BDNF sensitizes the response of lamina II neurons to high threshold primary afferent inputs
Abstract
Brain-derived neurotrophic factor (BDNF) is up-regulated and released in the dorsal horn following peripheral inflammation and has therefore been implicated in spinal mechanisms of sensitization. Despite these observations, the mechanisms associated with such a role for BDNF are not yet fully determined. Here, we investigate the effect of BDNF on dorsal root-evoked synaptic transmission in lamina II neurons. In a transverse spinal cord slice preparation from neonatal rats (P1–15), the whole cell patch-clamp technique was used to record from these neurons. Brief application of BDNF (50–200 ng/mL) facilitated the evoked synaptic currents; they remained enhanced even after BDNF was washed out. A significant minority of cells was minimally affected by BDNF and consistent with this, not all neurons in lamina II were immunoreactive for the tyrosine kinase (trk) B receptor. No facilitation was elicited when N-methyl-d-aspartate (NMDA) receptors were blocked with D-APV, when the postsynaptic NMDA receptors were selectively blocked with intracellular MK-801, or when postsynaptic neurons were loaded with BAPTA. Additionally, inhibiting phospholipase C (PLC) or protein kinase C (PKC) prior to BDNF application completely blocked facilitation. However, once synaptic current underwent BDNF-induced facilitation, the PKC inhibitors failed to reverse the effect, suggesting that PKC is needed for initiation, but not maintenance of BDNF-induced facilitation. These results demonstrate that BDNF functions at the spinal level to enhance synaptic efficacy in an NMDA receptor-dependent manner and requires the action of the PLC/PKC pathway. This action of BDNF may contribute to central sensitization and exaggerated pain states.
Introduction
The spinal cord dorsal horn processes sensory information entering the central nervous system (CNS). The substantia gelatinosa (SG; lamina II) is specifically implicated in pain processing, as nociceptive afferents (C fibers) terminate primarily in this region (Willis & Coggeshall, 1991). Many substances including the nociceptive neuropeptides substance P and calcitonin gene related peptide (CGRP) are present in these afferent terminals and contribute to the central sensitization that is an important component of nociceptive processing (Millan, 1999;Woolf & Salter, 2000).
The neurotrophin BDNF is up-regulated in small trkA-expressing sensory neurons after peripheral inflammation induced by nerve growth factor (NGF) or intraplantar complete Freund's adjuvant (Apfel et al., 1996; Michael et al., 1997). It has been suggested to function as a neuromodulator of synaptic transmission (Michael et al., 1997; Kerr et al., 1999) and spinal nociception (Thompson et al., 1999; Bennett, 2001). BDNF is released into the dorsal horn following electrical stimulation or capsaicin activation of these fibers (Lever et al., 2001) and binds to trkB receptors, which are present on spinal neurons throughout the superficial dorsal horn (Bradbury et al., 1998).
Although there is considerable evidence indicating an important role for BDNF/trkB in persistent pain in various injury models (Kerr et al., 1999; Mannion et al., 1999), the evidence that BDNF facilitates transmission from unmyelinated afferent fibers to their target neurons in lamina II of the spinal cord is only circumstantial at present. Furthermore, a recent report suggests the possibility of an inhibitory effect of BDNF on transmission into the dorsal horn via reduction in the release of substance P from small diameter sensory afferents (Pezet et al., 2002). In other CNS regions, especially the hippocampus, activity-dependent release of BDNF is linked to long-term potentiation (LTP) (e.g. Kang & Schuman, 1995; Canossa et al., 1997; Gottschalk et al., 1998; Gooney & Lynch, 2001; Ying et al., 2002). A recent study in neonatal rats showed that BDNF elicits an initial facilitation and subsequent prolonged inhibition of monosynaptic transmission to motoneurons (Arvanian & Mendell, 2001). However, these actions of BDNF are restricted to the first postnatal week.
In view of these incomplete and potentially conflicting data on the effects of BDNF, and because SG neurons are among the first to receive and process incoming nociceptive information before its transfer to supraspinal centres, we have undertaken to document the action of BDNF on the monosynaptic response of lamina II neurons to their segmental input. It is also important to determine whether these effects are restricted to the initial postnatal week as in motoneurons. To answer these questions, we used a transverse slice preparation to record synaptic responses evoked in SG neurons following electrical stimulation of afferent fibers in the dorsal root. The results suggest that BDNF generally facilitates these responses via postsynaptic NMDA receptor and protein kinase C- (PKC-) dependent mechanisms and that these effects persist well beyond the first postnatal week.
These results have been reported in abstract form (Garraway et al., 2001; Garraway & Mendell, 2002).
Materials and methods
Preparation of spinal cord slices
All experimental procedures were approved by the Institutional Animal Care and Use Committee at SUNY, Stony Brook. Neonatal rats (Sprague–Dawley postnatal days 1–15) were used. Animals between P3 and P9 were anaesthetized with halothane, while those older than P9 were anaesthetized with 10% urethane (2 g/kg body weight i.p.). All animals were decapitated and spinal segments L1–L6 were quickly removed. The isolated spinal cord was embedded in Agar, 2.5% w/v (Type E, Sigma) and sliced on a motorized advance vibroslice (Campden Instruments) in 500–600 µm transverse sections. Short dorsal rootlets remained attached to the spinal segments to allow for electrical stimulation of primary afferents. For animals younger than P10, cooled (≈4 °C) normal artificial cerebrospinal fluid (ACSF) containing (in mm): NaCl, 125; KCl, 2.5; CaCl2, 2; MgCl2, 1; glucose, 11; NaH2PO4, 1.25; NaHCO3, 26; at a pH of 7.3 and oxygenated with 95%O2 : 5%CO2 was used, while in older animals oxygenated high sucrose-containing ACSF containing (in mm): sucrose, 259; KCl, 2.5; glucose, 11; NaH2PO4, 1.25; NaHCO3, 26; at a pH of 7.3 was used.
