contact with the inner hair cells and expressed the GluR2 subunit of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors, further confirming the type I nature of the neurons transiently expressing the enzyme. The end of the tyrosine hydroxylase expression was not due to cell death because the immunoreactive neurons did not show TUNEL-labelled nuclei. Finally, all the type I neurons expressed the tyrosine hydroxylase mRNA at postnatal day 12, suggesting that the expression of the enzyme is a maturational step common to all these neurons and that the expression of the protein is not synchronized. Because the period of transient expression of tyrosine hydroxylase in type I neurons parallels the periods of maturation of evoked exocytosis in inner hair cells and of appearance and maturation of the cochlear potentials, we propose that the expression of the enzyme indicates the onset of hearing in individual type I primary auditory neurons. This enzyme expression could rely on a Ca2+ activation of its encoding gene subsequent to a sudden and massive Ca2+ entry through voltage-activated Ca2+ channels.
Introduction
In the last two decades, numerous reports have described a neuronal expression of tyrosine hydroxylase [TH: tyrosine 3-monooxygenase, l-tyrosine, tetrahydropteridine: oxygen oxidoreductase (3-hydroxylating), EC 1.14.16.2], the rate-limiting enzyme in the catecholamine (CA) biosynthesis pathway (Nagatsu et al., 1964; Levitt et al., 1965), in the absence of the other CA-synthesizing enzymes (see Jaeger & Joh, 1983; Berger et al., 1985; Nagatsu et al., 1990, 1996b; Battaglia et al., 1995; Marsais & Calas, 1999; Ershov et al., 2002, and references herein).
It is tempting to assume that these neurons synthesize l-dihydroxyphenylalanine (L-DOPA) because neurons synthesizing L-DOPA as an end-product have been described (Okamura et al., 1988; Kitahama et al., 1988, 1990; Mons et al., 1991; Karasawa et al., 1992). Indeed, L-DOPA has been proposed to play neuromodulator and neurotransmitter roles in several nervous regions (see Misu et al., 1996). By contrast, TH-immunoreactive (TH-ir) neurons lacking the other CA-synthesizing enzymes have been shown to lack GTP-cyclohydroxylase I, which synthesizes the necessary TH co-factor tetrahydrobiopterin (Fujii et al., 1994; Nagatsu et al., 1996a; Marsais & Calas, 1999), thus precluding any L-DOPA synthesis. In agreement, TH-ir neurons not expressing L-DOPA have been described (Kummer et al., 1990; Kitahama et al., 1990; Mons et al., 1991).
The function of this TH expression in the absence of the other CA-synthesizing enzymes essentially remains a matter of hypothesis (Schussler et al., 1995; Ugrumov et al., 2002). It appears to be related to stimuli-induced cellular activation (Asmus & Newman, 1994; Marsais & Calas, 1999; Bezin et al., 2000; Abramova et al., 2002; Marsais et al., 2002) and is most often found transiently expressed during the last stages of maturation of the central nervous system. This period of transient expression of TH roughly corresponds to the first month of the postnatal maturation in rodents: rats (Jaeger & Joh, 1983; Berger et al., 1985; Battaglia et al., 1995), mice (Satoh & Suzuki, 1990; Nagatsu et al., 1990, 1996b) and hamsters (Vincent & Hope, 1990), and it seems to be a general step in the nervous system maturation as it has also been described in monkeys and humans (Gaspar et al., 1987; Komori et al., 1991; Fujii et al., 1994; Ikemoto et al., 1998, 1999; Kitahama et al., 1998).
Indeed, its period of expression in the central auditory system, during the first postnatal weeks (Jaeger & Joh, 1983; Harper & Wallace, 1995; Nagatsu et al., 1996b), corresponds to the period of structural and physiological maturation of the system (Cant, 1998; Sanes & Walsh, 1998). We now show here that TH is also transiently expressed, in the absence of the other CA-synthesizing enzyme, in the rat primary auditory neurons during the first postnatal weeks, when the final steps of the synaptic maturation of hair cells (Lenoir et al., 1980; Pujol et al., 1980) and the onset of hearing (Uziel et al., 1981; Puel & Uziel, 1987) occurs. We further propose that this transient TH expression indicates the reception of the first stimuli transduced and transmitted by the sensory inner hair cells to the type I primary auditory neurons.
