Selective modifications in GAD67 mRNA levels in striatonigral and striatopallidal pathways correlate to dopamine agonist priming in 6-hydroxydopamine-lesioned rats
Abstract
The present study investigated long-term alterations in striatal gene expression after single exposure of unilaterally 6-hydroxydopamine-lesioned rats to different dopamine agonists (priming). Rats were primed with the D1 agonist SKF38393 (10 mg/kg), the D2/D3 agonist quinpirole (0.2 mg/kg), the dopamine precursor L-DOPA (50 mg/kg) or with vehicle (drug-naive), and GAD67, dynorphin and enkephalin mRNAs were evaluated in the striatum by in situ hybridization, 3 days after priming. To evaluate GAD67 mRNA in striatonigral and striatopallidal neurons, identified as enkephalin (−) and (+) neurons, double-labelling in situ hybridization was used. Drug-naive lesioned rats showed an increase in GAD67 mRNA in enkephalin (−) and (+) neurons, an increase in enkephalin and a decrease in dynorphin mRNAs. Priming with either SKF38393 or quinpirole further increased GAD67 mRNA in enkephalin (−) and (+) neurons, however, while SKF38393 produced a high and unbalanced activation toward enkephalin (−) neurons, after quinpirole the increase was of low intensity and similar in the two pathways. Dynorphin mRNA was increased by SKF38393 but not by quinpirole, whereas enkephalin mRNA was not changed by either priming. L-DOPA produced a high and similar increase in GAD67 mRNA in enkephalin (−) and (+) neurons. Priming differentially affected peptides and GAD67 mRNA in striatopallidal and striatonigral neurons depending on the dopamine agonist used. The degree of enduring overactivity of the striatopallidal and striatonigral pathways may be related to the ability of L-DOPA and D1 or D2/D3 receptor agonists to prime motor behavioural responses and to produce dyskinetic side-effects.
Introduction
Unilateral lesion of nigrostriatal dopamine neurons with 6-hydroxydopamine (6-OHDA) in rats offers a useful model for studying drugs active on Parkinson's disease. This model has been widely used to study neuronal adaptive processes, which develop on striatal output neurons after single and repeated exposure to dopamine agonists (Engber et al., 1991; Cenci et al., 1998; Henry et al., 1999).
Striatal γ-aminobutyric acidergic (GABAergic) spiny-neurons are distinguished into two main populations: the striatonigral (direct) and striatopallidal (indirect) pathway. Direct pathway neurons contain dynorphin and substance P and mainly express the D1 dopamine receptor, whereas indirect pathway neurons contain enkephalin and mainly express the D2 receptor (Cuello et al., 1981; Vincent et al., 1982; Gerfen et al., 1990; Le Moine & Bloch, 1995). The majority of medium spiny-neurons contain two isoforms of GABA-synthesizing enzyme, glutamic acid decarboxylase, GAD67 and GAD65. GAD67, being responsible for most GABA synthesis, is involved in general metabolic activity, whereas GAD65 appears to be related to the synthesis of GABA for vesicular release (Gonzales et al., 1991; Mercugliano et al., 1992; Esclapez et al., 1994; Soghomonian & Martin, 1998).
6-OHDA lesion of the dopaminergic nigrostriatal pathway and dopamine agonists administration induce adaptive changes in GAD67, dynorphin and enkephalin mRNAs in the striatum ipsilateral to the dopamine neuron lesion (Young et al., 1986; Voorn et al., 1987; Gerfen et al., 1990; Li et al., 1990; Soghomonian et al., 1992), which have been correlated with motor impairment in Parkinson's disease and with motor side-effects after L-DOPA or dopamine agonists treatments.
In the unilateral 6-OHDA rat model of Parkinson's disease, a single exposure to a dopamine agonist is capable of enhancing turning behaviour induced by a challenge with a D2 agonist or with an otherwise ineffective dose of a D1 agonist, given days apart (Morelli & Di Chiara, 1987; Morelli et al., 1989). This model of behavioural sensitization, called ‘priming’, is heterologous as well as homologous, as both D1 or D2 agonists are able to increase the behavioural response to the subsequent dopamine agonist challenge. Neurochemical studies reported that acute administration of a dopamine agonist after priming increases the metabolic activity in the substantia nigra/entopeduncular nucleus (Morelli et al., 1993), whereas in the striatum dopamine agonist-induced cAMP production, DARPP-32 phosphorylation, dynorphin, c-fos and GAD67 mRNA were modified as compared with drug-naive rats (Barone et al., 1994; Pinna et al., 1997; Pollack et al., 1997; Van de Witte et al., 1998; Consolo et al., 1999). Priming is a time-dependent phenomenon (Morelli et al., 1989), absent at 6 h, maximal at 3 days and decreased at 10 days, suggesting that adaptive changes in striatal neurons might be responsible for the expression of the increased D1 and D2 turning behaviour response. Thus, priming is a useful model for studying adaptive changes, which develop after exposure to dopamine receptor agonists.