Electrophysiology
Slices were incubated at 32 °C for at least 1 h in normal ACSF. Spinal cord slices were then transferred and affixed to a recording chamber using platinum ‘U’ frames across which was glued a parallel array of nylon fibers (Edwards et al., 1989). The chamber was maintained at room temperature (approximately 20 °C). Patch electrodes were prepared from 1.5 mm outer diameter capillary tubes (World Precision Instruments or A-M systems) pulled in a two-stage process (Narishige PC10) producing resistance values ranging from 6 to 10 MΩ and filled with recording solution containing (in mm): K-gluconate, 135; CaCl2, 1; MgCl2, 2; EGTA, 5; HEPES, 5; phosphocreatine, 5; Mg-ATP, 4; GTP, 1; pH 7.3. In some cases, QX-314 (2–4 mm) was added to the recording solution to block fast sodium currents. The recording chamber was continuously superfused with oxygenated normal ACSF at a flow rate of approximately 1 mL/minute. All chemicals for ACSF and intracellular solution were obtained from Sigma (St Louis, MO). The whole-cell ‘blind’ patch clamp recording technique (Blanton et al., 1989) was carried out using the Axopatch 200B amplifier (Axon Instruments) filtered at 5 kHz (low-pass Bessel). Voltage and current clamp data were acquired using pCLAMP acquisition software (v 8.1; Axon Instruments). Immediately following breaking into the cell to achieve the whole cell configuration in voltage clamp mode (at a holding potential of −80 mV), the resting membrane potential of the cell was determined. For the duration of the experiment, resting membrane potential and holding current were carefully monitored. Large changes, reflecting a decline in the health of the neuron, led to termination of the experiment.
Primary afferent stimulation
The attached dorsal roots were electrically stimulated using suction electrodes to evoke submaximal synaptic currents in the recorded SG neurons. To recruit C fibers, which terminate primarily in the SG, high intensity stimulation (refer to Thompson et al., 1990) was used to evoke synaptic responses in these neurons which were voltage clamped at −60 mV. In some neurons, the holding potential was changed to determine the reversal potential and hence the nature of the evoked synaptic responses. In most cases the responses were mainly excitatory, but in a few cases the early excitatory response was followed by a late occurring inhibitory component. The responses investigated here were excitatory postsynaptic currents (EPSC).
To further classify these excitatory currents, in some neurons (n = 5) we used the glutamate receptor antagonists 6-cyano-7-nitroquinoxaline (CNQX) 10 µm and 2-amino-5-phosphovaleric acid (D-APV) (40 µm) (Sigma-RBI, St Louis, MO). Primary afferent-evoked synaptic responses in SG neurons are primarily glutamatergic consisting of both early and late components mediated by α-amino-3-hydroxy-5-methylisoxasole-4-propionic acid (AMPA)/kainate (ka) and NMDA receptors (e.g. Yoshimura & Jessell, 1990), which are blocked by CNQX and D-APV, respectively.
In an additional seven neurons we investigated the effect of BDNF on synaptically evoked NMDA currents. In these experiments the NMDA-receptor mediated synaptic current was first isolated by adding a ‘cocktail’ consisting of CNQX (10 µm), bicuculline (5 µm) (Tocris Laboratories, Ballwin, MO, USA), CGP 35348 (10 µm) (Ciba Geigy, Basel), and strychnine (5 µm) (Sigma-RBI, St Louis, MO) to the superfusate. This blocked the AMPA/ka, GABAA, GABAB and glycine receptors, respectively, leaving a current that could be completely blocked by application of the NMDA receptor blocker D-APV.
Application of neurotrophin
A baseline of evoked EPSCs was collected at low stimulus frequencies (usually one per minute) for a period of approximately 10–12 min. BDNF (generously provided by Regeneron Pharmaceuticals, Tarrytown, NY) was added to the superfusate at 50, 100 or 200 ng/mL and applied for approximately 10, 20, 25 or 30 min. BDNF was then washed out, while synaptic currents were continually evoked for at least 20 min and in some cases up to 60 min. In some neurons, the effect of 200 ng/mL neurotrophin-3 (NT-3) (Regeneron Pharmaceuticals) on the evoked responses was also studied.
Analysis
Recordings were analysed off line using pCLAMP (v 8.1, Axon Instruments). The mean control value was calculated for responses before the test agents were applied. Individual synaptic responses (maximum amplitude of the monosynaptic component) plotted in 2-6 were normalized to this mean value denoted as 100%. The mean percent change in synaptic amplitude after a particular treatment (e.g. Fig. 2C) was calculated as the difference between the mean peak amplitude during drug application (the first three traces after drug application were not included) and the mean value of the synaptic responses before drug (control) [percentage change = 100 × (DrugAvg − ControlAvg)/ControlAvg)]. For the NMDA induced current (Fig. 4A and B), the amplitude of the evoked current was measured as the difference between the maximum inward current induced following NMDA application and the baseline current just prior to NMDA application. Unless otherwise stated, all per cent changes in synaptic amplitude are reported as mean ± SEM. All statistical analysis was carried out using Sigma Stat software (SPSS Inc).