Materials and methods
SDS-PAGE and Western blot analysis
Under deep sodium pentobarbital anesthesia, the otic capsules of ten postnatal day (PND) 12 rats were removed. The cochleas were then dissected from the capsule in ice-cold phosphate-buffered saline (PBS). The stria vascularis and the spiral ligament were carefully removed. The cochleas were then pooled and homogenized in 1% sodium dodecyl sulphate in 0.5 m Tris glycine buffer, pH 6.8. The proteins were electrophoresed on 12% acrylamide gels according to Laemmli (1970). The proteins were then transferred onto nitrocellulose membranes (Whatman, Maidstone, UK) and processed for TH detection using the Roche Molecular Diagnostics (Manheim, Germany) chemiluminescence kit according to the manufacturer's instructions. Briefly, the membranes were blocked with 1% blocking solution of the kit in Tris-buffered saline (TBS), then incubated for 1 h with the anti-TH antibodies diluted 1 : 7500 in the TBS containing 0.5% blocking solution. They were rinsed in TBS containing 0.1% Tween 20 (TBST) and incubated for 30 min in horseradish peroxidase (HRP)-conjugated anti-rabbit IgGs in the TBS containing 0.5% blocking solution. Finally, the membranes were rinsed in TBST, incubated for 1 min in luminol and exposed to X-OMAT-AR film (Kodak, Rochester, NY, USA). Prestained molecular weight standards from Gibco Life Technologies (Gaithersburg, MD, USA) were phosphorylase b (97 kDa), bovine serum albumin (62 kDa), ovalbumin (42 kDa), carbonic anhydrase (31 kDa), soybean trypsin inhibitor (21 kDa) and lysozyme (14 kDa).
Light and electron microscopic immunocytochemistry
Fixation and tissue processing
Both adult and postnataly developing rats were used for this study. The maturing rats were aged 0 (day of birth), 4, 8, 12, 16 and 20 PND and the adult rats 30 and 45 PND. Under a deep sodium pentobarbital anaesthesia, the rats were intra-aortically fixed with a perfusion of 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4. The cochleas were then dissected, post-fixed overnight in the same fixative, then rinsed several times in the phosphate buffer. During these rinses, the already ossified otic capsule and the stria vascularis of the cochleas from the oldest animals (from PND 8 onwards) were carefully removed. The tissues were then transferred to a phosphate buffer bath containing 20% sucrose for cryoprotection. Fourteen-micrometre-thick sections were cut with a Reichert-Jung 2800 cryostat microtome and stored at −20 °C until use.
Immunofluorescence
Our single and double immunofluorescence procedures were adapted from those already used in the laboratory (Eybalin et al., 2002). Briefly, after three 5-min rinses of the sections in PBS and a 1-h pre-incubation in 30% normal goat serum and 0.3% Triton X100, the sections were incubated overnight at 4 °C with the primary antibodies diluted in PBS containing 1% normal goat serum. They were then rinsed in PBS (3 × 8 min) and incubated for 2 h in goat anti-rabbit IgGs conjugated to Alexa 568 (Molecular Probes, Eugene, OR, USA) and goat anti-mouse IgGs conjugated to Alexa 488 (Molecular Probes) diluted 1 : 2000 in PBS. The sections were then rinsed in PBS (3 × 8 min) and mounted in Mowiol. They were first observed with a Leica DMRB microscope with an epifluorescence equipment. Selected sections were then observed with a Bio-Rad MRC1024 laser scanning confocal microscope equipped with a 15-mW krypton/argon laser. The fluorescence emissions were collected sequentially using the 488-nm and 568-nm lines for Alexa 488 and Alexa 568, respectively, in order to minimize bleed-through between the emitted fluorescences.
The rabbit anti-TH antibodies were purchased from Chemicon, Eugene Tech Inc. (Ramsey, NJ, USA) and Institut Jacques Boy (Reims, France). The Eugene Tech antibody was raised against bovine TH and the one from the Institut Jacques Boy against human TH. They were all used diluted 1 : 1000–1 : 3000. Both have previously been successfully used in cochlear immunocytochemistry (Jones et al., 1987; Eybalin et al., 1993).
To ascertain the catecholaminergic nature of the transiently TH-immunoreactive cells, we performed double labelling experiments coupling the TH antibodies to antibody against two other enzymes of the CA synthesis, aromatic amino acid decarboxylase (AADC, Sigma, Saint Louis, MO, USA, 1 : 1000) and dopamine-β-hydoxylase (DBH, 1 : 1000, Chemicon, Temecula, CA, USA). According to its data sheet, the AADC antibody only recognizes cell bodies and dendrites, but not axons. Sections from the brain were thus used as positive controls. To indentify which class of primary auditory neurons, type I or type II, expressed TH, we co-localized the enzyme with neurofilament 200 kDa (Chemicon, 1 : 4000) and peripherin (Chemicon, 1 : 500), two reliable markers of type II primary auditory neurons (Berglund & Ryugo, 1986, 1991; Dau & Wenthold, 1989; Hafidi et al., 1993; Despres et al., 1994; Hafidi, 1998). Finally, because inner hair cells are glutamatergic (Eybalin, 1993), we co-localized TH with the GluR2 subunit of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors (Chemicon, 1 : 1000).
Negative controls were carried out by omission of the primary antibodies in the first incubation step, or by their substitution with rabbit or mouse IgGs. No fluorescence could be observed under these conditions in the spiral ganglion and the organ of Corti. However, up to PND 12–16, non-sensory non-neural regions of the cochleas such as the spiral limbus or the spiral ligament displayed an unspecific fluorescence. In the double immunofluorescence labellings, we further assayed the species specificity of the secondary antibodies. Sections were incubated with only one primary antibody, then with both anti-rabbit and anti-mouse secondary antibodies. In all cases, only one fluorescence was observed revealing the species of the first antibody.
