A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord
Abstract
In the rat lumbar spinal cord the major supraspinal targets for lamina I projection neurons are the caudal ventrolateral medulla (CVLM), lateral parabrachial area (LPb) and periaqueductal grey matter (PAG). In this study we have estimated the number of lamina I neurons retrogradely labelled from each of these sites in the L4 segment, as well as the proportion that can be labelled by injecting different tracers into two separate sites. Our results suggest that this segment contains approximately 400 lamina I projection neurons on each side, and that approximately 85% of these can be labelled from either the CVLM or the LPb on the contralateral side. Around 120 lamina I cells in L4 project to the PAG, and over 90% of these cells can also be labelled from the CVLM or LPb. Most lamina I neurons projecting to CVLM or LPb are located in the contralateral dorsal horn, but in each case some cells were found to have bilateral projections. We also examined horizontal sections to investigate morphology and the expression of the neurokinin 1 (NK1) receptor in cells labelled from CVLM, LPb or PAG. There were no consistent morphological differences between these groups, however, while cells with strong or moderate NK1 receptor-immunostaining were labelled from LPb or CVLM, they seldom projected to the PAG. These results suggest that many lamina I cells project to more than one site in the brain and that those projecting to PAG may represent a distinct subclass of lamina I projection neuron.
Introduction
Lamina I of the spinal cord receives a substantial input from nociceptive primary afferents and contains neurons that project to brain regions that are important in nociception (Willis & Coggeshall, 1991; Craig, 1995; Villanueva & Bernard, 1999). In the rat, approximately 80% of lamina I projection neurons express the neurokinin 1 (NK1) receptor (Marshall et al., 1996; Todd et al., 2000). Selective ablation of NK1 receptor-expressing neurons in the superficial laminae causes a reduction in pain-related behaviours in both inflammatory and neuropathic models (Mantyh et al., 1997; Nichols et al., 1999), suggesting an essential role for lamina I projection neurons in chronic pain states.
In the rat lumbar cord, the spinothalamic projection from lamina I is sparse (Lima & Coimbra, 1988; Burstein et al., 1990a; Marshall et al., 1996); however, many lamina I neurons can be retrogradely labelled from the brainstem (Menétrey et al., 1982, 1983; Cechetto et al., 1985; Hylden et al., 1989; Lima & Coimbra, 1989; Lima et al., 1991). In a previous study we found that the largest numbers of labelled lamina I neurons were seen following tracer injections into the caudal ventrolateral medulla (CVLM) or lateral parabrachial area (LPb), while a smaller number was observed with injections into the periaqueductal grey matter (PAG) (Todd et al., 2000). In each case, most labelled cells were on the contralateral side, but there was also a small ipsilateral population.
Although many lamina I neurons project to more than one supraspinal target (e.g. Kevetter & Willis, 1983; McMahon & Wall, 1985; Hylden et al., 1989), we know little about the extent of overlap between the populations that are retrogradely labelled from CVLM, LPb and PAG, or whether labelled neurons on the ipsilateral side have bilateral projections. To address these issues, we have injected the tracers cholera toxin B subunit (CTb) and Fluorogold into combinations of these targets, and determined the proportions of single-labelled and double-labelled lamina I neurons. This approach provides important information about the organization of supraspinal projections from lamina I, and also allows us to estimate the total number of projection neurons in this lamina.
Lamina I projection neurons can be classified into three morphological types: pyramidal, fusiform and multipolar (or flattened) cells (Lima et al., 1991; Zhang et al., 1996; Zhang & Craig, 1997). Lima & Coimbra (1989) and Lima et al. (1991) reported that in the rat, the proportions of retrogradely labelled neurons in each morphological class differed according to projection target. In contrast, Craig and colleagues have suggested that morphology more accurately predicts function, as pyramidal cells in the cat were found to be thermoreceptive-specific, whereas fusiform and multipolar cells responded to noxious stimuli (Han et al., 1998). In support of this, Yu et al. (1999) reported that relatively few pyramidal neurons in the monkey expressed the NK1 receptor. We have therefore compared the proportion of retrogradely labelled neurons belonging to each morphological type for the three injection sites, and determined whether NK1 receptor expression varied systematically with projection target or morphology.
