Erythropoietin protects the in vitro blood–brain barrier against VEGF-induced permeability
Abstract
The blood–brain barrier (BBB) ensures the homeostasis of the brain microenvironment, mostly through complex tight junctions between brain endothelial cells that prevent the passage of hydrophilic molecules from blood to brain and vice versa. A recent study has shown in vivo that systemic administration of erythropoietin (Epo) protects against brain injury. Using an in vitro model of the bovine BBB, we observed that the expression of the Epo receptor is modulated by its ligand and hypoxic stimuli such as vascular endothelial growth factor (VEGF) treatment. In addition, Epo protects against the VEGF-induced permeability of the BBB, decreases the levels of endothelial nitric oxide synthase and restores junction proteins. The kinetic transport experiments revealed the capacity of Epo to cross the in vitro BBB in a saturable and specific way. Our results suggest a new mechanism for Epo-induced neuroprotection, in which circulating Epo controls and maintains the BBB through an Epo receptor signalling pathway and the re-establishment of cell junctions.
Introduction
Erythropoietin (Epo) was first described as a cytokine that acts as a major regulator of erythropoiesis. The site of Epo production switches during development from foetal liver to adult kidney, with low expression levels in adult liver (Moritz et al., 1997). Because Epo and the erythropoietin receptor (EpoR) are expressed in rodent and human brain tissue, as well as in cultured neurons and astrocytes, and Epo affects neuronal cells, the biological role of Epo is not limited to haematopoiesis (Liu et al., 1994; Masuda et al., 1994; Digicaylioglu et al., 1995; Morishita et al., 1997). Epo gene expression in the brain is regulated by hypoxia-inducible factor-1, which is activated by a variety of stressors, including hypoxia (Gage & Stanton, 1996). Several independent research groups have reported that Epo protects cultured neurons against glutamate toxicity (Morishita et al., 1997; Kawakami et al., 2001; Wen et al., 2002) and prevents neuronal apoptosis after cerebral ischaemia and metabolic stress (Siren et al., 2001). These studies, performed using neurons and neuron-like cells, have confirmed the protective potential of Epo against various insults such as excitotoxicity, serum deprivation and growth factor deprivation. Results from in vivo experiments using experimental models of global and focal ischaemia, blunt trauma, immune-mediated inflammation, excitotoxin-elicited seizures, subarachnoid haemorrhage and toxin-induced Parkinsonism suggest that Epo is a beneficial agent in these situations (Brines et al., 2000; Siren et al., 2001; Grasso et al., 2002).
The blood–brain barrier (BBB) plays a crucial role in protecting the central nervous system (CNS). The high selectivity of the BBB interface relies on its physical barrier properties, determined by complex tight junctions (TJ) between brain capillary endothelial cells and low levels of pinocytosis, as well as metabolic characteristics such as the rich amounts of specific enzymes expressed at the endothelium (Drewes, 2001). Expression of EpoR in brain capillary endothelial cells and the ability of systemically administered Epo to cross the BBB have been reported in vivo (Brines et al., 2000; Grasso et al., 2002). However, the mechanism by which exogenous Epo mediates its neuroprotective effects across the BBB is not fully understood.
In the present study, using an in vitro model of the BBB, we investigated the protein expression and localization of EpoR in bovine brain endothelial cells (BBECs) after Epo and vascular endothelial growth factor (VEGF) exposures. In addition, we examined the influence of recombinant human Epo (rHuEpo) on the BBB status. Our results indicate that: (i) Epo has a protective role against the VEGF-induced hyperpermeability; (ii) the transport kinetic of rHuEpo through BBEC monolayers is saturable and EpoR dependent; and (iii), the mechanism involves an EpoR-induced pathway resulting in the decrease of endothelial nitric oxide synthase (eNOS) levels and the re-establishment of cell junctions.