Semi-quantitative analysis of TH-positive neurons in the spiral ganglion at different postnatal ages
Eight animals per each age group, PND0, PND4, PND8, PND12, PND16 and PND20 were killed by intra-aortic perfusion and the cochleas dissected and processed for cryostat sectioning.
The sections used for counting TH-positive neurons in the spiral ganglion, were processed using an avidin–biotin–peroxidase technique (Vectastain Elite kit; Vector Laboratories, Burlingame, CA, USA) according to the manufacturer's instructions. The immunocytochemical procedures were the same as those used for immunofluorescence, with the exceptions that blocking of eventual endogenous biotin, biotin receptors and avidin-binding sites was performed using the Vector avidin–biotin blocking kit and that the 3–3′-diaminobenzidine (DAB) end-product was re-inforced using a DAB-enhancing solution (Vector).
The total number of auditory neurons and the total number of TH-ir cells were counted in each animal in two different sections of the middle coil spiral ganglion using a SAMBA 2000 device (SAMBA Technologies, Meylan, France). The sections were spaced by more than 100 µm in order to avoid counting the same cell twice. The data obtained were then statistically processed using a two-way analysis of variance.
Electron microscopy immunocytochemistry
Rats aged 8, 12 and 14 PND were intracardially perfused under deep sodium pentobarbital anaesthesia with 4% paraformaldehyde−0.2% glutaraldehyde in 0.1 m phosphate buffer, pH 7.4. After an overnight post-fixation in 4% paraformaldehyde at 4 °C, the cochleas were rinsed several times in the phosphate buffer and dissected from the otic capsule.
The pre-embedding immunocytochemical procedure was similar to that we have previously used (Eybalin et al., 1993). Briefly, after an in toto incubation with the anti-TH antibody diluted 1 : 3000 in PBS−1% normal goat serum (24 h, 4 °C), the cochleas were incubated for 1 h with HRP-conjugated goat Fab fragments against anti-rabbit IgGs (ABCYS, Paris, France) diluted 1 : 100 in PBS. After the cytochemical detection of HRP with DAB (Sigma), the cochleas were osmicated and processed for Epon embedding. Pale gold thin sections were taken on a Reichert Ultracut ultramicrotome using a diamond knife and observed uncontrasted at 50 kV on an Hitachi H7100 electron microscope.
Here also, the negative controls were carried out by omission of the primary antibodies in the first incubation step, or by their substitution with rabbit IgGs. No DAB deposit could be observed in these conditions.
Combined TUNEL and immunocytochemistry of tyrosine hydroxylase
Sections from PND8, 12 and 16 rat cochleas were first processed for the detection of apoptotic nuclei according to the FITC-ApopTag kit instructions (Serologicals Inc., Gaithersburg, MD, USA). Briefly, the sections were rinsed twice for 5 min in PBS and pre-incubated for 5 min in the equilibration buffer provided with the kit. They were then incubated for 1 h at 37 °C with terminal deoxynucleotidyl transferase, which catalysed the 3′ addition of digoxigenin-11-dUTP to the DNA. The reaction was then stopped by placing the sections for 30 min at 37 °C in the stop/wash buffer of the kit. The sections were given three 5-min rinses in PBS at room temperature and incubated for 30 min in FITC-conjugated anti-digoxygenin F(ab)2′ diluted in the blocking solution and rinsed three times each for 5 min in PBS. After the completion of the TUNEL procedure, the sections were processed for TH immunofluorescence according to the above described procedures. The secondary goat anti-rabbit IgGs was conjugated to Cy3 (Jackson Immunoresearch, West Grove, PA, USA, 1 : 1000). After the end of the immunofluorescence procedure, the sections were mounted in Mowiol and observed on the 1024 confocal microscope using the 488-nm and 568-nm excitation lines.
In situ hybridization
Six PND12 male rats were anaesthetized and perfused as for immunocytochemical studies. The cochleas were dissected and post-fixed in the same fixative. The cochleas were then rinsed several times in PBS containing 0.1% diethylpyrocarbonate. During these rinses, the otic capsule and the stria vascularis were carefully removed. The cochleas were then transferred to an RNAse-free phosphate buffer bath containing 20% sucrose for cryoprotection. Sections 14 µm thick were cut, parallel to the modiolus plane, with a Reichert-Jung 2800 cryostat microtome and stored at −80 °C until use.