Materials and methods
Animals
All experiments were approved by the Ethical Review Process Applications Panel of the University of Glasgow, and were performed in accordance with the UK Animals (Scientific Procedures) Act 1986. Thirteen adult male Wistar rats (240–340 g; Harlan, Loughborough, UK) were deeply anaesthetized with a mixture of ketamine and xylazine (73.3 and 7.3 mg/kg i.p., respectively, supplemented as necessary) and placed in a stereotaxic frame. Each rat received two injections through glass micropipettes into different brainstem targets. In each case, one injection consisted of 200 nL 1% CTb (Sigma, Poole, U.K) and the other of 50 nL 4% Fluorogold (Fluorochrome Inc, Englewood, CO, USA). Where the injection sites were at different rostrocaudal levels, Fluorogold was injected into the more rostral site, as Bice & Beal (1997a) reported that injection of Fluorogold could reduce the number of spinal neurons labelled by a second tracer injected rostral to the Fluorogold injection site. Each injection was targeted at one of three sites: the caudal part of the PAG, the LPb, or the CVLM. Injections into the CVLM were aimed at the region between the lateral reticular nucleus and the adjacent part of the spinal trigeminal nucleus (Lima et al., 1991; Craig, 1995; Todd et al., 2000). In all but one case, different pipettes were used for each tracer, while in the remaining case (experiment 1, Table 1) the pipette was rinsed thoroughly between injections. At the end of each injection the pipette was left in place for 5 min in an attempt to minimize leakage of tracer back up the track. Details of the injection targets for each animal are given in Table 1. All animals made an uneventful recovery from anaesthesia. After survival periods of 3 or 4 days the rats were anaesthetized with pentobarbitone (300 mg i.p.) and perfused with a fixative containing 4% freshly de-polymerized formaldehyde. Lumbar spinal cord segments were removed and stored in fixative for between 4 and 24 h and the brainstem was cryoprotected with 30% sucrose overnight.
| Experiment number | CTb injection site | Fluorogold injection site | Survival period (days) |
|---|---|---|---|
| 1 | CVLM (L) | LPb (L) | 3 |
| 2 | CVLM (L) | LPb (L) | 3 |
| 3 | CVLM (L) | LPb (L) | 3 |
| 4 | CVLM (L) | PAG (L) | 4 |
| 5 | CVLM (L) | PAG (L) | 3 |
| 6 | CVLM (L) | PAG (L) | 3 |
| 7 | LPb (L) | PAG (L) | 3 |
| 8 | LPb (L) | PAG (L) | 3 |
| 9 | LPb (L) | PAG (L) | 3 |
| 10 | CVLM (L) | CVLM (R) | 3 |
| 11 | CVLM (L) | CVLM (R) | 4 |
| 12 | LPb (L) | LPb (R) | 3 |
| 13 | LPb (L) | LPb (R) | 4 |
- Details of the injection sites for the 13 experiments. In each case, animals received one injection of CTb (200 nL 1% solution) and one of Fluorogold (50 nL 4% solution). These injections were made into the following targets, as listed above: the caudal ventrolateral medulla (CVLM), lateral parabrachial area (LPb) or periaqueductal grey matter (PAG). In experiments 1–9 injections were made into two different targets on the left side of the brain, while in experiments 10–13 injections were placed bilaterally. (L), left side; (R), right side.
Immunocytochemistry
Spinal cord segments were notched so that left and right sides could subsequently be distinguished, and then cut into 70 µm thick sections with a Vibratome. All of these were treated with 50% ethanol for 30 min to enhance antibody penetration (Llewellyn-Smith & Minson, 1992).