The purpose of the present study was to assess adaptive long-lasting changes in identified striatal neuronal populations, i.e. striatonigral and striatopallidal neurons, resulting from dopamine agonist exposure. GAD67, enkephalin and dynorphin mRNAs were measured after priming with L-DOPA, a D1 or a D2/D3 agonist given 3 days previously. Doses of SKF38393 and quinpirole were chosen based on their ability to elicit a contralateral turning of similar intensity. Single-cell analysis of striatal GAD67 mRNA was performed in enkephalin (+) and (−) neurons, which, as shown by previous studies (Gerfen et al., 1995), represent the indirect striatopallidal and the direct striatonigral pathways, respectively.
Materials and methods
Experimental procedures
6-OHDA lesion
In order to lesion the dopaminergic nigrostriatal pathway, 6-OHDA HCl (8 µg in 4 µL of 0.05% ascorbic acid in saline) was injected unilaterally in the medial forebrain bundle of chloral hydrate-anaesthetized rats, at coordinates A 2.2, L 1.5, V 7.9, according to the Atlas of Pellegrino et al. (1979). Rats were pretreated with 10 mg/kg (i.p.) of desipramine, to prevent damage to noradrenergic neurons. Rats were screened for the lesion by measuring the ipsilateral spontaneous turning behaviour during the dark period. Only rats showing at least 10 ipsilateral rotations in 3-min observation from day 3 to day 5 post-lesion were included in the experiments.
All experiments were carried out in accordance with the European Communities Council Directive (86/609/EEC).
Drug treatment
Fourteen days after 6-OHDA lesion, rats were randomly assigned to the experimental groups (N = 5 for each group) and placed in hemispherical bowls connected to automated rotameters. After 30 min of habituation, rats were injected either with benserazide (30 mg/kg i.p.) plus the dopamine precursor L-DOPA (50 mg/kg, i.p.), with the D1 agonist SKF38393 (10 mg/kg, i.p.), the D2/D3 agonist quinpirole (0.2 mg/kg s.c.), or with vehicle (drug-naive). Contralateral turning was measured by automated rotameters for 2 h following the drug injection.
Tissue section preparation
Three days after dopamine agonist priming, rats were killed with CO2, the brain was rapidly removed and frozen in dry ice. Coronal sections (12 µm) were cut on a cryostat, mounted on gelatin-coated slides and stored at −20 °C. Slides were then post-fixed in a 4% paraformaldehyde/0.9% NaCl solution, acetylated in 0.25% acetic anhydride/0.1 m triethanolamine/0.9% NaCl (pH 8), dehydrated in an ascending series of alcohol, delipidated in chloroform, rehydrated in a descending series of alcohol, air-dried and stored at −20 °C.
In situ hybridization
Antisense [35S]-labelled and digoxigenin-labelled riboprobes were generated by in vitro transcription from cDNA clones encoding for tyrosine hydroxylase (TH), GAD67, dynorphin and enkephalin. Plasmids were linearized with BamH 1 (TH), Sal1 (GAD67) and EcoR1 (dynorphin and enkephalin) restriction enzymes (Promega). In vitro transcription was performed using T7 (TH), T3 (GAD67) and SP6 (enkephalin and dynorphin) RNA polymerases (Promega), in the presence of [35S]UTP or [DIG]UTP (Roche Molecular Biochemicals). Buffer, 100 µL, containing 2 × 106 cpm of radioactive probe, and 3 µL of digoxigenin probe for double-labelling experiments were added to each slide. Hybridization was carried out at 55 °C overnight and slides were washed as described (Carta & Gerfen, 1999). For autoradiographic analysis, slides were air-dried and apposed to X-ray films. Double-labelled slides were processed for digoxigenin probe visualization (Carta & Gerfen, 1999). After washing, slides were incubated in Tris-buffered saline containing 3% normal goat serum, 0.3% Triton X-100 and rabbit IgG directed against digoxigenin coupled to alkaline phosphatase (1 : 1000) for 4 h. Sections were then rinsed 3 × 10 min in Tris-buffered saline and placed in a buffer (in mm: Tris HCl, 100; NaCl, 100; MgCl2, 50) containing nitroblue tetrazolium salt (0.34 mg/mL) and 5-bromo-4-chloro-3-indolyl-phosphate toluidinium salt (0.18 mg/mL) for 12 h. Thereafter, sections were rinsed in water and air-dried. Slides were then dipped in a photographic emulsion (LM-1 Amersham), dried and allowed to expose in the dark at −20 °C. After 3–4 weeks slides were developed (developer Kodak D-19), dehydrated and coverslipped.