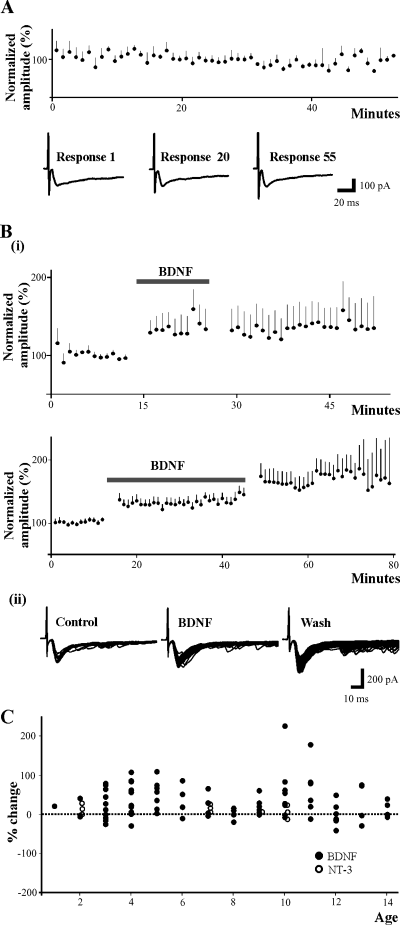
BDNF induces synaptic facilitation in most lamina II neurons. (A) EPSC amplitude plotted vs. time (minutes) in controls. In the absence of BDNF, synaptic responses evoked at low frequency stimulation (1/min) remain stationary for the duration of the recording (approximately one hour). Representative traces taken at times 1, 20 and 55 min are shown for one cell. (Bi) In the presence of BDNF, EPSCs evoked at low frequency undergo a significant 30% increase in amplitudes compared to control values. BDNF-induced facilitation is observed in neurons following at least 10 min (n = 10) and up to 30 min (n = 20) application of BDNF (thick horizontal line). The facilitation is not reversed during wash of BDNF irrespective of whether it was applied for 10 min or 30 min, but is maintained for the duration of the recording, generally 30 min and up to one hour after BDNF superfusion is ended. (Bii) An example showing the evoked synaptic responses of an individual neuron during control, BDNF and wash. Individual traces collected every minute are superimposed. There is an additional increase in EPSC amplitude during wash. (C) Graph showing percentage change in EPSC amplitude during BDNF or NT-3 application vs. age of the animal (days). BDNF-evoked synaptic facilitation does not depend on the age of the animal, but occurs in most neurons regardless of whether they were obtained from 1-week-old (n = 40) or 2-week-old (n = 42) animals. Note the modest effects of NT-3 by comparison with BDNF.
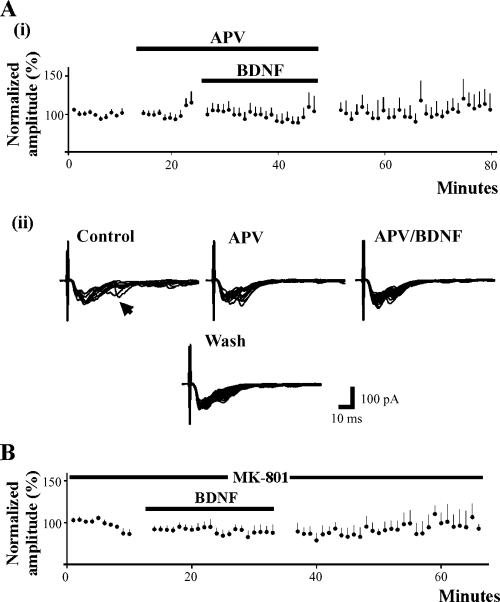
NMDA receptor antagonists block BDNF-induced facilitation. (Ai) Bath application of APV (10 min) prior to and during BDNF application blocks the expression of synaptic facilitation (n = 14). (Aii) An example of a cell tested with D-APV is shown. In the presence of D-APV, the later component of the EPSC (occurring at approximately 25 ms; arrow head) is blocked although the peak of the response is unchanged, demonstrating that the NMDA receptor dependent component is blocked while the AMPA/ka receptor-mediated component is unchanged. (B) Selective blockage of the postsynaptic NMDA receptor with MK-801 in the patch electrode prevents BDNF-induced facilitation (n = 14), suggesting that postsynaptic NMDA receptors are required for BDNF-induced facilitation.
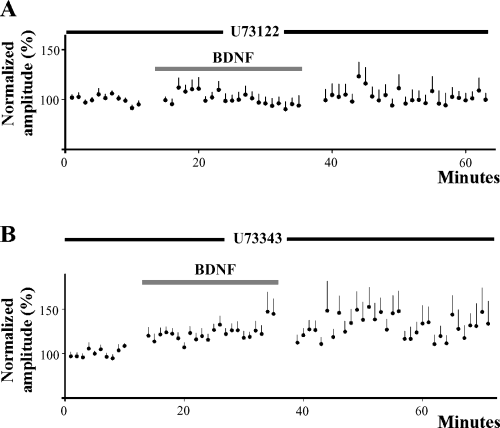
Phospholipase C is required for BDNF-induced facilitation. (A) Inhibition of the phospholipase C pathway in the postsynaptic neuron with U73122 prevents facilitation of synaptic currents during BDNF application (n = 12). EPSC amplitude remains unchanged during wash. (B) BDNF induces synaptic facilitation (24%, n = 18) when the analogue U73343 is incorporated into the patch electrode. Synaptic facilitation is not reversed during wash and is produced in neurons obtained from either 1- or 2-week-old animals.
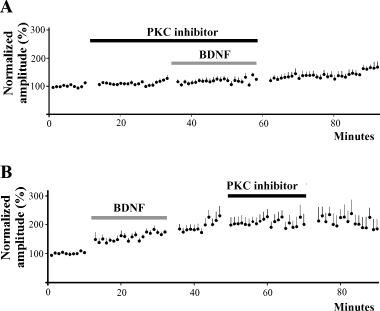
Protein kinase C plays a role in mediating BDNF facilitation. (A) Bath application of PKC inhibitors bisindolylmaleimide-1 or chelerythrine chloride for 20 min prior to and during application of BDNF (20 min) prevents EPSC facilitation (n = 15). There is a small increase in EPSC amplitude during wash. (B) The PKC inhibitors fail to reverse established BDNF-induced facilitation (n = 11). EPSC amplitudes remain elevated during and after 20 min application of bisindolylmaleimide-1 or chelerythrine chloride.