Materials and methods
Materials
Reagents were purchased from Sigma (St Louis, MO, USA) unless otherwise stated. Rabbit polyclonal antibodies (pAbs) were used to detect the following antigens: ZO-1 and occludin (Zymed, San Francisco, CA, USA); VE-cadherin (Bender MedSystem, Vienna, Austria); and Epo receptor (M-20 clone, Santa Cruz Biotechnology Inc., CA, USA). rHuEpo was from Laboratorios PENSA (Barcelona, Spain) and human recombinant VEGF from Oncogene Research products (Cambridge, MA, USA). eNOS monoclonal antibody was purchased from Transduction Laboratories (Lexington, KY, USA). The monoclonal antibody against VEGF receptor-2 (VEGFR) was purchased from Sigma. Low-density lipoprotein (LDL) was isolated from human plasma by sequential ultracentrifugation and was labelled with DiI (1,1′-dioctadecyl-3, 3,3′, 3′-tetramethyl-indocarbocyanine percholate) as described (Stephan & Yurachek, 1993). Dil-labelled LDL was kindly provided by Dr Sonia Benitez (Sant Pau Hospital, Barcelona, Spain). Transwell polyester membrane inserts were from Costar (Corning, NY, USA). Radioactive products were from Amersham Pharmacia Biotech (Buckinghamshire, UK).
Cell culture
Fresh bovine brains were obtained from the local slaughterhouse (Mercabarna, Barcelona) under veternarry supervision. Bovine brain endothelial cells were isolated from brain grey matter by mechanical methods as described previously (Cecchelli et al., 1999) and were seeded at 50 000 cells/cm2 onto 24-mm diameter Transwell filter inserts with 0.4-µm pores, coated with rat tail type I collagen (20 µg/mL). Cells were grown in DMEM, 10% horse serum, 10% newborn calf serum, 2 mm glutamine, 0.5 µg/mL gentamicine and basic fibroblast growth factor (1 ng/mL).
Astrocytes were isolated from newborn rat cerebral cortex. Briefly, the rats were killed by cervical dislocation and after removing the meninges, brain tissue was forced gently through an 82-µm nylon sieve. Astrocytes were plated on 6-well microplates in 2 mL of DMEM supplemented with 10% foetal calf serum (Cecchelli et al., 1999).
The in vitro model of bovine BBB used in this study was obtained by coculturing BBECs on porous supports with astrocytes in the lower compartment. Under these conditions, BBECs differentiate after 12 days into a confluent polarized monolayer that closely mimics the in vivo BBB (Cecchelli et al., 1999). To control the BBB status of BBEC polarized cultures, transendothelial electrical resistance (TEER), paracellular permeability and TJ networks were checked in each experiment before use. The experiments were approved by the Ethical committee of Barcelona University.
Transendothelial transport studies
Once the BBB was formed, BBEC-seeded supports were transferred to new receiving wells to avoid any interference from the astrocyte population. Cells were washed carefully with Hank's balanced salt solution (HBSS) buffer (KCl 540 µm; KH2PO4 44 µm; NaCl 13.68 mm; NaHPO4 33 µm; CaCl2 1.3 mm; MgCl2 1.1 mm) and 10 U/mL of rHuEPO in HBSS buffer was added either to the upper or lower compartment (2.5 mL). At the indicated time points (0, 0.5, 1, 2 and 4 h) 200 µL samples were recovered from each compartment and frozen at 80 °C until further analysis by ELISA (Roche Diagnostic, S.L.). The specificity of Epo transport through EpoR was determined by adding 10 µg EpoR antibody to the upper or lower compartment for 1 h before and during the kinetic experiments. VEGFR antibody was applied similarly in control wells as negative control. [3H]inulin (2.2 nmol/mL) and DiI-labelled LDL (50 µg/mL) were applied apically as controls for passive paracellular diffusion and receptor-mediated transport, respectively. For the luminal uptake of Dil-LDL, BBECs were previously incubated for 24 h in medium with 10% lipoprotein deficient serum. Dil-LDL fluorescence was determined in a Fluoroskan Ascent CF spectrofluorometer with excitation and emission wavelengths set at 530 and 590 nm, respectively. In addition, ApoB content of LDL was determined in a Hitachi 911 autoanalyser. The apparent permeability coefficient was calculated according to the formula Papp = (dQ/dt/)/(A.Co), as described previously (Artursson, 1990), where dQ/dt represents the amount of compound transported per minute (nmol/min), A is the surface area of the filter (cm2) and Co is the initial concentration of compound (nmol/mL). The Papp of LDL was calculated as Papp of ApoB.