Data analysis
Analysis of autoradiograms
Sections from middle striatal levels for each rat (9.2 mm anterior to the interaural line; Paxinos & Watson, 1998) were examined for mRNA evaluation.
To obtain the average density of autoradiogram grey values, quantitative analysis of labelling was performed using the image analysis program Scion Image. The average grey value from white matter was subtracted from striatal value to correct for background labelling.
For measurement of GAD67 mRNA the striatum was divided into four quadrants of the same size: dorsolateral, dorsomedial, ventrolateral and ventromedial. For dynorphin and enkephalin mRNA the total striatum was evaluated.
Analysis of double-labelling
In order to identify the neuronal population in which GAD67 mRNA changes occurred, striatal sections were double-labelled with a non-radioactive probe against enkephalin mRNA and a radioactive probe against GAD67 mRNA. Three rats from each experimental group were randomly selected for double-labelling. Three different double-labelling experiments were performed, one for each primer used. Silver grains over enkephalin (+) neurons (indirect, putative D2 pathway), identified by the alkaline phosphatase reaction-product, or enkephalin (−) neurons (direct putative D1 pathway) were counted separately. Adjacent images from the selected area were digitized at 400 × through a Zeiss microscope. Three-hundred–three-hundred and fifty neurons from the dorsolateral portion of each striatum were analysed. For each cell, grains were counted automatically with an image analyser (Zeiss, KS 300) inside an area of fixed size (diameter 35 µm). Cells containing different numbers of grains, grouped by bins of 2, were tabulated as a frequency distribution.
Statistics
For analysis of autoradiograms, results on mRNA levels obtained from 6-OHDA drug-naive and primed rats were expressed as a percentage of the unlesioned striatum of drug-naive 6-OHDA-lesioned rats. Data were analysed with a one-way analysis of variance followed by Tukey-honest (HSD) post-hoc test.
For double-labelling experiments, data from each group were expressed as a frequency distribution. Because data were not normally distributed, the median value was used and a non-parametric test was applied for statistical analysis. For each frequency distribution the median value was calculated as reported in Tables 1 and 2. Mann–Whitney U-test was used to compare frequency distribution of each treatment with the corresponding control group. Treatment with different primers could not be compared, as slides were processed separately.
| Median number of grains per cell | ||
|---|---|---|
| Unlesioned | 6-OHDA lesion | |
| ENK (−) | 25.33 ± 1.15 | 29.33 ± 0.67* |
| ENK (+) | 27.33 ± 0.67 | 42.00 ± 1.16* |
- Statistical significance was obtained by comparison of frequency distributions with the non-parametric Mann–Whitney test. *P < 0.001 vs. the same neuronal population in the unlesioned striatum.
| Median number of grains per cell after treatment with: | ||||
|---|---|---|---|---|
| 6-OHDA | SKF38393 | Quinpirole | L-DOPA | |
| ENK (−) | 29.33 ± 0.67 | 39.33 ± 2.4*** | – | – |
| ENK (+) | 42.00 ± 1.15 | 48.67 ± 1.77*** | – | – |
| ENK (−) | 18.67 ± 0.67 | – | 20.00 ± 0.51* | – |
| ENK (+) | 19.33 ± 1.33 | – | 21.33 ± 0.66** | – |
| ENK (−) | 34.00 ± 1.15 | – | – | 44.67 ± 0.67*** |
| ENK (+) | 51.33 ± 0.67 | – | – | 60.67 ± 0.65*** |
- Statistical significance was obtained by comparison of frequency distributions with the non-parametric Mann–Whitney test. ***P < 0.001; **P < 0.01; *P < 0.05, vs. the same neuronal population in the 6-OHDA striatum of drug-naive rats. Note that different primers could not be compared as slides were processed separately.
Drugs
6-OHDA–HCl, L-DOPA, desipramine and 1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol (SKF38393) were purchased from Sigma; quinpirole was purchased from Research Biochemicals International.
Results
The extent of 6-OHDA-induced lesion was assessed by TH mRNA in the substantia nigra pars compacta. Only rats showing a loss of TH mRNA (measured as optical density) above 98% in the 6-OHDA infused side as compared with the intact side were included in the analysis (data not shown).