The sections were treated with protease K (1 µg/mL; Sigma) for 10 min and rinsed twice in PBS for a total of 10 min at room temperature. The hybridization buffer consisted of 50% deionized formamide (Sigma), 4× sodium chloride/sodium citrate (SSC), 1× Denhardt's solution (Sigma) and 10% dextran sulphate (Sigma) in 0.1% diethylpyrocarbonate-treated H2O. The hybridizations were carried out overnight at 40 °C with 3′-tailed digoxigenin (DIG) oligoprobe (Eurogentec, Liege, Belgium) at 100 ng/mL. The antisense oligoprobe had the following sequence 5′-TCTGGGTCAAAGGCTCGGACCTCAGGCTCCTCTGAC-3′, complementary of nucleotides 1223–1258 of the rat nucleotide sequence (GenBank accession number: NM012740). After the hybridization, the non-specifically bound probe was removed in 4× SSC (15 min), followed by two rinses for 30 min in 0.1× SSC at 40 °C and 5 min in the washing solution (Roche Diagnostics). To block non-specific staining, the sections were immersed in a blocking solution (1 h, Roche Diagnostics). The probe was detected with a sheep anti-DIG antibody conjugated to alkaline phosphatase (Digoxigenin Detection Kit; Roche Diagnostics) diluted 1 : 500 in the blocking solution. The sections were then rinsed (1 h) in the washing solution and immersed (5 min) in the detection solution (Roche Diagnostics). After an overnight incubation with NBT/BCIP (Roche Diagnostics) in the dark at room temperature, the sections were briefly washed in PBS, dehydrated, cleared in xylene and mounted with Permount.
The negative controls were carried out by hybridizing the sections with a DIG-labelled sense probe or with an unlabelled antisense probe. None of these procedures resulted in a specific signal.
Results
Expression of tyrosine hydroxylase in the postnatally maturing spiral ganglion
The Western blot analysis of the rat PND12 cochleas showed a single immunoreactive band in the molecular weight range of ∼56 kDa (Fig. 1).

Immunoblot analysis of PND12 rat cochleas showing a single tyrosine hydroxylase immunoreactive band at ∼56 kDa.
At PND0, a TH-ir was already seen in thin unmyelinated fibres invading the modiolus, the spiral ganglion and the osseous spiral lamina (data not shown). This immunoreactivity was particularly noticeable at PND4, before declining to the adult level at around PND12. This immunostaining pattern was retrieved in sections displaying polyclonal AADC-ir and DBH-ir. Indeed, co-localization experiments between TH and DBH showed that both TH(+)/ DBH(+) (Fig. 2B) and TH(+)/ DBH(−) fibres were present in these cochlear regions.
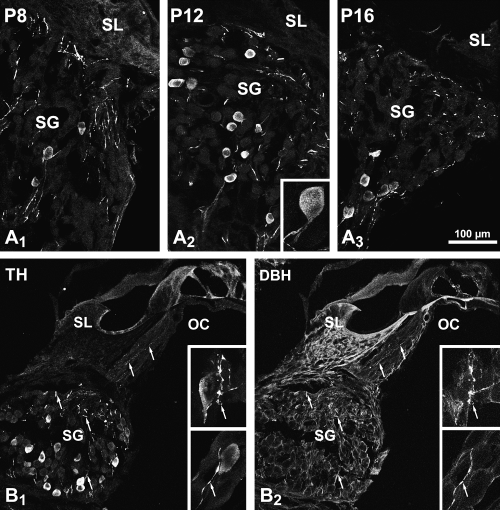
(A) Confocal microscopy showing TH-immunoreactive neurons in the second coil (medial) spiral ganglion at PND8 (P8, A1), 12 (A2) and 16 (A3). The number of immunoreactive neurons was higher at PND12 than at the two other ages. The insert in A2 shows a high magnification of an immunoreactive neuron and its central process. The low magnification views are two-dimensional (2-D) projections of ten optical sections of 0.5 µm thickness and the high magnification view in A2 is a 2-D projection of 35 optical sections of 0.5 µm thickness. (B) Double labelling confocal microscopy in the second coil of a PND12 rat cochlea. The TH-immunoreactive neurons in B1 do not display DBH-ir in B2. By contrast, all the thin TH-immunoreactive fibres (arrows) are also DBH-ir. The inserts show high-magnification views of pairs of immunolabelled neurons and fibres. The low magnification images are 2-D projections of 20 0.5-µm-thick optical sections and the high magnifications images the 2-D projections of 15 0.3-µm-thick optical sections. Scale bar, 100 µm in the low-magnification images and 30 µm in the inserts.
Some rare TH-ir perikarya were first observed in the basal coil spiral ganglion by PND4 (data not shown). By PND8, their number had increased in this coil. However, by PND12 and thereafter, basal coil perikarya no longer exhibited a TH-ir. At PND8 (Fig. 2 A1), TH-ir perikarya were found in the middle and apical coils. Their number increased by PND12 (Fig. 2 A2 and B1) and decreased thereafter (Figs 2, A3). In the adult, they could still be seen on rare occasions at the most apical level. In no case were TH-ir cell bodies immunoreactive to AADC (data not shown) or DBH (Fig. 2 B2). At all the stages studied, we noted that the TH-ir was never seen in cell bodies intensely immunofluorescent to neurofilament 200 kDa (Fig. 3A–C) or expressing a peripherin-ir (Fig. 3D–G).