To evaluate Epo protection on BBB status, Papp of [3H]inulin, as marker of cell barrier integrity, was determined after different treatments indicated in the figure legends.
Immunofluorescence staining of BBECs
Confluent BBECs monolayers grown on Transwell filters for 12 days in the presence of astrocytes were exposed for 24 h to 100 ng/mL of VEGF (basolateral part), 10 U/mL of rHuEPO (apical part) or both. After treatments, the culture medium was removed and monolayers were rinsed with phosphate-buffered saline (PBS). BBEC-seeded supports were transferred to new receiving wells fixed with 3% paraformaldehyde in microtubule stabilizing buffer (MTSB; 80 mm K-PIPES, pH 6.5; 5 mm EGTA; 2 mm MgCl2) for 5 min at room temperature and permeabilized using 3% paraformaldehyde-100 mm Na4BO7 (pH 11) plus 0.2% saponin for 10 min. Monolayers were then processed for indirect immunofluorescence staining with pAbs against occludin (1 : 50), ZO-1 (1 : 100) or VE-cadherin (1 : 100), incubated with FITC-conjugated anti-rabbit IgGs (Dako, A/S, Denmark) and analysed by confocal microscopy (Reinsh et al., 1998).
Protein extraction and Western blot analysis
To study EpoR localization, confluent BBEC monolayers grown on Transwell filters were stimulated for 24 h with Epo (10 U/mL), and further incubated for 20 min at 4 °C in PBS containing 0.5 mg/mL Sulfobiotin (Sulfo-NHS-LC-Biotin, Pierce, Rockford, IL, USA) applied either apically or basolaterally. Cells were lysed for 30 min at 4 °C with lysis buffer A (1% Triton X-100, 150 mm NaCl, 50 mm Tris-HCl pH 7.5) containing protease inhibitors (Arcasoy et al., 2002). Lysates were centrifuged at 16000 g for 10 min at 4 °C, and supernatants were collected and incubated with streptavidin agarose beads for 4 h at 4 °C. Beads were washed five times with cold buffer (1% Triton X-100, 150 mm NaCl, 10 mm Tris pH 7.4) containing protease inhibitor cocktail. Proteins were eluated with 5 × concentrated electrophoresis sample buffer (125 mm Tris pH 6.8, 4% sodium dodecyl sulphate (SDS), 10% glycerol, 0.006% bromophenol blue, 2% β-mercaptoethanol), and boiled for 5 min. EpoR expression was analysed by Western blotting. VEGFR expression was analysed as biotinylation control.
To study protein expression after each treatment total extracts were obtained by lysing cells with buffer B (1% SDS 150 mm NaCl, 50 mm Tris-HCl pH 7.5) containing protease inhibitors.
For Western blotting, 20 µg of proteins was separated by SDS-polyacrylamide gel electrophoresis and transferred onto nitrocellulose (Schleicher & Schuell, Dassel, Germany), and protein expression was analysed as described elsewhere (Bazzoni et al., 2000). Densitometry analyses of Western blot are shown in graphs. The band intensity was normalized using actin as a loading control and was represented as percentage of expression of each protein.
Statistics
Data are expressed as means ± standard error. Significance of differences was examined using the Student's t-test. P-values < 0.05 were considered to be significant.