Priming was elicited by administration of SKF38393 (10 mg/kg i.p.; total contralateral turns 276 ± 38.63), quinpirole (0.2 mg/kg s.c.; total contralateral turns 298.25 ± 15.21) or L-DOPA (50 mg/kg i.p.; total contralateral turns 576 ± 50.03). mRNA expression was evaluated 3 days after priming.
GAD67, dynorphin and enkephalin mRNAs expression
In drug-naive rats, GAD67 mRNA expression was increased in the 6-OHDA-lesioned striatum, as compared with the contralateral unlesioned striatum (Fig. 1). Analysis of the four striatal portions showed an increase in the dorsomedial, dorsolateral and ventrolateral striatum only (Fig. 1). 6-OHDA lesion altered striatal levels of both dynorphin and enkephalin mRNAs. Dynorphin mRNA was significantly decreased, whereas enkephalin mRNA was increased in the 6-OHDA-lesioned as compared with the intact contralateral striatum (Fig. 2).
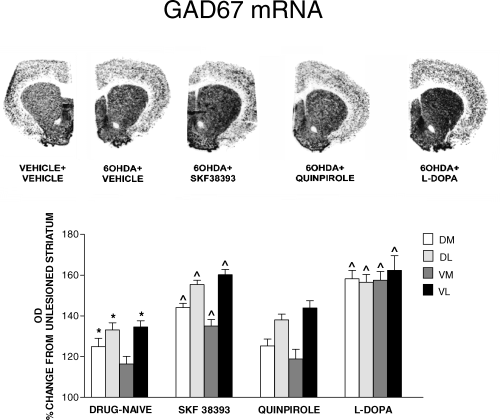
In situ hybridization film autoradiograms showing mRNA expression for GAD67 in the 6-OHDA-lesioned striatum of rats drug-naive or primed, 3 days previously, with SKF38393 (10 mg/kg i.p.), quinpirole (0.2 mg/kg s.c.) or L-DOPA (50 mg/kg i.p.). Histograms report quantification of GAD67 mRNA levels, measured as mean density values (mean ± SEM) and expressed as percentage of control (unlesioned contralateral striatum). GAD67 mRNA was measured in four striatal portions (DL, dorsolateral; DM, dorsomedial; VL, ventrolateral; VM, ventromedial.). *P < 0.05, drug-naive 6-OHDA-lesioned vs. unlesioned striatum. ^P < 0.05, vs. drug-naive 6-OHDA-lesioned striatum.
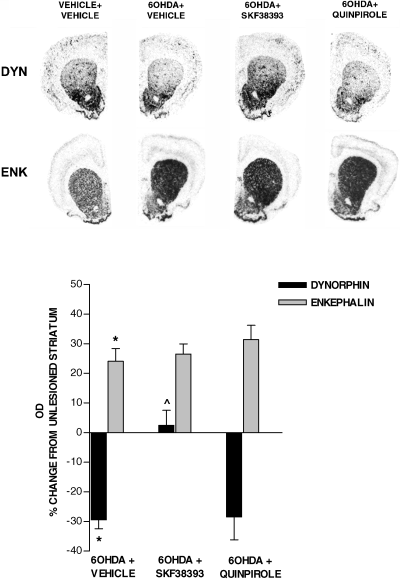
In situ hybridization film autoradiograms showing mRNA expression for dynorphin and enkephalin in the 6-OHDA-lesioned striatum of rats drug-naive or primed, 3 days previously, with SKF38393 (10 mg/kg i.p.) or quinpirole (0.2 mg/kg s.c.). Histograms report quantification of dynorphin and enkephalin mRNA levels, measured as mean density values (mean ± SEM), and expressed as percentage of control (unlesioned striatum). *P < 0.05 6-OHDA-lesioned vs. unlesioned striatum. ^P < 0.05 vs. drug-naive 6-OHDA-lesioned striatum.
Priming with the D1 agonist SKF38393 (10 mg/kg i.p.) and the dopamine precursor L-DOPA (50 mg/kg i.p.) induced an increase of GAD67 mRNA above 6-OHDA lesion levels in all striatal quadrants, whereas priming with quinpirole (0.2 mg/kg s.c.) did not alter GAD67 mRNA levels in the striatum (Fig. 1). SKF38393 (10 mg/kg i.p.) reversed dynorphin mRNA to a level similar to the intact striatum, whereas it did not produce any effect on enkephalin mRNA levels (Fig. 2). Priming with quinpirole (0.2 mg/kg s.c.) failed to alter striatal peptides mRNA levels (Fig. 2). Peptides mRNA levels were not modified in the unlesioned contralateral striatum by any treatment (data not shown).