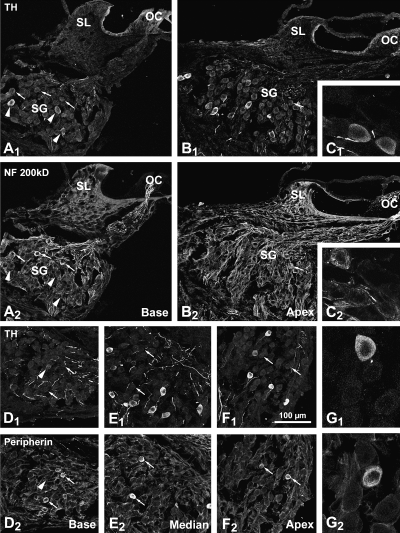
(A–C) Double labelling confocal microscopy showing the lack of colocalization of TH and neurofilament 200 kDa immunoreactivities in the upper basal (A) and apical (B) coils of a PND12 rat cochlea. In the basal coil, more or less intensely TH-ir neurons can be seen (arrowheads). These neurons do not belong to the population of neurons showing an intense immunoreactivity to neurofilament 200 kDa (arrows). In the apical coil, which shows a delayed maturation with respect to the basal coil, only one weakly neurofilament 200 kDa-immunoreactive neuron can be seen (arrow). It does not display TH-ir. (C) High-magnification image of three neurons. Two of theses are immunoreactive to tyrosine hydroxylase, the third to neurofilament 200 kDa. (D–F) Double labelling confocal microscopy showing the lack of co-localization of TH and peripherin immunoreactivities in the basal (D), medial (E) and apical (F) coils of a PND12 rat spiral ganglion. In the basal coil (D), only a weakly TH-ir neuron remains (arrowhead). This neuron does not show a peripherin-immunoreactivity (arrows). In the medial (E) and apical (F) coils, both populations of immunoreactive neurons can be seen, although peripherin-immunoreactive neurons appear less intensely labelled in the apical coil. No neuron displays a double immunolabelling. Note that the peripherin-immunoreactive neurons are slightly smaller in size than the TH-ir neurons. (G) High-magnification image of a group of neurons; one is TH-ir (in G1), another is peripherin-immunoreactive (in G2). The difference in size between the two immunoreactive neurons is more obvious here. These figures are 2-D projections of 20 (A, B and D–F) and ten (C and G) 0.5-µm-thick optical sections. Scale bar in F1 (100 µm) applies to all the figures with the exception of C and G, where it is 30 µm.
Because the TH-ir cells were found over a longer period in the middle coil than in the basal coil, we focused our quantitative analysis on this middle coil. This cell count showed that 0.79 ± 0.31% (± SEM) of the total number of primary auditory neurons displayed a TH-ir in the PND4 group. The figures increased to 8.32 ± 0.81% in the PND8 group, reached a peak of 13.99 ± 1.24% by PND12, then decreased to 4.92 ± 1.36% and 3.97 ± 0.55% in the PND16 and PND20 groups, respectively. In the adult spiral ganglion, no TH-ir neurons were observed in this coil. A Kruskal–Wallis analysis of variance on ranks showed that the differences in the median values were greater than would be expected by chance and that there was a statistically significant difference (P = 0.001) between the age groups. All the multiple comparison procedures performed using the Student–Newman–Keuls method showed a significant difference between the pairs of age groups (P < 0.05), with the exception of the PND16–PND20 pair analysis.
TH mRNA localization in the spiral ganglion
Because the quantitative analysis showed that the highest number of TH-ir neurons was found at PND12, we focused our in situ hybridization analysis of the expression of TH mRNA in spiral ganglion neurons at this postnatal stage.
The digoxigenin staining that revealed TH mRNA expression was observed in the cytoplasm of most primary auditory neurons in all the cochlear coils (Fig. 4). Satellite glial cells surrounding them, Schwann cells in the osseous spiral lamina and fibrocytes of the spiral limbus did not express the mRNA (Fig. 4).
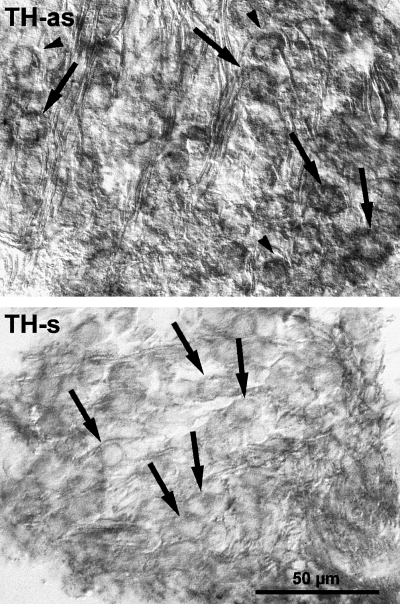
Sections through a PND12 rat spiral ganglion hybridized with digoxigenin-labelled tyrosine hydroxylase antisense (TH-as) and sense (TH-s) oligoprobes. The primary auditory neurons moderately express the TH mRNA (arrows). No hybridization signal is seen in the satellite glial cells (arrowhead). The scale bar (50 µm) applies to both figures.