Results
EpoR is expressed in endothelial cells from brain capillaries
Because expression levels of EpoR detected by immunofluorescence were too low (data not shown), to define EpoR distribution in greater detail, 12-day-BBEC polarized monolayers were either basolaterally or apically labelled with biotin; EpoR expression was followed by streptavidin precipitation and Western blot analysis. Notably, EpoR was present at the apical cell surface (Fig. 1A), whereas no specific signal was detected when biotin labelling was performed in the basal compartment. As control of biotinylation, VEGFR was detected and found in both apical and basolateral cell surfaces. Further Western blot analyses on Epo-treated BBECs at different time points showed that EpoR expression was upregulated by its ligand to maximal levels after 1 h (40% higher than time 0) and maintained for at least 24 h (Fig. 1B). Furthermore a 20% of EpoR expression was observed under VEGF vs. control. However EpoR expression levels in the cotreatment were similar to Epo treatment, suggesting a lack of synergistic effect between VEGF and Epo (Fig. 1C).
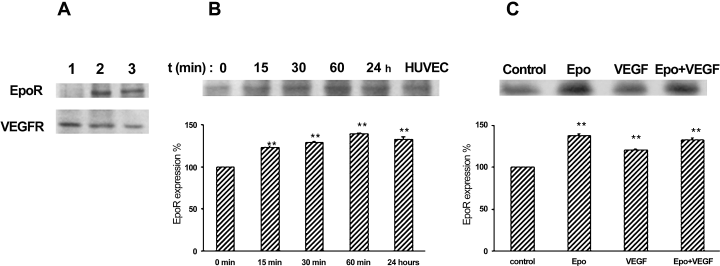
EpoR is expressed at the apical part of BBECs. (A) Monolayers were labelled for 20 min with biotin, either basolaterally (1) or apically (2). Lysates were incubated with streptavidin agarose beads and analysed by Western blot with EpoR and VEGFR antibody. Extracts from HUVEC cells were run in parallel as control (3). (B) EpoR time-dependent expression upon rHuEpo treatment (10 U/mL) was analysed by Western blot with anti-EpoR antibody. (C) EpoR expression was analysed by Western blotting with anti-EpoR antibody on total lysates from monolayers incubated for 24 h with Epo (10 U/mL; upper side), VEGF (100 ng/mL; lower side) or both. Graphs represent EpoR expression levels after different treatments, calculated as a percentage taking either time 0 or untreated control expression levels as reference (100%). To determine the significance of the observed effects, the mean of each treatment group was compared with control using Student's t-test, **P < 0.01. Data shown are representative of three separate experiments.
Apical to basolateral transport of Epo across BBEC monolayers
rHuEpo was added to the luminal or basal compartment of the culture, and its progressive transfer across the cell monolayer was monitored (Fig. 2A). The Papp value of apical to basal Epo transport (Epo A–B) was 0.42 × 10−3 ± 0.04 × 10−3 cm/min whereas the basal to apical (Epo B–A) was 0.13 × 10−3 ± 0.006 × 10−3 cm/min, compared with the Papp of LDL, which was 1.02 × 10−3 ± 0.007 × 10−3 cm/min, representative of receptor-mediated transport.
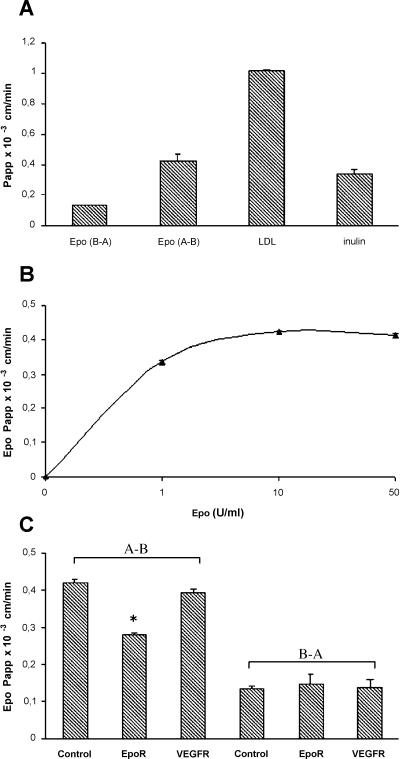
Transport of rHuEpo across the in vitro BBB. Graphs represent Papp values obtained under each experimental conditions (A) rHuEpo (10 U/mL) was added to the apical or basolateral side of BBEC monolayers. Samples were recovered from upper and lower compartments at 0, 0.5, 1, 2 and 4 h. The amount of rHuEpo present in each fraction was determined by ELISA, and its transport was evaluated as Papp coefficient. Epo (A–B), apical to basolateral; Epo (B–A), basolateral to apical. LDL (50 µg/mL) and inulin (2.2 nmol/mL) were applied in parallel as control. (B) Papp values of Epo obtained when increasing amount (1, 10, 50 U/mL) were applied apically. (C) Papp values of Epo after incubation with antibody against EpoR (EpoR) or VEGFR (VEGFR). Experiments were performed in triplicate transwells. Graphs represent results from three independent experiments.