Double-labelling experiments
An enkephalin mRNA probe was used to identify enkephalin (+) neurons in the striatum. About 50% of striatal neurons project to the globus pallidus and mainly express D2 receptors and enkephalin, whereas the other 50% project to the substantia nigra and mainly express the D1 receptor (Gerfen et al., 1990; Le Moine & Bloch, 1995). We therefore assumed that enkephalin (+) and (−) neurons represented the indirect striatopallidal and the direct striatonigral pathway, respectively.
Figure 3 shows neurons double-labelled for enkephalin (dark grey) and GAD67 (silver grains) mRNAs, from the contralateral unlesioned and 6-OHDA-lesioned striatum of drug-naive rats. In the unlesioned striatum, GAD67 mRNA labelling was present in both enkephalin (−) and enkephalin (+) neurons (Fig. 3 and Table 1). Non-parametric analysis of frequency distributions showed an increase in the number of silver grains per cell in the striatum ipsilateral to 6-OHDA lesion as compared with the contralateral unlesioned striatum, in both enkephalin (−) and enkephalin (+) cells (P < 0.001) (Fig. 3 and Table 1).
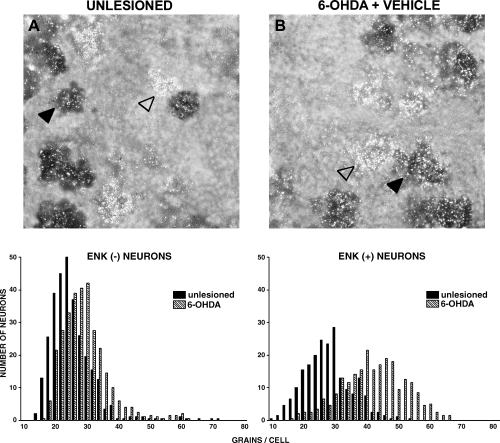
Photomicrographs showing neurons from (A) the unlesioned striatum, and from (B) the 6-OHDA-lesioned striatum of drug-naive rats. Sections were double-labelled with an 35S probe for GAD67 mRNA (silver grains) and a digoxigenin probe for enkephalin mRNA (dark cells). Examples of enkephalin (−) (empty arrow) and enkephalin (+) (filled arrow) neurons are shown. Lower panels show the frequency distribution of the amount of GAD67 mRNA (expressed as number of silver grains per neuron), in enkephalin (−) and enkephalin (+) neurons. For each neuronal population, histograms were compared with the non-parametric Mann–Whitney test. Results are reported in Table 1.
Priming with SKF38393 (10 mg/kg i.p.) significantly increased the number of grains/cell in the 6-OHDA-lesioned striatum as compared with the 6-OHDA-lesioned striatum of drug-naive rats (Fig. 4 and Table 2). The increase was observed in both enkephalin (−) neurons and in enkephalin (+) neurons (P < 0.001). Quinpirole (0.2 mg/kg s.c.) priming produced a slight but significant increase in GAD67 mRNA labelling in both enkephalin (−) (P < 0.05) and (+) neurons (P < 0.01) (Fig. 5 and Table 2). L-DOPA (50 mg/kg i.p.) produced a robust increase in the number of silver grains on both enkephalin (−) and enkephalin (+) neurons (P < 0.001) (Fig. 6 and Table 2).
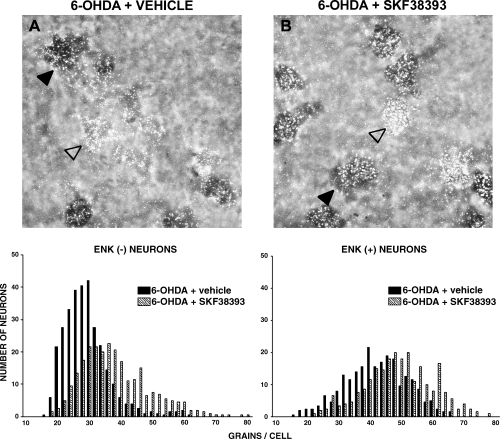
Photomicrographs showing neurons from the 6-OHDA-lesioned striatum of (A) rats drug-naive or (B) primed, 3 days previously, with SKF38393 (10 mg/kg i.p.). Sections were double-labelled with an 35S probe for GAD67 mRNA (silver grains) and a digoxigenin probe for enkephalin mRNA (dark cells). Examples of enkephalin (−) (empty arrow) and enkephalin (+) (filled arrow) neurons are shown. Lower panels show the frequency distribution of the amount of GAD67 mRNA (expressed as number of silver grains per neuron), in enkephalin (−) and enkephalin (+) neurons. For each neuronal population, histograms were compared with the non-parametric Mann–Whitney test. Results are reported in Table 2.