The fourth lumbar (L4) segment was cut into transverse sections. Approximately half of these were incubated for 1–2 days in a mixture of goat antibody against CTb (List Biological Laboratories, Campbell, CA, USA; diluted 1 : 5000) and rabbit antibody against Fluorogold (Chemicon International, Harrow, UK, diluted 1 : 5000), and for 1 day in species-specific secondary antibodies (antirabbit and antigoat IgG) conjugated to fluorescein or cyanine 5.18 (both raised in donkey, obtained from Jackson Immunoresearch, West Grove, PA, and diluted 1 : 100). These sections were then reacted with propidium iodide in the presence of RNAse (as described previously, Todd et al., 1998) to provide a fluorescent nuclear stain, mounted in antifade mounting medium (Vectashield, Vector laboratories, Peterborough, UK) and stored at −20 °C until needed. Some sections were incubated for 1–2 days in the mouse monoclonal antibody NeuN (against a neuronal nuclear protein, Chemicon International, diluted 1 : 1000), followed by 1 day in donkey antimouse IgG conjugated to cyanine 5.18 (Jackson Immunoresearch, 1 : 100), and 30 min in the fluorescent nuclear stain 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich, Poole, Dorset, UK, 1 µg/mL in phosphate-buffered saline). They were then coverslipped in Vectashield and stored at −20 °C.
The third lumbar (L3) segment was cut into horizontal sections, which were incubated for 1–2 days in the following cocktail of primary antibodies: goat antiCTb and rabbit antiFluorogold (as above), and guinea-pig antiserum against NK1 receptor (Polgár et al., 1999; diluted 1 : 1000). These were revealed with species-specific secondary antibodies (antirabbit, antigoat and antiguinea pig IgG) conjugated to rhodamine, cyanine 5.18 and fluorescein (all raised in donkey, Jackson Immunoresearch, diluted 1 : 100), mounted in Vectashield and stored at −20 °C.
The NK1 receptor antiserum was raised against a 15-amino acid sequence at the C terminus of the rat NK1 receptor. Staining with this antiserum is blocked by preincubation with the immunizing peptide, and it has been shown that the antibody stains identical structures to those detected by a well-characterized rabbit antiNK1 receptor antibody (Vigna et al., 1994; Polgár et al., 1999). The antibody to Fluorogold was used because Fluorogold is not detected with the Krypton-Argon laser on our Bio-Rad MRC1024 confocal microscope.
The region of the brainstem that included the injection sites was cut into 100 µm thick coronal sections with a freezing microtome. These were collected in five complete series: at least one of these series was mounted in Vectashield (for detection of Fluorogold with epi-fluorescent illumination and a UV filter set), and at least one was reacted with goat antiCTb (1 : 50 000) using an immunoperoxidase method (Todd et al., 2000). All injection sites were photographed, and in each case the spread of tracer from the injection site was plotted onto drawings of the brainstem (Paxinos & Watson, 1997).
Confocal microscopy and neuronal analysis
Spinal cord sections were examined with either a Bio-Rad MRC1024 or Radiance 2100 confocal laser scanning microscope, equipped with Krypton-Argon (MRC1024) or Argon/Green HeNe/Red diode/Blue diode (Radiance 2100) lasers (Bio-Rad, Hemel Hempstead, UK).
Transverse sections of the L4 segment that had been reacted with antibodies against CTb and Fluorogold and stained with propidium iodide were used to estimate the number of retrogradely labelled neurons (containing CTb, Fluorogold or both tracers) in lamina I. In cases where both injections were on the same side (experiments 1–9, Table 1), only cells on the contralateral side of the spinal cord were counted, while in experiments with bilateral injections (experiments 10–13) counts were obtained from both sides of the spinal cord. From each experiment, ten Vibratome sections were randomly selected and lamina I was scanned through the full section thickness with a 20× dry lens to reveal CTb, Fluorogold and propidium iodide. All retrogradely labelled cells in these sections were initially identified, and the presence or absence of CTb and Fluorogold in each cell was noted. In order to correct for the over-counting that results from the presence of transected cells at the section surfaces, cells were only included in the sample if their nucleus (stained with propidium iodide) was entirely contained within the Vibratome section, or if part of the nucleus was present in the first optical section in the z-series (corresponding to the top of the Vibratome section). They were excluded if part of the nucleus was present in the last optical section in the z-series. In this way the mean number of retrogradely labelled cells containing CTb, Fluorogold or both tracers per 70-µm section was determined for each experiment.