The effect of increasing Epo concentrations (1–50 U/mL) on its transfer through BBEC monolayers was evaluated (Fig. 2B) and revealed a saturable mechanism. To determine the specificity of Epo transport through EpoR, experiments were performed in the presence of antibodies against EpoR or VEGFR (control). As shown in Figure 2C, a decrease of 35% of the initial apical to basal transport was observed in presence of the anti-EpoR antibody, whereas VEGFR antibody had no effect, suggesting the specific involvement of EpoR in the traffic of Epo.
Soluble Epo protects against the VEGF-induced permeability of BBEC monolayers
Cells were treated for 24 h in the absence (control) or in the presence of either VEGF (basolateral) or rHuEpo (apical), or both, and BBEC monolayer permeability was evaluated using inulin as marker for barrier status. VEGF treatment induced an increase (40%) of inulin Papp value, compared with untreated monolayers, whereas the Epo-VEGF cotreatment did not, thus suggesting that Epo was able to protect against VEGF-induced permeabilization (Fig. 3).
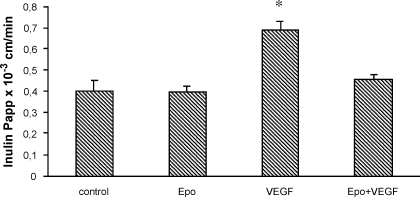
rHuEpo restores VEGF-induced permeability. BBECs were incubated for 24 h with Epo (10 U/mL; upper side), VEGF (100 ng/mL; lower side) or both. Monolayer permeability in various conditions was measured using inulin and its corresponding Papp values as described in Materials and methods. Data are the mean from triplicate samples and are representative of five independent experiments. *P < 0.05, Student's t-test, compared with control.
Epo protects in vitro BBB against VEGF by restoring eNOS levels
To determine whether the Epo-induced decrease in eNOS levels reduced the VEGF-increased permeability, eNOS expression in treated BBEC monolayers was evaluated by immunoblotting. Incubation with rHuEpo for 24 h markedly downregulated eNOS protein levels, whereas VEGF caused a clear upregulation, and the combination of both treatments maintained eNOS at control levels (Fig. 4).
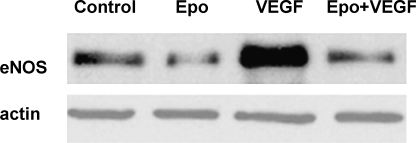
rHuEpo prevents VEGF-induced upregulation of eNOS. BBECs were treated as described in Figure 1C. Samples were analysed by Western blot with anti-eNOS antibody. Blots were then stripped and reproved with anti-actin to verify equal loading. Data shown are representative of three separate experiments.
VEGF-induced changes in the distribution pattern of interendothelial junction proteins were reverted by Epo
Inter-endothelial junctions play a key role in the regulation of vascular permeability. VEGF-induced signal transduction altered the formation or distribution of adherens and TJ (Esser et al., 1998; Antonetti et al., 1999). Polarized control cells showed a regular linear labelling of ZO-1, VE-cadherin and occludin. Upon Epo treatment, the staining at cell–cell junctions further increased for VE-cadherin. VEGF-treated cells presented a less regular and reduced immunoreactivity at regions of cell–cell contact for all three proteins. After VEGF/Epo cotreatment, cells did not display altered patterns (Fig. 5A).