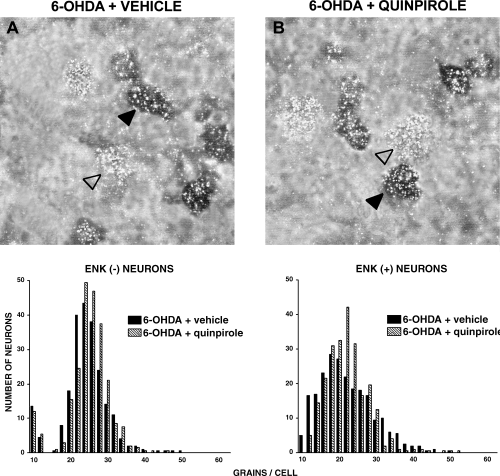
Photomicrographs showing neurons from the 6-OHDA-lesioned striatum of (A) rats drug-naive or (B) primed, 3 days previously, with quinpirole (0.2 mg/kg s.c.). Sections were double-labelled with an 35S probe for GAD67 mRNA (silver grains) and a digoxigenin probe for enkephalin mRNA (dark cells). Examples of enkephalin (−) (empty arrow) and enkephalin (+) (filled arrow) neurons are shown. Lower panels show the frequency distribution of the amount of GAD67 mRNA (expressed as number of silver grains per neuron), in enkephalin (−) and enkephalin (+) neurons. For each neuronal population, histograms were compared with the non-parametric Mann–Whitney test. Results are reported in Table 2.
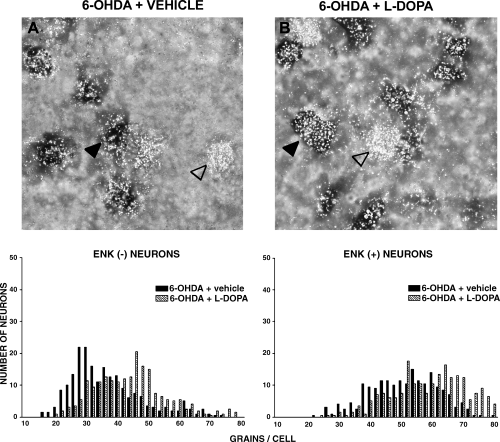
Photomicrographs showing neurons from the 6-OHDA-lesioned striatum of (A) rats drug-naive or (B) primed, 3 days previously, with (C) L-DOPA (50 mg/kg i.p.). Sections were double-labelled with an 35S probe for GAD67 mRNA (silver grains) and a digoxigenin probe for enkephalin mRNA (dark cells). Examples of enkephalin (+) (empty arrow) and enkephalin (−) (filled arrow) neurons are shown. Lower panels show the frequency distribution of the amount of GAD67 mRNA (expressed as number of silver grains per neurons), in enkephalin (−) and enkephalin (+) neurons. For each neuronal population, histograms were compared with the non-parametric Mann–Whitney test. Results are reported in Table 2.
Discussion
Previous studies reported that about 50% of striatal neurons project to the globus pallidus and express enkephalin, whereas the other 50% project to the substantia nigra (Gerfen et al., 1990; Le Moine & Bloch, 1995). Double-labelling of striatal enkephalin (+) and (−) neurons therefore provides a means to evaluate selective modifications in the indirect enkephalin (+) striatopallidal and the direct enkephalin (−) striatonigral pathway (Gerfen et al., 1995).
The present study shows that in 6-OHDA-lesioned rats, changes in GAD67 mRNA in selective striatal neurons, but not changes in dynorphin and enkephalin mRNA, correlate with long-term behavioural modifications that characterize priming with L-DOPA or selective D1 or D2/D3 dopamine agonists.
GAD67, enkephalin and dynorphin mRNA in drug-naive 6-OHDA-lesioned rats
Lesion of the nigrostriatal dopaminergic pathway with 6-OHDA, in accordance with previous results (Gerfen et al., 1990; Soghomonian et al., 1992), induced an increase in GAD67 and enkephalin mRNAs and a decrease in dynorphin mRNA in the striatum. Single-cell analysis showed that striatal GAD67 mRNA was increased in both enkephalin (−) and enkephalin (+) neurons. GAD67 mRNA expression is considered an index of GABAergic striatal efferent neuron activity (Litwak et al., 1990; Soghomonian & Martin, 1998), thus the present results suggest that the basal activity of both striatonigral and striatopallidal neurons rises following dopamine neuron denervation. In agreement with the present results, several studies suggest that striatal neuron activity increases following dopamine depletion by showing an increased electrical activity of GABAergic neurons (Calabresi et al., 1993) and an enhanced release of GABA (Tossman et al., 1986; Lindefors et al., 1989). The activation of both striatal efferent pathways does not correlate to the opposite influence of the 6-OHDA lesion on the enkephalin and dynorphin mRNAs, which are, respectively, increased and decreased. Evaluation of GAD67 mRNA levels therefore appears to correlate better than dynorphin and enkephalin mRNA levels with functional studies showing that the activity of GABA neurons in the striatum is increased by the 6-OHDA lesion.