TH-immunoreactive neurons are not TUNEL-positive
In order to verify if the disappearance of the TH-ir was not due to the death of the neurons that expressed the enzyme, we attempted to localize TH in TUNEL-labelled dying cells. Small TUNEL-labelled nuclei were observed in the spiral ganglia at the three ages studied (PND8, PND12 and PND16), together with TH-ir neurons (Fig. 5A). However, no co-localization of the two markers could be observed at any age studied, whatever the coil under study.
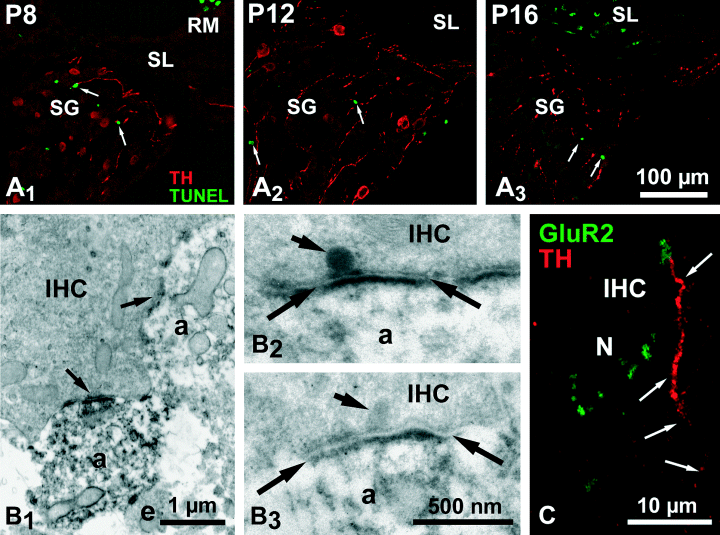
(A) Double labelling confocal microscopy in the PND8 basal (A1) and PND12 (A2) and 16 (A3) medial coils spiral ganglion of the rat showing that the TH-ir neurons (in red) do not possess TUNEL-labelled nuclei (in green) whatever the age studied. The images are 2-D projections of ten 0.5-µm-thick optical sections. (B) Ultrastructural immunocytochemistry of inner hair cell (IHC) basal poles of PND8 rat cochlea. B1 is a low-magnification view of the close apposition (arrows) between an inner hair cell and two TH-ir radial dendrites (a). B2 and B3 are higher magnifications of two synapses between an inner hair cell and immunoreactive radial dendrites (a). The plane of the sections passes through the synaptic ribbons (short arrows). The post-synaptic density on the dendrites is bracketed by the arrows. (C) Double labelling confocal microscopy of a radial dendrite synapsing with an inner hair cell (IHC) in a PND12 rat cochlea. The radial dendrite, which displays a TH-ir (in red), has an enlarged GluR2-immunoreactive terminal (in green). Note the presence of other GluR2-immunoreactive terminals devoid of TH-ir surrounding the basal pole of the hair cell. N, nucleus of the inner hair cell. This figure is a 2-D projection of four optical sections of 0.25 µm thickness. The scale bar in A3 (100 µm) also applies to A1 and A2.
Expression of TH in dendrites connecting the inner hair cells
Electron microscopic examination of the inner spiral bundle area in the PND8–PND14 cochleas showed thick unvesiculated TH-ir fibres climbing through the bundle to contact the inner hair cell base (Fig. 5B), establishing fully differentiated synapses with a synaptic ribbon on the presynaptic side (Fig. 5 B2 and B3). However, this population of TH-ir fibres was clearly minor with respect to the unreactive fibres synapsing with inner hair cells. TH-ir vesiculated varicosities were also found throughout the bundle (data not shown). At the confocal microscopy level, it appeared that thick TH-ir fibres climbing radially in the direction of the inner hair cells appeared terminated by GluR2-ir puncta, suggesting that they were in contact with these glutamatergic hair cells (Fig. 5C).
Discussion
Using immunocytochemistry, we have localized TH-ir in rat primary auditory neurons and in unmyelinated fibres of the spiral ganglion. This immunoreactivity probably revealed the true TH as the immunoblot analysis showed a single immunoreactive band at ∼56 kDa, corresponding to the enzyme molecular weight. In addition, according to our in situ hybridization analysis, the mRNA coding the enzyme was expressed in nearly all the primary auditory neurons.
We further showed using a cell counting analysis that the TH expression was transient. It began between birth and PND4 in the basal coil of the cochlea. It then extended as a wave to the upper coils, following the base to apex gradient of maturation of the cochlea (Pujol & Marty, 1970; Lenoir et al., 1980), and disappeared by the fourth week of life. The maximum density of the TH expression in primary auditory neurons was observed by PND12, when approximately 14% of the neurons were TH-ir.