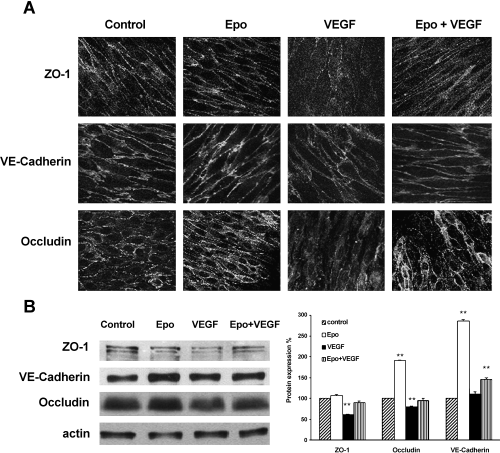
Effect of rHuEpo on the distribution and expression of junction proteins. (A) BBEC monolayers were treated as described in Figure 1C, fixed, permeabilized and analysed by immunofluorescence staining and confocal microscopy for the expression of ZO-1, VE-cadherin and occludin. (B) Lysates from control and treated monolayers were analysed by Western blot with antibodies against ZO-1, VE-cadherin or occludin. Actin was used to verify equal loading. Representative data from three independent experiments are shown. Graph represents the expression levels of each protein after different treatments, calculated as percentage taking control expression levels as 100% reference. **P < 0.01, Student's t-test, compared with control.
Effect of Epo on the expression of adherens and TJ proteins
Alterations in the expression of cell–cell junction proteins were further examined by Western blotting from Epo, VEGF, Epo/VEGF and control cells (Fig. 5B). As shown by densitometric quantification of normalized protein levels represented in graphs (Fig. 5B), the total level of VE-cadherin was increased upon Epo treatment (about 185% increase), although no further change was detected upon VEGF treatment. By contrast, ZO-1 and occludin were decreased after VEGF treatment (40% and 20% compared with control cells, respectively), and Epo cotreatment was able to restore basal levels. Epo alone was also able to increase the levels of occludin by 90%.
Discussion
Complex TJ are responsible for the integrity of the BBB. They continuously connect the endothelial cells, thereby separating the extracellular compartments of the neuronal parenchyma and microvasculature. The BBB restricts the free exchange of most solutes between plasma and the extracellular fluid of the brain. Furthermore, although BBECs contain no direct trans-endothelial passageways, such as fenestration or channels, specific transport mechanisms ensure that the CNS receives an adequate supply of nutrients. Such carriers and receptors have been identified elsewhere (Descamps et al., 1996; Dehouck et al., 1997). Previous studies based on polymerase chain reaction assays have reported that EpoR is present in the BBB (Yamaji et al., 1996), and Epo and its receptor have been shown to function as primary mediators of the normal physiological response to hypoxia. This study provides the first direct evidence that functional EpoR is present at apical membranes of BBB cells, and that its expression is regulated by its ligand with a maximal expression at 1 h, and by hypoxic stimuli such as VEGF. In transfected and normal haematopoietic cells, the majority of newly made EpoRs are retained in the endoplasmic reticulum, destined for degradation (Hilton et al., 1995). It is also known that EpoR is degraded rapidly with a half-life of about 1 h (Neumann et al., 1996). The rapid modulation of total levels of EpoR in the presence of Epo observed in this present study could be explained by an Epo-induced inhibition of EpoR degradation.
Although it was first accepted that Epo does not cross the intact BBB because of its size and glycoprotein structure, results available on this aspect are contradictory. Whereas Brines et al. (2000) reported that rHuEpo might cross the BBB using receptor-dependent endocytosis followed by translocation into the brain (Brines et al., 2000; Grasso et al., 2002) other studies demonstrated that neither intravenous rHuEpo nor endogenous Epo produced by the kidney were able to cross the normal BBB (Marti et al., 1997; Buemi et al., 2000).