Horizontal sections from the L3 segments of six experiments (experiments 4–9) were used to analyse morphology and NK1 receptor-immunostaining of retrogradely labelled neurons. This plane of section was used, as it is parallel to the major dendritic arborization of lamina I projection neurons, and therefore most clearly reveals their somato-dendritic morphology (Lima et al., 1991; Zhang et al., 1996; Zhang & Craig, 1997; Todd et al., 2002). In each case the Vibratome section containing the largest number of retrogradely labelled neurons on the contralateral side was selected and scanned sequentially with three lines of the laser through a 40× oil-immersion lens to reveal CTb, Fluorogold and NK1 receptor. In this way, confocal image stacks (1 µm z-separation) through the cell bodies and dendritic trees of all retrogradely labelled neurons in each of these sections were obtained. Cells were excluded from the sample if they were so close to one surface of the Vibratome section that substantial parts of their proximal dendrites (and/or cell bodies) were not present on the section, as this made it impossible to allocate them to one of the three morphological classes. Drawings of the cell bodies and proximal dendrites of all of the retrogradely labelled neurons included in the sample were made with Neurolucida for Confocal software (MicroBrightField, Inc., Colchester, VT, USA) and the presence or absence of CTb and Fluorogold were recorded for each cell. These drawings were used to analyse morphology. For each cell included in the sample, the morphology was assessed independently by two observers and cells were allocated to one of the following classes: fusiform, multipolar or pyramidal (Zhang et al., 1996; Zhang & Craig, 1997). In cases of disagreement, the cells were examined by a third observer, and in these cases the cell was allocated to a group if two of the observers agreed. A small number of cells could not be classified, as they showed features that were transitional between two of the three morphological classes, while a few could not be allocated to any of these classes because of their atypical appearance (Zhang et al., 1996; Zhang & Craig, 1997). These cells were defined as ‘unclassified’. The presence or absence of NK1 receptor-immunoreactivity on the plasma membrane of the soma and dendrites of each cell was recorded (Todd et al., 2000, 2002). NK1 receptor-immunoreactive projection neurons were classified as weakly (+), moderately (++) or strongly (+++) immunoreactive. The analysis of morphology and NK1 receptor-immunoreactivity was performed by observers who were unaware of whether the cells were retrogradely labelled with CTb, Fluorogold or both tracers.
In order to estimate the total number of neurons in lamina I at the L4 level, two transverse Vibratome sections of the L4 segment that had been immunoreacted with NeuN and stained with DAPI were selected at random from each of three rats (experiments 5, 10 and 12). The sections were scanned through a 40× oil-immersion lens to reveal NeuN and DAPI. In each case, z-series consisting of 16 optical sections at 1 µm z-spacing were obtained from the entire mediolateral extent of lamina I on both sides of the spinal cord. Drawings of these sections were made with Neurolucida for Confocal, and the boundaries of lamina I were added as described previously (Todd et al., 1998). A modification of the optical disector method (Sterio, 1984) was then used to determine the total number of neurons in an 11-µm thick slice through lamina I, by examining all optical sections and counting all of the neurons with nuclei that had a bottom surface between the third and fourteenth optical sections in the z-series (Todd et al., 1998).