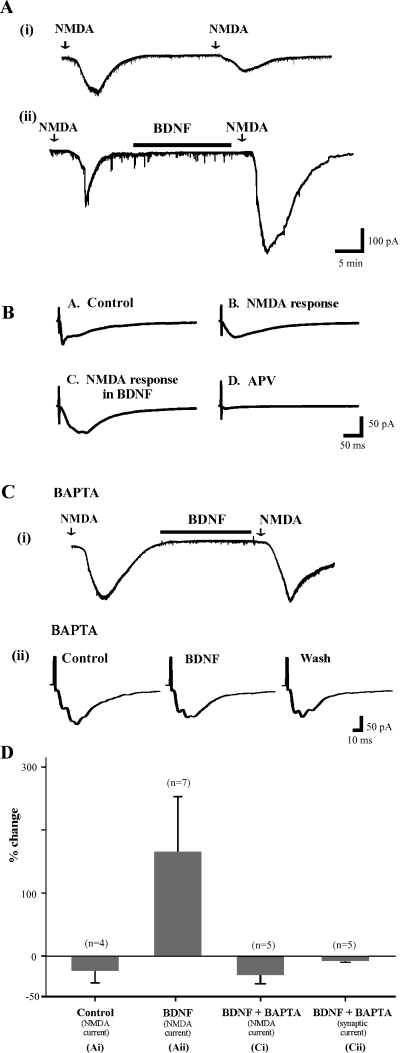
BDNF facilitates NMDA receptor mediated currents. (A) Currents evoked in lamina II cell by exogenous NMDA in the presence of TTX to block synaptic activation of the recorded cell (see Materials and methods). Cells clamped at −60 mV. (Ai) A second superfusion of NMDA ≈30 min after the first results in a smaller response. (Aii) If BDNF is superfused in the interval between the two NMDA stimuli, the second response is facilitated and prolonged. (B) BDNF facilitates pharmacologically isolated NMDA-receptor mediated synaptic current. Both the amplitude and duration of the response are increased by BDNF. (Ci) Inclusion of BAPTA in the patch pipette prevents facilitation of the response to exogenous NMDA by BDNF. (Cii) BAPTA in the patch pipette also prevents BDNF-induced facilitation of synaptically evoked AMPA/ka currents. (D) Histograms summarizing the results of experiments displayed in A and C.
TrkB immunohistochemistry
For immunohistochemical examination of the distribution of trkB in the superficial spinal cord, neonatal rats (P3, n = 2; P12, n = 2) were deeply anaesthetized with halothane and perfused transcardially with heparinized phosphate buffered saline (PBS), followed by 4% paraformaldehyde in PBS. Lumbar spinal cord was removed and placed in 4% paraformaldehyde for 1–2 h. It was then transferred to 30% sucrose in PBS and stored at 4 °C. The tissue was then mounted in frozen tissue mounting medium and cryostat sectioned at 16–20 µm. Sections were thaw-mounted onto gelatin/chrom-alum and poly l-lysine substrated slides. Sections were incubated in 1 : 30 normal goat serum in PBS with 0.4% Triton X-100 (GS-PBS-T) for 1 h to block nonspecific antibody binding. Sections were then incubated overnight in 1 : 2000 rabbit anti-trkB (full-length and truncated; BD Transduction Laboratories; #T14930). The following day the sections were rinsed multiple times with 1% GS-PBS-T, followed by 3 h in 1 : 100 goat anti-rabbit-AlexaFluor 594 (Molecular Probes). The sections were then incubated overnight in 1 : 1500 mouse anti-NeuN (Chemicon), a monoclonal antibody that specifically labels neuronal nuclei (Mullen et al., 1992). Procedures the following day were similar to those for anti-trkB. The goat anti-mouse-AlexaFluor 488 (Molecular Probes) antiserum was preadsorbed against normal rat serum prior to incubation with tissue sections. After final rinses in 1% GS-PBS-T, sections were covered with a glycerol-based mounting medium with p-phenylenediamine to prevent fading, and coverslipped. Control sections incubated without primary antisera showed no signal. Sections were viewed with a Zeiss Axioskop microscope equipped with epifluorescence illumination through fluorophore-specific filters (Omega Optical). Images were captured with a Spot RT digital camera using ImagePro Plus software (Media Cybernetics) running on a PC.
Results
The substantia gelatinosa was identified in transverse sections as a semi-translucent band across the superficial dorsal horn under the light microscope. The patch electrode was visually placed in this region and advanced blindly to record from SG neurons. A total of 197 neurons were recorded under different conditions. An average membrane potential of −55 ± 0.9 mV was estimated for 185 of these neurons after adjusting for the liquid junction potential.
Characterization of evoked responses
High intensity electrical stimulation was required to evoke synaptic responses in these neurons by comparison with the activation of ventral root potentials known to be elicited by large diameter afferents (Fig. 1A). This confirmed that small diameter fibers activate the synaptic responses in SG neurons. The amplitude of the synaptic current evoked in SG neurons was dependent on both stimulation intensity and duration (Fig. 1A). Consistent with previous studies utilizing similar methods, the primary afferent-evoked responses are for the most part glutamatergic (Yoshimura & Jessell, 1990; Yoshimura & Nishi, 1992). They were blocked to a great extent by CNQX, suggesting that EPSCs were mediated primarily by AMPA/ka glutamate receptors (Fig. 1C). D-APV decreased the duration of the synaptic responses of some neurons, suggesting that there is an NMDA receptor-mediated component in some of the evoked EPSCs (Fig. 3, Aii, arrow head; also see Fig. 4B).