Recent studies have shown long-term changes in excitatory striatal inputs after dopamine depletion, such as an increase in striatal glutamate release, mean percentage of asymmetrical synapses and in phosphorylation and level of glutamate receptor subunits (Oh et al., 1998; Meshul et al., 1999; Ganguly & Keefe, 2001; Jonkers et al., 2002). This hyperactive glutamate transmission might contribute to keep striatal neurons tonically overactive, therefore the enhanced basal activity observed in the present study might represent an adaptive response of GABAergic neurons resulting from an increased excitatory input to the striatum.
Classical basal ganglia organization scheme indicates that stimulation of the GABAergic direct striatonigral pathway, which mainly contains D1 receptors, inhibits substantia nigra efferent neuron activity. In contrast, stimulation of the GABAergic indirect striato-pallido-nigral pathway, which mainly contains D2 receptors, raises substantia nigra neuronal activity. An unbalanced activity of these striatal output neurons, which would cause an overactivation of the substantia nigra, is considered to be responsible for the motor impairment produced by dopamine depletion (DeLong, 1990; Chase & Oh, 2000). The present results support this hypothesis, as evaluation of frequency distributions of GAD67 mRNA-labelled neurons and their medians suggest that 6-OHDA lesion produced a large increase in GAD67 mRNA levels in striatopallidal neurons, whereas it only produced a slight increase in striatonigral neurons. Thus, dopamine depletion, although increasing basal activity of both striatal efferent pathways, brings them to a different, unbalanced activity, which favours an overstimulation of substantia nigra.
GAD67, enkephalin and dynorphin in primed 6-OHDA-lesioned rats
In the priming model, a single administration of a dopamine D1 or D2/D3 receptor agonist, or the dopamine precursor L-DOPA, results in homologous and heterologous potentiation in turning behaviour upon administration of a dopamine agonist, given days apart (Morelli & Di Chiara, 1987, Morelli et al., 1989).
In the present study, administration of either the D1 agonist SKF38393 or the D2/D3 agonists quinpirole or L-DOPA produced, 3 days after priming, an increase in GAD67 mRNA as compared with drug-naive 6-OHDA-lesioned rats, in both striatonigral and striatopallidal neurons.
The ability of a selective dopamine agonist to recruit both striatal efferent pathways might represent the mechanism underlying the heterologous nature of priming, as suggested by previous studies on c-fos induction (Pollack & Yates, 1999). Priming by bringing the basal activity of the direct (D1 receptor containing) pathway to a higher level might directly facilitate subsequent D1 receptor stimulation. Similarly, priming would indirectly facilitate subsequent D2 receptor stimulation. In fact, the sensitized behavioural response observed after a D2 agonist challenge might be related to the priming-dependent overactivation of the direct pathway, which is disclosed when D2 receptor stimulation removes the negative influence of the hyperactive indirect pathway.
Analysis of autoradiography showed that after SKF38393 and quinpirole priming, GAD67 mRNA was primarily increased in the dorsal and lateral striatal subregions. Cortical projections innervate the striatum in a topographic manner, the dorsal and lateral striatum being mostly innervated by motor cortical areas (Gerfen & Wilson, 1996). Thus, the pattern of GAD67 mRNA increase is in line with the motor behaviour sensitization observed in priming.
Priming with SKF38393 and L-DOPA produced intense activation of GAD67 mRNA above the lesion in enkephalin (−) and (+) neurons. In contrast, quinpirole priming, although it elicited a contralateral turning of similar intensity as compared with SKF38393, produced a low increase in GAD67 mRNA in both neuronal populations. The increase in GAD67 mRNA after quinpirole administration reached statistical significance only at the single-cell analysis. Silver grains counting in emulsion-dipped slides provides a more sensitive method as compared with autoradiography and may explain the discrepancy between the two experimental approaches. The ability of priming to induce long-term changes in striatal efferent neurons is therefore dependent on the type of dopamine receptor stimulated.