Data analysis
Because of the case-by-case variation in the number of retrogradely labelled projection neurons, all of the statistical analyses were performed with nonparametric tests. The Kruskall–Wallis anova was used to compare the proportion of cells that were NK1 receptor-immunoreactive between different projections in the L3 horizontal sections of experiments 4–9, and also the proportion of neurons in different projection populations that belonged to each morphological class. To analyse NK1 receptor-immunostaining intensity, the negative, + , ++ and +++ values were transformed to a 0–3 score, and the general linear model with Tukey's test posthoc was then used to investigate whether intensity of NK1 receptor-immunostaining varied systematically by projection target and morphology. For all tests, a P-value of < 0.05 was considered to be significant.
Results
Injection sites
The spread of tracer within the brainstem in each experiment is illustrated in 1-3, and representative injection sites for each target nucleus are shown in Fig. 4. Injections into the PAG were targeted at its caudal part (1, 2, experiments 4–9, Fig. 4C). In each case Fluorogold largely filled one side of the PAG at the level of the injection, but did not spread across the midline or into the parabrachial nuclei. In several cases there was spread dorsally into the overlying superior colliculus, and/or laterally into the medial part of the cuneiform nucleus. Parabrachial injections 1-3, experiments 1–3, 7–9, 12–13, Fig. 4B) filled most or all of the LPb at the level of the injection site. There was generally spread into the medial parabrachial area, and often into the Kölliker-Fuse and/or cuneiform nuclei, but not into the PAG. In experiment 13, there was a necrotic core in the superior cerebellar peduncle at the centre of the Fluorogold injection (Fig. 3). Injections into the CVLM (1, 3, experiments 1–6, 10–11, Fig. 4A) filled the whole of the lateral reticular nucleus, or else its lateral half, at the level of the injection, and extended into surrounding areas including the regions dorsal to the lateral reticular nucleus, and between the lateral reticular and spinal trigeminal nuclei. In some cases there was spread into the spinal trigeminal nucleus. In experiments 10 and 11 there was a small necrotic core at the centre of the Fluorogold injections. In experiment 1, in which the same pipette was used for both tracers, there was no deposition of the first tracer (CTb) at the second injection site (LPb).
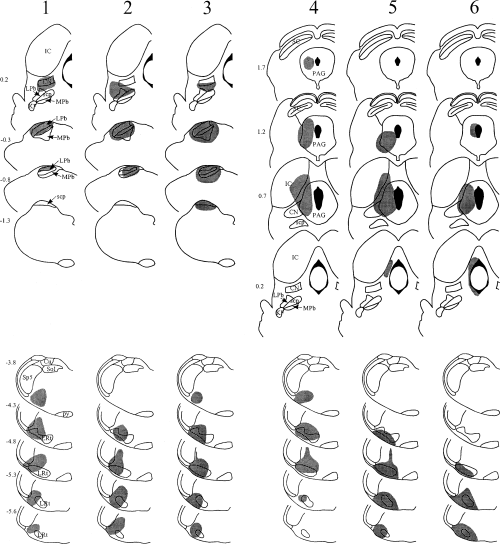
Diagrams to show the spread of tracer (shaded area) in experiments 1–3 (Fluorogold injected into the LPb, CTb into the CVLM) and 4–6 (Fluorogold injected into the PAG, CTb into the CVLM). Each vertical column represents a single experiment, and the experiment number (corresponding to those in Tables 1–3) is shown at the top of the column. The upper part of each column shows a series of drawings at different rostrocaudal levels through the rostral injection site, and the lower part shows drawings through the caudal injection site. Numbers at the top left of each drawing give the approximate position of the section anterior or posterior (–) to the ear-bar. Drawings are based on those in Paxinos & Watson (1997). CN, cuneiform nucleus; Cu, cuneate nucleus; IC, inferior colliculus; KF, Kölliker-Fuse nucleus; LPb, lateral parabrachial area; LRt, lateral reticular nucleus; mcp, middle cerebellar peduncle; MPb, medial parabrachial area; PAG, periaqueductal grey matter; py, pyramid; SC, superior colliculus; scp, superior cerebellar peduncle; sol, tractus solitarius and its nucleus; Sp5, spinal trigeminal nucleus.