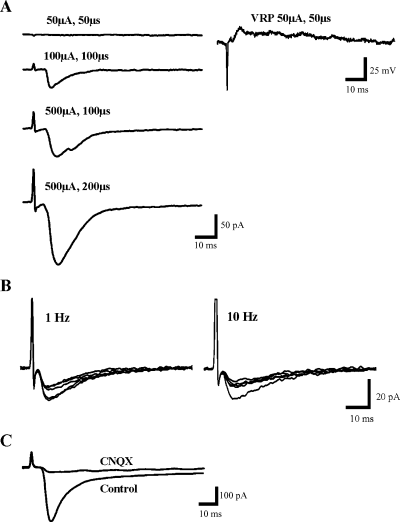
Characteristics of control synaptic responses. (A) High intensity electrical stimulation is needed to evoke synaptic responses in SG neurons (left column). In contrast ventral root potentials (right column) can be evoked by low intensity electrical stimulation (50 µA, 50 µs). The size of the dorsal root-evoked EPSC in SG neurons is dependent on both stimulation intensity and duration, becoming larger with increases of both. (B) Increasing stimulus frequency from the usual 1/min to 1 Hz and 10 Hz leads to progressive decline of the EPSC amplitude but no change in latency or shape. (C) Evoked EPSC is largely blocked by CNQX indicating that AMPA/ka receptors mediate most of this response.
The latency of these synaptic responses was typically approximately 5 ms and fluctuated only minimally over successive stimulus presentations (1, 2, 3). Raising the stimulus frequency resulted in a progressive decline in the amplitude of the response with little change in latency or shape (Fig. 1B). There was no hint that short latency components disappeared and reappeared on later trials that would suggest that they were polysynaptic. Thus we conclude that the responses had a monosynaptic component in agreement with the well known projection of small diameter afferent fibers into lamina II (Willis & Coggeshall, 1991).
Effects of BDNF
In the absence of BDNF, the amplitude of evoked synaptic currents was stable for the duration of recording, lasting from 30 to 60 min (n = 6) (Fig. 2A). After administration of BDNF, the monosynaptic component of the EPSC generally exhibited an immediate jump in amplitude averaging approximately 30% followed by a gradual increase to approximately 40% above control levels after approximately 25 min (P < 0.001, Signed Rank test). The immediate increase in synaptic amplitude was observed for all four durations of BDNF application. Figure 2 (Bi) shows the changes in synaptic amplitude induced in neurons following 10 min (n = 10) and 30 min (n = 20) of BDNF application, although similar results were noted with BDNF application of 20 min (n = 26) and ≈25 min (n = 26) (not shown). Also, the facilitatory effect was similar for all three concentrations of BDNF used. When BDNF was then washed out, the mean EPSC amplitude remained constant in amplitude (Fig. 2, Bi). However, there was larger apparent variability at later times due to a decrease in the number of cells in the population, as some were lost after one hour. In some cases, facilitation continued to increase during wash. Despite the overall average increase in EPSC amplitude in the population of 82 neurons tested, BDNF had variable effects with 20 cells exhibiting a small decrease in EPSC (mean = −13 ± 2%) while the majority (n = 61) exhibited a larger increase (mean = 46 ± 5%). One cell exhibited no change in amplitude. BDNF itself did not induce any inward current in any of the neurons tested.
Previously, NT-3 was found to evoke long-term potentiation-like facilitation of monosynaptic EPSPs in spinal motoneurons from rats 1–5 days old, but not in rats older than 7 days (Arvanov et al., 2000). Similarly, the actions of BDNF on synaptic transmission in motoneurons were observed only in animals < 1-week-old (Arvanian & Mendell, 2001). In the present experiments, BDNF produced facilitation in cells from 1- to 2-week-old-animals that was similar to that observed in cells from 0 to 1-week-old-animals [average peak change of 34 ± 6% in 40 neurons (P1–7 days) and 29 ± 8% in 42 neurons (P8–14)] (Fig. 2C; closed circles), suggesting that the effect of BDNF on synaptic responses in SG neurons is not dependent on the age of the animal, at least during the first 2 weeks of postnatal development. In both age groups, the increase in synaptic responses was statistically significant (P < 0.01). This graph also demonstrates that by comparison, NT-3 had at most very modest effects on EPSC amplitude (open circles).
Effect of NMDA receptor antagonists and agonist
Because the NMDA receptor is implicated in, and plays several critical roles in various forms of plasticity such as windup, long-term potentiation and central sensitization (reviewed in Dickenson et al., 1997; Millan, 1999; Ali & Salter, 2001; also see South et al., 2003), we next investigated whether the NMDA receptor is involved in mediating BDNF-induced facilitation. To understand its role, several types of experiments were conducted. First, we studied whether the receptor plays a role in initiating BDNF-induced synaptic facilitation. In these experiments, we first applied 40 µm D-APV following baseline recording for approximately 7–10 min and then added BDNF in the continued presence of D-APV. Although D-APV blocked long latency components (25 ms) of the EPSC, it had no effect on the monosynaptic component of the response (Fig. 3, Aii). In a total of 14 neurons tested in the presence of D-APV, BDNF induced no significant change (1 ± 5%; P > 0.1) in the amplitude of the evoked monosynaptic responses (Fig. 3, Ai), nor was there any synaptic facilitation during wash. This finding suggests that global blockage of the NMDA receptor prevented BDNF-evoked synaptic facilitation in SG neurons.
We next investigated whether the NMDA receptors involved in the modulatory effects of BDNF are postsynaptic. In these experiments, MK-801 (5 µm; Sigma-RBI, St Louis, MO) an open channel blocker selective for the NMDA receptor, was incorporated into the recording solution, thereby blocking the NMDA receptors in the recorded neuron (e.g. Berretta & Jones, 1996; Arvanov et al., 1999; Arvanian & Mendell, 2001). Under these conditions, we found that the evoked monosynaptic response was not facilitated but rather underwent a small nonsignificant 7% decrease in the presence of BDNF (n = 14). This finding suggests that NMDA receptors in the recorded lamina II neuron are required for BDNF-induced synaptic facilitation in these neurons (Fig. 3B).