The evidence that different primers induce different degrees of activation of striatal neurons is in line with previous studies by our group (Morelli & Di Chiara, 1987), where D2-mediated turning in D2-primed rats was less intense than D1-mediated turning in D1-primed rats, indicating that D1 priming is more effective than D2 priming. The high activation of striatal neurons produced by the D1 agonist and L-DOPA priming, as compared with the low activation produced by D2 priming, might be an interesting correlate of the different ability of drugs selective for the D1 or D2 receptor to induce dyskinetic movements (Reichmann, 2000; Rascol et al., 2001; Calon et al., 2002). Accordingly, an excessive activation of striatonigral neurons has been correlated to the onset of dyskinetic movements produced by long-term L-DOPA treatment in humans (Engber et al., 1991; Cenci et al., 1998; Henry et al., 1999).
D1 and D2 receptors have opposite effects on striatal output neurons (Gerfen, 1992; Le Moine & Bloch, 1995). Therefore, a direct effect of receptor stimulation can neither explain GAD67 mRNA increase in striatal neurons nor the recruitment of both pathways found after priming with selective D1 or D2 dopamine agonists. It is likely that different, non-dopaminergic mechanisms might mediate GAD67 mRNA increase in striatal output neurons after priming. Glutamate transmission plays a pivotal role in striatal motor function (Di Chiara et al., 1994), and altered glutamatergic inputs induce neurochemical changes and modifications in levels of GAD67 mRNA in striatal neurons, suggesting that the expression of this mRNA is under glutamatergic control (Cenci & Bjorklund, 1993; Salin & Chesselet, 1993; Berretta et al., 1997; Van Bree et al., 1999; Giorgi et al., 2001). Moreover, it was reported that L-DOPA increases striatal glutamate transmission in terms of release and receptor functionality (Oh et al., 1998; Jonkers et al., 2002), and that D1 and D2 receptors regulate striatal glutamate release (Exposito et al., 1999). Thus, glutamate might represent a good candidate to explain the increase in GAD67 mRNA observed in the present study. Indirect activation of glutamate transmission by dopamine agonists during priming induction might keep glutamate receptors in an overactive state, which would render overactive both striatonigral and striatopallidal neurons after priming. In line with this hypothesis, blockade of NMDA receptors by MK801, which blocks the NMDA channel in an use-dependent way, prevents the development of priming (Morelli et al., 1996; Pollack & Haisley, 2001; Van de Witte et al., 2002).
Priming also affected peptide mRNAs in the striatum. Priming with SKF38393 modified dynorphin but not enkephalin mRNA, whereas quinpirole failed to modify either peptide mRNAs. These results, together with previous studies showing that L-DOPA priming affected striatal dynorphin mRNA but was unable to modify enkephalin mRNA (Van de Witte et al., 1998), suggest that peptide changes do not account for the homologous and heterologous origin of priming, as only SKF38393 and L-DOPA, but not quinpirole, modified mRNA for dynorphin and none of the treatments modified enkephalin mRNA.
As suggested by previous studies (Steiner & Gerfen, 1995; Van de Witte et al., 2000; Henry et al., 2001), alterations in dynorphin mRNA may be secondary to the altered behavioural response observed in sensitization phenomena and may not reflect the activity state of striatonigral neurons. Glutamate receptor activation is influenced by dynorphin levels, as shown by Caudle & Dubner (1998), who described that low concentrations of dynorphin stimulate NMDA receptor current, whereas high concentrations have inhibitory actions. Thus, decrease of dynorphin mRNA after 6-OHDA lesions may facilitate the action of glutamate, which would keep striatonigral GAD67 mRNA elevated. In contrast, the increase in dynorphin mRNA observed after priming with L-DOPA or a D1 agonist would tend to counteract the high activity of the striatonigral pathway (Van de Witte et al., 2000). Priming with quinpirole, in contrast, by producing low activation of the direct striatonigral pathway, would produce no compensatory modifications in dynorphin mRNA. Therefore, priming correlates with GAD67 mRNA changes, but not with dynorphin mRNA, which would rather be a marker of striatonigral neurons response to dopamine D1 or D1/D2 agonists.
In conclusion, the adaptive changes observed in GAD67 mRNA, but not in peptide mRNAs, in striatonigral and striatopallidal neurons after priming appear related to the sensitized homologous and heterologous behavioural long-term changes observed in this model of behavioural sensitization. Moreover, the degree of activation of the two striatal efferent pathways could be related to the ability of D1 and/or D2 receptor stimulation to prime motor behavioural responses and to produce dyskinetic side-effects.
Acknowledgement
We thank Dr C.R. Gerfen for the generous gift of the GAD67, dynorphin and enkephalin cDNAs.
Abbreviations
-
- 6-OHDA
-
- 6-hydroxydopamine
-
- GABA
-
- γ-aminobutyric acid
-
- GAD
-
- glutamic acid decarboxylase
-
- TH
-
- tyrosine hydroxylase.