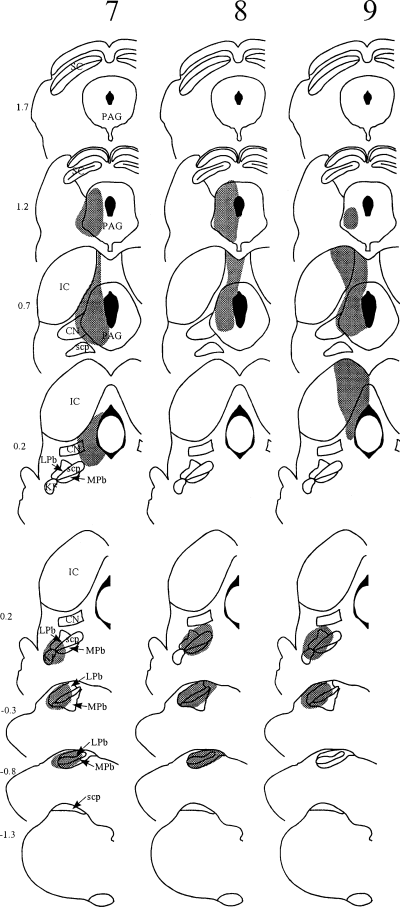
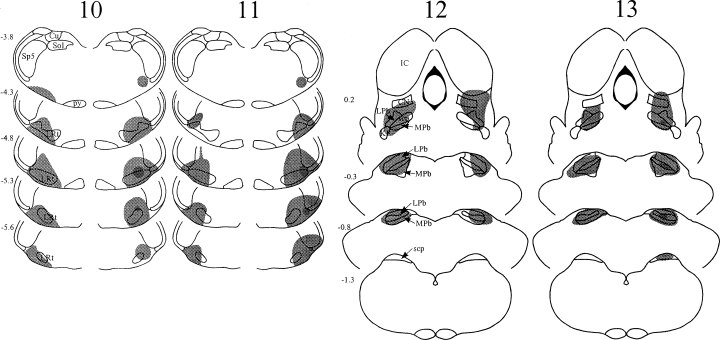
Diagrams to show the spread of tracer (shaded area) in experiments 10–11 (bilateral injections into CVLM) and 12–13 (bilateral injections into LPb). In each case, CTb was injected on the left side (left side of diagram) and Fluorogold on the right. In experiments 10, 11 and 13, the Fluorogold injection site contained a small necrotic core, and this is shown as a darker area near the centre. Abbreviations as in Fig. 1.
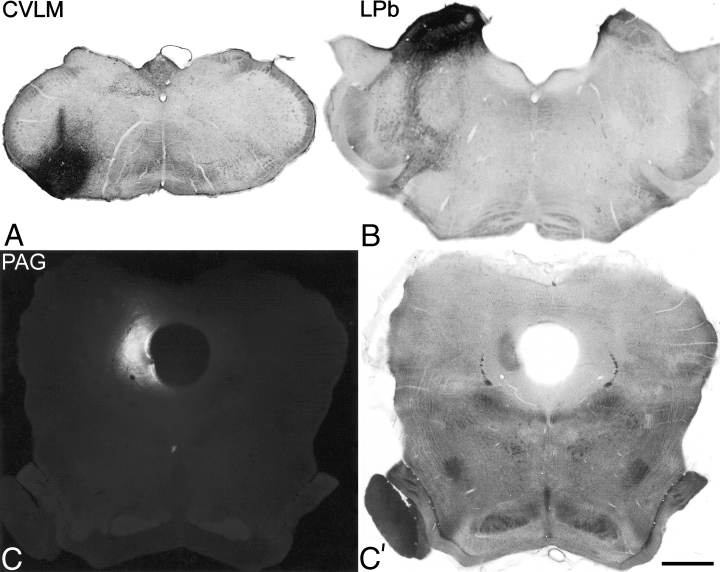
Photomicrographs of representative injection sites for each of the brainstem targets. Transverse sections through the brainstem near the centre of the injection are shown for: (A) the CVLM (experiment 3), (B) the LPb (experiment 8), (C and C′) the PAG (experiment 6). The injections in A and B consisted of CTb, and the sections have been reacted to reveal this with an immunoperoxidase method. The PAG injection involved Fluorogold, and this is shown in a photomicrograph taken with a UV filter set in (C). (C′) is the same field taken with bright-field optics. Scale bar, 1 mm.