The availability of an in vitro model to study the passage of macromolecules through the monolayers of BBECs on porous membranes enabled us to investigate the characteristics of the passage of rHuEpo through BBEC monolayers. Because of the fact that a close correlation exits between the values of the brain uptake index obtained in vitro in this model and those obtained in vivo for a large number of drugs (Dehouck et al., 1994), this cellular system is accepted as a good model able to mimic the BBB in vivo (Dehouck et al., 1997). The restrictive paracellular pathway presented by BBECs monolayers cocultured with astrocytes allowed us to discriminate with sufficient sensitivity between drugs with different permeabilities. The predictivity of the model is reinforced by using marker molecules as internal controls within each transport experiment; thus, inulin has been used as a representative for low brain penetration solutes, with Papp value of 0.34 × 10−3 ± 0.03 × 10−3 cm/min. The apical-to-basolateral rHuEpo kinetic uptake through BBECs showed a Papp value of 0.42 × 10−3 ± 0.04 × 10−3 cm/min whereas the basolateral-to-apical uptake value was three-times less. The apical-to-basolateral transport was saturable and partially inhibited by an antibody against EpoR, supporting a receptor-dependent mechanism. However, as a complete reduction was not observed, we cannot exclude the participation of other transporter proteins involved in Epo transfer through BBB.
These results indicated that: (i) Epo was directed to the basal compartment through an active receptor-mediated transport mechanism; and (ii), Epo permeability values in vitro were predictive of a medium–low brain penetrating drug.
Our data are in agreement with previous results obtained for LDL, a protein with a molecular weight similar to Epo, in an in vitro model with similar characteristics (Dehouck et al., 1997). Finally, the trace amounts of apoliprotein B that were detected by Salen et al. (1987) in cerebrospinal fluid from healthy patients using a very sensitive technique confirm that, at most, small amounts of apolipoprotein B normally pass through the BBB.
Experimental studies have described a potent neuroprotective effect of Epo during cerebral ischaemia. In cultured neurons, Epo prevents glutamate-induced cell death in a dose-dependent manner (Morishita et al., 1997). A lateral ventricular infusion of rHuEpo ameliorates neuron survival in a model of ischaemia (Siren et al., 2001). Moreover, Epo stimulates neuronal functional viability (Shingo et al., 2001) and the systemic administration of rHuEPO reduces mortality rates, ameliorates functional recovery and prevents brain ischaemic damage (Brines et al., 2000; Grasso et al., 2002). Continuous microvessel endothelial cells are essential for the integrity of the BBB, and several pathological conditions such as local ischaemia and brain tumours disrupt the BBB, which might lead to an ischaemia-induced brain edema. The increase in paracellular permeability during hypoxia is one of the main effects of CNS damage, resulting in the infiltration of cytokines and cells from the immune system, thus increasing the inflammation response. Hypoxia-induced permeability is mediated by VEGF via the release of NO (Fischer et al., 1999). The inhibitory effects of Epo on NO production and eNOS expression (Wang & Vaziri, 1999) led us to determine the effect of Epo on the VEGF-induced permeability of the BBEC monolayer. VEGF coincubation of BBB cells with Epo restored control permeability levels by 40%, suggesting a protective role not only for the brain capillary endothelium but also, indirectly, for the CNS environment. Although the role of BBB as a functional and anatomical barrier is of great importance for the maintenance of a constant environment for optimal CNS physiology (Huber et al., 2001), this is the first evidence of the protective effect of circulating Epo on the BBB and offers new therapeutics properties of rHuEpo.
Thus, data from both transport and paracellular permeability experiments suggest that the Epo-induced protective effect on the hypoxic BBB is mediated from the apical surface of capillary endothelial cells through EpoR and a specific signalling pathway.
The eNOS protein was first described as constitutive. However, recent studies have demonstrated that is regulated by a number of biophysical, biochemical and hormonal stimuli, both under physiological and pathological conditions. Several pathological stimuli, such as hypoxia, chronic exercise and the growth state, up-regulate eNOS expression in endothelial cells (Moncada et al., 1991). On the other hand, effects of Epo on eNOS are controversial. Indeed, some authors described a decrease on eNOS expression (Wang & Vaziri, 1999), while others demonstrated an increase or even no change in NO production (Banerjee et al., 2000). Our results demonstrated that in this in vitro model of BBB, Epo inhibits eNOS expression, as previously reported for human aortic endothelial cells (Wang & Vaziri, 1999). These contradictory results could be explained by the fact that eNOS expression was evaluated either at protein level, or mRNA level, or NO production (Wang & Vaziri, 1999; Banerjee et al., 2000) which do not necessarily correlate. Finally, we cannot exclude the possibility that Epo could induce a biphasic effect on eNOS expression as described in some reports such as for oxLDL (Liao et al., 1995).