Thirdly, to further substantiate that postsynaptic NMDA receptors are involved in BDNF-induced facilitation, we studied the effect of BDNF on the postsynaptic inward current induced by NMDA. In these experiments, 50 µm of NMDA was applied in the presence of 0.5 or 1.0 µm tetrodotoxin (TTX; used to block all presynaptic input to the recorded SG neurons). NMDA induced inward current in all neurons tested. In seven neurons, BDNF (in the presence of TTX) was applied for 15–20 min following the recovery from the NMDA-induced depolarization. NMDA was then applied for a second time for 1 min at approximately 30–40 min after the first application. The second application of NMDA produced a much larger inward current following BDNF application (163 ± 89%; Fig. 4, Aii and D). In four additional neurons without BDNF superfusion there was no facilitation of the second NMDA induced current (Fig. 4, Ai and D), suggesting that BDNF is responsible for the facilitation of NMDA induced inward current evoked in postsynaptic SG neurons.
To confirm that the effect of BDNF was exerted on synaptic NMDA receptors we then looked at the effects of BDNF on synaptic currents mediated by the NMDA receptor (see Materials and methods). In five of seven neurons tested, BDNF applied for 20 min produced an increase and/or prolongation of the evoked synaptic current (Fig. 4B). This potentiated synaptic current was blocked virtually completely by D-APV, the NMDA receptor antagonist. In two of nine cells application of the cocktail completely blocked the evoked synaptic response suggesting that NMDA receptors do not mediate the EPSC in all lamina II neurons, at least at −60 mV.
Intracellular calcium
Because NMDA receptors are highly permeable to calcium (Ca2+), we next investigated the involvement of intracellular Ca2+ in mediating BDNF-induced facilitation. In ten neurons, the calcium chelator BAPTA-K4+ (20 mm; Sigma-RBI, St Louis, MO) was incorporated into the recording electrode, thus loading the postsynaptic SG neuron with BAPTA. In five cells the effect of BDNF on the NMDA-induced inward current was studied, while in the other five, the same procedure was used to investigate the effect of BDNF on the evoked EPSC. With BAPTA in the electrode, neither the NMDA-induced current (average change: −29 ± 14%) nor the amplitude of the EPSC (average change: −7 ± 3%) was facilitated by BDNF (Fig. 4, Ci, Cii and D). This finding suggests that an increase in intracellular Ca2+ is necessary for BDNF-induced facilitation of glutamate-evoked transmission in SG neurons.
Effect of U73122, a phospholipase C inhibitor
Neurotrophins are known to exert their actions by activating various intracellular signalling mechanisms, including the phospholipase C (PLC) pathway (reviewed in Patapoutian & Reichardt, 2001). We therefore investigated whether PLC is involved in mediating the facilitatory actions of BDNF. In these experiments, the PLC inhibitor, U73122 (5 µm; Calbiochem, San Diego, CA), was incorporated into the recording solution. A total of 12 cells were tested and in these neurons BDNF produced no change in synaptic amplitude (1 ± 5%) (Fig. 5A). In contrast, when U73343 (Calbiochem, San Diego, CA), an analogue of U73122 with no inhibitory capability, was added to the recording solution at the same concentration, significant synaptic facilitation was evoked by BDNF (24 ± 6%; n = 18; P < 0.01) (Fig. 5B). As in experiments with BDNF alone, the facilitation was not reversed during wash. These findings suggest that the postsynaptic PLC signalling pathway plays a role in synaptic facilitation evoked by BDNF.
Effect of protein kinase C inhibitors
Because protein kinase C (PKC) is a downstream effector of PLC, we investigated whether it plays a role in the actions of BDNF. The membrane-permeable PKC inhibitors bisindolylmaleimide-1 (30 nm; Calbiochem) or chelerythrine chloride (1 µm; Calbiochem, San Diego, CA) were bath applied for at least 10 min (usually 20 min) following collection of control synaptic responses. Neither had a significant effect on the amplitude of the synaptic currents (Fig. 6A). This was followed by application of BDNF for 20 min during the continued presence of the PKC inhibitor. In 15 cells tested, BDNF failed to produce synaptic facilitation, causing an average change in synaptic amplitude of 2 ± 4% (P > 0.1) (Fig. 6A). In a second set of experiments, we first added BDNF to evoke synaptic facilitation, before adding the PKC inhibitor. The 11 neurons studied underwent synaptic facilitation of 57 ± 12% in the presence of BDNF. We allowed a 10-minute wash period during which time the synaptic responses continued to increase, before adding bisindolylmaleimide-1 or chelerythrine chloride for 20–25 min. In the presence of the PKC inhibitor, the synaptic facilitation was not reversed. There was a steady increase in synaptic amplitude during and following BDNF application despite the presence of the PKC inhibitor (Fig. 6B). Thus, it appears that inhibiting PKC activity can suppress the ability of BDNF to facilitate responses, although it does not affect synapses that have already undergone BDNF-induced facilitation.
Localization of trkB in dorsal horn
BDNF did not facilitate EPSCs in all cells examined (Fig. 2C). In order to determine whether this result might be due to a lack of expression of trkB in some cells, we examined the localization of trkB protein in the spinal cord. TrkB was found in many cells of the SG and lamina I. These cells were shown to be neurons by their expression of the NeuN antigen (Mullen et al., 1992). However, it was also very clear that an appreciable number of neurons lacked trkB immunoreactivity (Fig. 7).