Quantitative results from the L4 sections
Quantitative data for retrogradely labelled lamina I neurons were obtained from ten randomly selected 70-µm thick transverse sections from the L4 segment in each animal, and these are shown in Table 2. Examples of retrogradely labelled lamina I neurons in transverse sections of L4 are shown in Fig. 5. The mean number of retrogradely labelled contralateral lamina I neurons following injections into the CVLM was 9.51 cells per section (± 1.31, SD) while the corresponding values for LPb and PAG injections were 9.9 (± 1.25) and 3.28 (± 0.95), respectively (Table 2). Labelled lamina I neurons on the ipsilateral side were only examined in experiments 10–13, and these gave values of 2.48 (± 0.55) ipsilateral cells per section for CVLM injections and 3.0 (± 0.82) ipsilateral cells per section for LPb injections (Table 2). We have found that the average length of the L4 segment in the rat is 2.5 mm (A.J. Todd and E. Polgár, unpublished observations), and from this we estimate that the numbers of cells in L4 that are labelled from contralateral CVLM, LPb and PAG are approximately 340, 354 and 117, respectively. Corresponding values for the ipsilateral CVLM and LPb are 89 and 107 (Table 2).
| Experiment number | Injection sites | CVLM contralateral | LPb contralateral | PAG contralateral | CVLM ipsilateral | Lpb ipsilateral | Double-labelled neurons (n) | Total neurons (n) |
|---|---|---|---|---|---|---|---|---|
| 1 | CVLM/LPb | 103 | 101 | – | – | – | 79 | 125 |
| 2 | CVLM/LPb | 93 | 103 | – | – | – | 83 | 113 |
| 3 | CVLM/LPb | 125 | 110 | – | – | – | 103 | 132 |
| 4 | CVLM/PAG | 77 | – | 28 | – | – | 25 | 80 |
| 5 | CVLM/PAG | 97 | – | 49 | – | – | 47 | 99 |
| 6 | CVLM/PAG | 83 | – | 23 | – | – | 22 | 84 |
| 7 | LPb/PAG | – | 106 | 37 | – | – | 36 | 107 |
| 8 | LPb/PAG | – | 91 | 34 | – | – | 32 | 93 |
| 9 | LPb/PAG | – | 122 | 26 | – | – | 26 | 122 |
| 10 (right) | CVLM/CVLM* | 95 | – | – | 20 | – | 19 | 96 |
| 10 (left) | CVLM/CVLM* | 99 | – | – | 21 | – | 17 | 103 |
| 11 (right) | CVLM/CVLM* | 93 | – | – | 26 | – | 24 | 95 |
| 11 (left) | CVLM/CVLM* | 86 | – | – | 32 | – | 32 | 86 |
| 12 (right) | LPb/LPb* | – | 80 | – | – | 23 | 21 | 82 |
| 12 (left) | LPb/LPb* | – | 85 | – | – | 23 | 21 | 87 |
| 13 (right) | LPb/LPb* | – | 91 | – | – | 39 | 33 | 97 |
| 13 (left) | LPb/LPb* | – | 101 | – | – | 35 | 29 | 107 |
| [Cells per section | (mean ± SD) | 9.51 ± 1.31 | 9.9 ± 1.25 | 3.28 ± 0.95 | 2.48 ± 0.55 | 3.0 ± 0.82] | ||
| [Cells per segment† | – | 340 | 354 | 117 | 89 | 107] |
- Counts of labelled neurons in the 13 experiments. In each case, cells were counted in ten randomly selected 70 µm transverse sections from the L4 segment. Columns 3–7 give the total number of cells labelled from each injection site, column 8 the number of double-labelled cells, and column 9 the total number of cells labelled. *For experiments 10–13 data from both sides of the spinal cord are included. In each of these cases the upper row shows results from the right side of the spinal cord and the lower row those from the left side. Note that cells on the side of the spinal cord ipsilateral to the injection site were only examined in these experiments. †Based on a length of 2.5 mm for the L4 segment.