VEGF has also been described to increase endothelial permeability by affecting the expression or distribution of junction proteins such as VE-cadherin, ZO-1 and occludin (Esser et al., 1998; Antonetti et al., 1999; Fischer et al., 2002). VE-cadherin is selectively expressed in endothelial cells (Dejana, 1997) and plays a key role in the regulation of endothelial cell permeability. In addition, TJ proteins are the main structural elements of the BBB, especially occludin, which together with claudins and JAM, are the integral transmembrane components of the TJ (Tsukita et al., 1999). Immunofluorescent staining of VE-cadherin, occludin and ZO-1 revealed a decrease in these proteins at the endothelial junctions after VEGF treatment, while incubation with rHuEpo prevented this effect. Moreover, treatment with rHuEpo alone produced a localized increase in the staining of these proteins at the contact area.
The involvement of rHuEpo on adhesion protein expression, alone or in combination with VEGF was also determined. VE-cadherin and occludin total levels were increased following Epo treatment; similar results have been described for other junction proteins after hypoxic/reoxygenation (Mark & Davis, 2002), primary stimuli leading to increase the levels of Epo. Recently it has been evidence the properties of Epo to up-regulate genes encoding for proteins involved in vascular functions, in human endothelial cells (Fodinger et al., 2000). Analysis of mRNA levels should clarify the mechanism through which rHuEpo regulates junction protein expression. The VEGF-induced decrease in junction protein staining was associated with a decreased in occludin and ZO-1 content, while it had no significant on VE-cadherin levels. The cotreatment was able to restore the expression of ZO-1 and occludin at control levels and maintain a small increase in the VE-cadherin levels.
In conclusion, this in vitro model that closely mimics the in vivo situation enables us to apprehend the cellular mechanism of Epo interaction with the BBB. Our results suggest that Epo acts through a specific transport across the BBB. Moreover circulating Epo protects BBB permeability of the microvascular endothelium by activating an EpoR-dependent intracellular signalling pathway. This would inhibit eNOS expression and stabilize junctions by regulating junction protein expression. Our data are in agreement with recent reports (Chong et al., 2002) pointing out Epo as a potential vascular protectant, and opening up new therapeutic approaches for cerebral vascular diseases. In the course of this work Epo administration has been already object of a clinical trial assessing the safety and efficacy of rHuEpo for the treatment of ischemic stroke in man (Ehrenreich et al., 2002).
Acknowledgements
We thank Dr C. de Castellarnau for helpful discussions, S. González and R. García for technical assistance, J. M. Avinyó for reagents and Robin Rycroft for the revision of the manuscript. O.M. is a postdoctoral fellow from the Ministerio de Educación, Cultura y Deporte. This study was supported by grants from the John Hopkins Center for Alternatives to Animal Testing, Programa de Apoyo de Capacitación Tecnológica de la Industría (PACTI; grant number COO1999-AX044) and CIDEM (grant number R + DCOOP01-10).
Abbreviations
-
- BBB
-
- blood–brain barrier
-
- BBEC
-
- bovine brain endothelial cells
-
- CNS
-
- central nervous system
-
- eNOS
-
- endothelial nitric oxide synthase
-
- Epo
-
- erythropoietin
-
- EpoR
-
- Epo receptor
-
- LDL
-
- low density lipoprotein
-
- Papp
-
- apparent permeability coefficient
-
- PBS
-
- phosphate-buffered saline
-
- rHuEpo
-
- recombinant human Epo
-
- TJ
-
- tight junctions
-
- VEGF
-
- vascular endothelial growth factor
-
- VEGFR
-
- VEGF receptor-2.