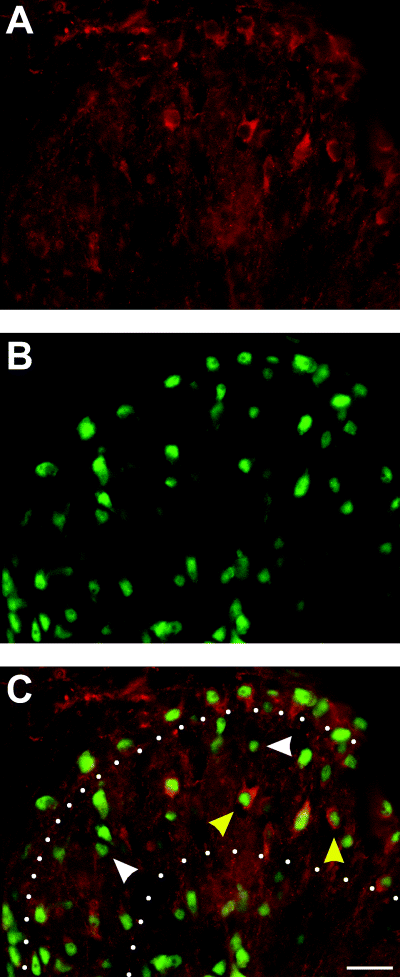
TrkB positive neurons present in lamina II. P12 Spinal cord dorsal horn labelled for trkB (red; A and C) and NeuN (green; B and C), a specific marker of neuronal nuclei. Yellow arrows in C indicate neurons expressing trkB, white arrows indicate neurons lacking trkB. White dots in C demarcate lamina II. Dorsal is at the top, and lateral is to the left. Note that only some neurons in both lamina I and II express trkB. Calibration bar, 20 µm.
Discussion
The present study was prompted by evidence from numerous laboratories indicating a role for the BDNF/trkB system in sensitizing nociceptive input to the spinal cord. Several pieces of evidence pointed in this direction, specifically the up-regulation of BDNF in NGF-sensitive sensory afferents after inflammation (Apfel et al., 1996; Michael et al., 1997), its colocalization with calcitonin gene related peptide and substance P in central terminals of nociceptive afferents (Luo et al., 2001), the presence of trkB receptors in the superficial dorsal horn (Bradbury et al., 1998; Mannion et al., 1999) and the pronociceptive effects of BDNF when applied to the spinal cord, specifically its enhancement of small fibre evoked ventral root reflexes and c-fos expression (Kerr et al., 1999). These findings combined with an up-regulation of trkB in the spinal cord (Narita et al., 2000), an enhanced level of BDNF in the spinal cord and dorsal root ganglia in various models of chronic pain (Cho et al., 1997; Kerr et al., 1999; Fukuoka et al., 2001; Ha et al., 2001; Miletic & Miletic, 2002) as well as the antinociceptive effects of BDNF sequestration in the spinal cord (Kerr et al., 1999) are all indicative of an important role for BDNF in the nociceptive pathway. The present findings provide a crucial link for the role of BDNF in pain processing by demonstrating directly that it facilitates the monosynaptic response of lamina II neurons to their afferent input.
Our experiments reveal a number of important features of BDNF action. Firstly, it produces synaptic facilitation, which requires functional postsynaptic NMDA receptors, shown previously to be expressed in lamina II neurons (Bardoni et al., 1998; Bardoni et al., 2000; Bardoni, 2001). A similar dependence has been reported for long-lasting synaptic modification produced by BDNF in other regions of the nervous system including spinal motoneurons (Arvanian & Mendell, 2001) and hippocampus (Kovalchuk et al., 2002; Manabe, 2002). Secondly, the results with BAPTA indicate increased intracellular calcium is also necessary for BDNF actions. Thirdly, intracellular signalling via PLC and its downstream effector PKC is required for the BDNF effects to occur. Activity in this pathway leads to phosphorylation of several membrane proteins including the NMDA receptor (Ben Ari et al., 1992; Dray & Urban, 1996) and thereby to lasting alterations in synaptic efficacy. Consistent with this idea, Slack & Thompson (2002) recently showed that BDNF modifies NMDA receptor activity by phosphorylation of the NR1 subunit and that this process involves PKC. Like the NMDA receptor, PKC contributes to the mechanisms underlying central sensitization and persistent pain (Coderre, 1992; Basbaum, 1999). PKC can also interact with other proteins such as the AMPA receptor to produce synaptic facilitation in the dorsal horn (Li et al., 1999). The ability of BDNF to trigger phosphorylation of NMDA receptors probably contributes to the persistence of these effects in excess of one hour after BDNF is washed out of the superfusate.
The results of these experiments indicate that the effect is neurotrophin specific. Unlike BDNF, NT-3 exerted only very modest effects on these synaptic responses. Furthermore, the unidirectional effect of BDNF sharply contrasts with its complex actions on the monosynaptic input to spinal motoneurons in similarly aged animals where it produced short-lasting postsynaptic facilitation followed by prolonged presynaptic inhibition (Arvanian & Mendell, 2001).
The predominance of facilitation in response to BDNF superfusion was not surprising in view of previous results indicating that BDNF enhances nociceptive responses in neonatal animals (Kerr et al., 1999). However, BDNF did not facilitate the response of all cells in lamina II. Our immunocytochemical data provide a possible basis for this finding, namely that not all neurons in this region express trkB, at least under these experimental conditions. This is consistent with the report of Mannion et al. (1999) indicating that expression of both the full length and truncated trkB receptor is submaximal in the spinal cord of rats not previously subjected to injury or C-fibre stimulation. Furthermore, even those cells whose responses were facilitated by BDNF may not have responded maximally. The test stimulus activated sensory fibers at 0.017 Hz during a control period lasting approximately 10 min and during the test period lasting up to approximately 60 min. This frequency is much lower than the 50 Hz used by Mannion et al. (1999). Bardoni et al. (1998) reported that all neurons from which they recorded in the superficial dorsal horn exhibited NMDA receptor-mediated responses. We have confirmed this finding in a subset of 11 cells tested with direct application of NMDA, although a small minority of cells (≈20%) failed to exhibit a NMDA receptor-mediated synaptic response at −60 mV. Thus heterogeneity in NMDA receptor expression among these neurons may also account for some variability in the effects of BDNF.