By contrast with most TH-ir unmyelinated fibres, which belonged to the sympathetic supply of the cochlea or to the lateral efferent innervation (Eybalin, 1993), TH-ir primary auditory neurons never expressed AADC nor DBH. We thus conclude that this transient TH expression was not related to a catecholamine neurotransmission, as has been proposed for same cases in the central nervous system (Jaeger & Joh, 1983; Berger et al., 1985; Gaspar et al., 1987; Nagatsu et al., 1990, 1996b; Satoh & Suzuki, 1990; Vincent & Hope, 1990; Komori et al., 1991; Fujii et al., 1994; Battaglia et al., 1995; Ikemoto et al., 1998, 1999; Kitahama et al., 1998; Ershov et al., 2002).
Nature of the neurons transiently expressing TH
Inner and outer hair cells are innervated by different classes of primary auditory neurons. Type I neurons, which account for 90–95% of the whole neuronal population of the spiral ganglion, innervate inner hair cells by their unique radial dendrites. Type II neurons, accounting for the remaining 5–10% of the spiral ganglion neurons, innervate outer hair cells by the multiple branches of their spiralling dendrites. Type I and type II primary auditory neurons can be distinguished by their intermediate filament content. Indeed, neurofilament 200 kDa and peripherin are predominantly expressed by type II neurons (Berglund & Ryugo, 1986, 1991; Dau & Wenthold, 1989; Hafidi et al., 1993; Despres et al., 1994; Hafidi, 1998). The TH-ir appeared in spiral ganglion neurons after type II neurons can be identified on morphological and immunocytochemical criteria (Schwartz et al., 1983; Romand & Romand, 1984, 1985, 1990; Hafidi & Romand, 1989; Hafidi et al., 1993). We thus propose that our results showing that TH is not co-localized with a strong expression of peripherin and neurofilament 200 kDa indicate that the TH-ir neurons do not belong to the type II class but rather to the type I class of primary auditory neurons. This conclusion is in agreement with the detection of TH-ir fibres in the inner spiral bundle radially travelling toward inner hair cell basal poles, establishing fully differentiated synapses as evidenced using immunoelectron microscopy, and bearing GluR2 subunits of AMPA receptors.
The rather low number of TH-ir neurons may have two explanations. TH was expressed synchronously by a subpopulation of type I neurons, which later down-regulated the enzyme or died. Alternatively, all the type I neurons expressed TH in an unsynchronized way, giving the appearance of an immunoreactive subpopulation. Apoptosis plays a significant role in the development of the spiral ganglion (Orita et al., 1999; Nikolic et al., 2000), which loses ∼20% of its neurons by the end of the first postnatal week (Rueda et al., 1987). That the TH expression begins after this period of neuronal loss and that TH-ir neurons did not have TUNEL-positive nuclei rule out the hypothesis that the expression of TH ends with the death of a neuronal subpopulation. Furthermore, our in situ hybridation data at PND12 showing the presence of the TH mRNA in most primary auditory neurons, perhaps all the type I neurons, largely fits with a transient TH expression affecting all the type I neurons in an unsynchronized way rather than being in line with a TH expression triggered in a neuronal subpopulation only.
Possible triggers of the transient expression of TH in the maturing cochlea
The expression of TH occurred during the period of onset of the cochlear function, i.e. when the first cochlear microphonic potentials and compound action potentials of the auditory nerve (CAP) can be recorded in response to natural stimuli. The microphonic potential is an evoked potential reflecting the receptor potential of sensory hair cells (Dallos, 1986) and the CAP an evoked potential reflecting the synchronized discharges of auditory neurons. In the rat, the microphonic potential appears around PND8, and the first CAP by PND11–12 (Uziel et al., 1981; Puel & Uziel, 1987). Before this onset period, the synapses between the inner hair cells and the radial dendrites of the type I neurons are already present (Pujol et al., 1980; Lenoir et al., 1980) and Ca2+-induced exocytosis can be elicited in inner hair cells (Beutner & Moser, 2001). This exocytosis increases during the first postnatal week to peak around PND6 and then decreases to mature values around PND14 (Beutner & Moser, 2001). During the 4–5 days following its appearance, the CAP shows a progressive increase in amplitude (Carlier et al., 1975; Shnerson & Pujol, 1981; Uziel et al., 1981; Puel & Uziel, 1987; Rybak et al., 1992), which is thought to reflect a greater synchronization of the auditory neuron discharges.
The TH expression in the type I primary auditory neurons just follows the peak of Ca2+-induced exocytosis in inner hair cells at PND6 and occurs at the same time as the first microphonic potentials, around PND8. This expression decreases after PND12, when the evoked exocytosis in inner hair cells reaches a mature level and it disappears after the CAP has acquired its mature characteristics, after PND16. During this period, dramatic changes have occurred pre- and post-synaptically at the junction between inner hair cells and radial dendrites (Knipper et al., 1997; Eybalin et al., 2001, 2002). Although we do not yet know the causes, it may well be that the transient expression of TH in the type I neurons is related to the onset of the hearing function and to the physiological and molecular changes that occur at the synapses between inner hair cells and radial dendrites. This would be in line with the fact that TH expression in adult neurons often occurs in response to stringent stimuli (Asmus & Newman, 1994; Marsais & Calas, 1999; Bezin et al., 2000; Abramova et al., 2002; Marsais et al., 2002).