Other reported physiological effects of BDNF may also have influenced the findings in these experiments. The first is the finding that BDNF inhibits substance P release into the dorsal horn (Pezet et al., 2002). These effects, mediated by GABA released from interneurons, might be expected to compete with the direct facilitation observed here. The action of BDNF on synaptic transmission to motoneurons in neonates also has mixed postsynaptic excitatory and presynaptic inhibitory effects with the inhibitory ones predominating (Arvanian & Mendell, 2001). BDNF has also been found to inhibit AMPA transmission in neurons of the solitary tract (Balkowiec et al., 2000). Such an effect in lamina II of the spinal cord might act to reduce the net facilitation or in some cases to elicit net inhibition.
A key finding arising from these studies is the importance of the NMDA receptor for BDNF-induced facilitation of the AMPA/ka receptor response. Although we have not explicitly explored the linkage between the NMDA receptor and AMPA receptor activity, there is considerable evidence indicating interaction. Specifically, stimulus-evoked long-term potentiation and long-term depression have been shown to involve movement of AMPA receptors in and out of the synapse controlled by NMDA receptor activity (Isaac et al., 1995). Evidence from several laboratories has also pointed to the ability of neurotrophins, including BDNF, to enhance AMPA/ka-mediated responses, an action that required the function of NMDA receptors (Levine et al., 1998; Arvanov et al., 2000; Malcangio & Lessman, 2003). Previous work has pointed to a central role for the NMDA receptor in various types of synaptic plasticity important in pain mechanisms including windup in the spinal cord (Dickenson, 1990; Dickenson et al., 1997) and central sensitization (Woolf, 1983; South et al., 2003).
The present experiments were carried out only in neonates (up to postnatal day 14), but the application of these findings to pain mechanisms in adults is supported by several previous studies. These indicate that sequestration of spinal BDNF reduces inflammatory hyperalgesaia in adults (Mannion et al., 1999; Kerr et al., 1999) suggesting the importance of BDNF potentiation of synaptic transmission into lamina II throughout the life of the animal. NMDA receptors are known to be functional in the superficial dorsal horn throughout life unlike in motoneurons where they are present at birth but become unresponsive after 1 week due to Mg2+ block (Arvanian & Mendell, 2001). The difference in NMDA responsiveness between motoneurons and cells in lamina II in the second postnatal week agrees with Kalb et al. (1992) who reported that during the second postnatal week, NMDA receptors are lost in all areas of the spinal grey matter, except the SG. The potential significance of these findings may extend beyond the role of BDNF as a component in normal hyperalgesia as there is considerable evidence that exogenously delivered BDNF can promote survival and elongation of axons in models of spinal cord injury (Menei et al., 1998). The present experiments suggest possible pronociceptive effects of BDNF, which would be important in considering its use as a therapeutic molecule.
Our results confirm the importance of increased postsynaptic calcium in order for BDNF to evoke facilitation of synaptic responses or NMDA induced current, in that no facilitation was induced by BDNF when postsynaptic neurons were loaded with the calcium chelator BAPTA. The source of this required Ca2+ is presently not known. One possibility is that it enters via the NMDA receptor. However, another possibility is that Ca2+ comes from intracellular stores released by activation of inositol 1,4,5-triphosphate (IP3), downstream from PLC that we have shown to be an essential intermediate in the action of BDNF.
Finally, to gain perspective, it is necessary to recall that in the spinal cord, as in the periphery, sensitization involves the action of many different molecular species. In the periphery, several agents (e.g. bradykinin, prostaglandins, NGF) work synergistically to produce sensitization of primary afferent fibers (reviewed in Millan, 1999; Shu & Mendell, 1999). In the spinal cord, it is instructive to compare the pronociceptive features of BDNF to those of the neuropeptides substance P and calcitonin gene related peptide. All three neurotransmitters/modulators are present in small diameter trkA+ nociceptors. Following chronic peripheral inflammation induced by NGF or Freund's adjuvant, all three peptides are up-regulated in afferent terminals and released in greater amounts into the spinal cord (Thompson et al., 1995; Malcangio & Bowery, 1996; Michael et al., 1997; Kerr et al., 1999; also see Sluka & Westlund, 1993; De Koninck et al., 1994; Malcangio et al., 2000; Woolf & Salter, 2000). Thus they are implicated in spinal nociception and central sensitization (Millan, 1999). Finally, they activate signalling mechanisms in postsynaptic cells that eventually lead to phosphorylation of membrane proteins resulting in synaptic facilitation. At least in the case of substance P and BDNF there is some evidence that PKC is involved in the signalling pathway (Basbaum, 1999; Wajima et al., 2000). As they are all implicated in central sensitization and spinal pain processing, it will be important to know what contribution each transmitter makes to these phenomena and/or to what extent they work independently or together to exert these actions.
Acknowledgements
This study was supported by the Christopher Reeve Paralysis Foundation grant to LMM. Additional support was made available from NIH 2 RO1 NS 16996 and 5 PO1 NS 39420 to LMM and a UNCF-Merck Science Initiative fellowship to SMG. We thank Regeneron Pharmaceuticals for generously supplying BDNF and NT-3. We also wish to thank Dr Victor Arvanian for critical discussions and suggestions regarding this study.
Abbreviations
-
- ACSF
-
- artificial cerebrospinal fluid
-
- AMPA
-
- α-amino-3-hydroxy-5-methylisoxasole-4-propionic acid
-
- BDNF
-
- brain-derived neurotrophic factor
-
- CNQX
-
- 6-cyano-7-nitroquinoxaline
-
- D-APV
-
- 2-amino-5-phosphovaleric acid
-
- EPSC
-
- excitatory postsynaptic currents
-
- ka
-
- kainate
-
- NGF
-
- nerve growth factor
-
- NMDA
-
- N-methyl-d-aspartate
-
- NT-3
-
- neurotrophin-3
-
- PBS
-
- phosphate buffered saline
-
- PKC
-
- protein kinase C
-
- SG
-
- substantia gelatinosa.