The times of onset of the cochlear function are based on the first recording of evoked potentials, which reflect synchronized discharges of individual units. They cannot account for the behaviour of these units before their synchronization. Because the TH-ir appears after the stimulated exocytosis in inner hair cells has peaked and disappears after the synchronization of the auditory nerve units, we hypothesize that this transient TH expression signals the reception by type I neurons of the first stimuli transduced and transmitted to radial dendrites by inner hair cells. A possible mechanism could be a sudden and massive Ca2+ entry in radial dendrites through voltage-activated Ca2+ channels at the onset of function of individual neurons, resulting in the activation of the TH gene (Nagatsu et al., 1989; Kilbourne et al., 1992; Kumer & Vrana, 1996; Menezes et al., 1996; Nagamoto-Combs et al., 1997; Brosenitsch et al., 1998; Brosenitsch & Katz, 2001). Beacuse this onset of function occurs at different times for each neuron, the number of neurons expressing TH would remain rather low during these few days.
Interestingly, neurons in the central auditory system seem to behave similarly than type I neurons. They also transiently express TH during the first postnatal weeks (Jaeger & Joh, 1983; Harper & Wallace, 1995; Nagatsu et al., 1996b), when the whole auditory pathway undergoes its structural and physiological maturation (Cant, 1998; Sanes & Walsh, 1998), here also characterized by dramatic changes at glutamatergic synapses (Winer & Larue, 1989; Zhou et al., 1995; Caicedo et al., 1998; Kubke & Carr, 1998; Caicedo & Eybalin, 1999; Lawrence & Trussell, 2000; Brenowitz & Trussell, 2001; Elezgarai et al., 2001; Joshi & Wang, 2002; Lohrke & Friauf, 2002).
Are there any functional implications of TH expression in type I primary auditory neurons?
The functional role of TH in non-CAergic neurons remains unclear, especially where these neurons do not synthesize L-DOPA (Schussler et al., 1995). In the opposite situation, where these neurons do synthesize L-DOPA, a modulation of catecholamine and glutamate neurotransmissions has been described in the striatum, together with neurotransmitter function in the lower brainstem (see Misu et al., 1996). Such temporary neuromodulatory roles may also occur in the cochlea as glutamate is the inner hair cell neurotransmitter and dopamine a lateral efferent neurotransmitter (see Eybalin, 1993). Another possibility is co-operation between neurons expressing TH, but not AADC, and neurons expressing AADC, but not TH, to produce dopamine, as has been proposed in the arcuate nucleus (Ugrumov et al., 2002); however, we found no evidence of AADC-ir neurons in our present study. In the absence of further investigations, a more parsimonious hypothesis may be that the onset of function induces a TH expression in type I primary auditory neurons through a Ca2+ activation of its gene transduction. This TH expression would indicate that individual neurons are ready to transmit natural auditory stimuli to the higher levels of the auditory system. In conclusion, we propose that the TH expression in type I primary auditory neurons can be used as an index of cochlear maturation, as is the case for the timing of appearance of the microphonic potential and the CAP.
Acknowledgements
We wish to thank Dr Marc Lenoir for helpful discussions, Dr John Bulger for editorial comments on the manuscript, and Dr Sandra Rebelo and Mrs Maria Manuela Pacheco for their collaboration and technical advice. The confocal and electron microscopy observations were performed in the Centre Régional d'Imagerie Cellulaire de Montpellier with the technical support of Nicole Lautredou-Audouy, Jérôme Taki and Patrice Minary. This work was supported by projects STRAD/C/SAV/304/92, PECS/C/SAV/87/95, Praxis XXI/PCS/C/CED/157/96 and Bolsa de curta duração da Fundação Calouste Gulbenkian.
Abbreviations
-
- AADC
-
- aromatic amino acid decarboxylase
-
- AMPA
-
- α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
-
- BCIP
-
- 5-bromo-4-chloro-3-indolyl phosphate
-
- CA
-
- catecholamine
-
- CAP
-
- compound action potential of the auditory nerve
-
- DAB
-
- 3–3′-diaminobenzidine
-
- DBH
-
- dopamine-β-hydoxylase
-
- DIG
-
- digoxigenin
-
- -ir
-
- immunoreactive/immunoreactivity
-
- L-DOPA
-
- l-dihydroxyphenylalanine
-
- NBT
-
- nitro blue tetrazolium
-
- PBS
-
- phosphate-buffered saline
-
- PND
-
- postnatal days
-
- SDS-PAGE
-
- sodium dodecyl sulphate-polyacrylamide gel electrophoresis
-
- SSC
-
- sodium chloride/sodium citrate
-
- TBS
-
- Tris-buffered saline
-
- TBST
-
- TBS with 0.1% Tween 20
-
- TH
-
- tyrosine hydroxylase
-
- TUNEL
-
- terminal deoxynucleotidyl transferase biotin-dUTP nick end labelling.