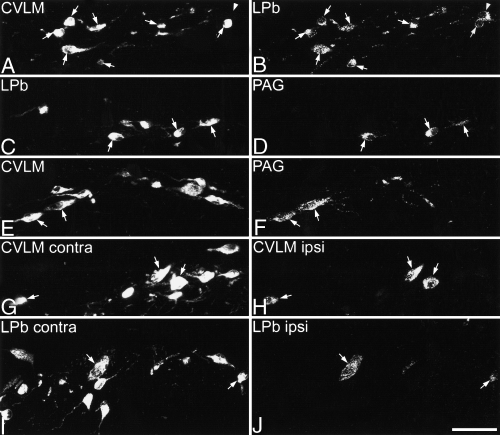
Examples of retrogradely labelled neurons seen in transverse sections from L4. In each case, the image on the left shows CTb labelling, and that on the right is the corresponding field scanned to reveal Fluorogold-immunoreactivity. (A and B) in this section from experiment 2 (CTb in CVLM, Fluorogold in LPb) several lamina I neuronal cell bodies from the contralateral side of the spinal cord are visible. Most are labelled with both CTb and Fluorogold, and these are indicated with arrows, while a lamina I neuron that is labelled with Fluorogold but not CTb is shown with an arrowhead. In these and subsequent images, small labelled profiles represent dendrites belonging to neurons with cell bodies that are not included in these optical sections. (C and D) Part of a section from experiment 9 (CTb in LPb, Fluorogold in PAG) shows several lamina I neurons on the contralateral side of the spinal cord that are labelled with CTb. Three of these (arrows) also contain Fluorogold. (E and F) Part of a section from experiment 5 (CTb in CVLM, Fluorogold in PAG) showing several contralateral CTb-labelled lamina I neurons, two of which are also Fluorogold-labelled (arrows). (G and H) This image from experiment 10 (CTb in left CVLM, Fluorogold in right CVLM) shows part of the right-hand side of the spinal cord (contralateral to the CTb injection). Numerous CTb-labelled neurons are visible, and three of these (arrows) are also labelled with Fluorogold from the ipsilateral CVLM. (I and J) In these images from experiment 13 (CTb in left LPb, Fluorogold in right LPb), part of the right side of the spinal cord (contralateral to the CTb injection) is shown. Numerous lamina I neurons labelled with CTb from the contralateral LPb are visible, and two of these (arrows) are also labelled with Fluorogold from the ipsilateral LPb. Scale bar, 50 µm. Images are projections of 10 (A and B), 17 (C–F), 13 (G and H) and 14 (I and J) optical sections at 1 µm z-spacing.
In experiments 1–3 the majority of retrogradely labelled lamina I neurons contained both CTb (transported from the CVLM) and Fluorogold (from the LPb) (Table 2, Fig. 5A and B). The mean proportion of cells labelled from CVLM that were also labelled from the LPb was 82.8% (range 76.7–89.2), while the mean proportion of cells labelled from the LPb that were also labelled from the CVLM was 84.1% (range 78.2–93.6) (calculated from data in Table 2).
From experiments 4–9 it was clear that the great majority of contralateral lamina I neurons that were retrogradely labelled from the PAG were also labelled from either the LPb or from the CVLM (Table 2, Fig. 5C–F). Of the PAG-labelled neurons, between 89.3 and 95.9% (mean 93.6%) were labelled from CVLM in experiments 4–6, while between 94.1 and 100% (mean 97.1%) were labelled from the LPb in experiments 7–9 (Table 2).
In experiments 10–13, in which bilateral injections were made into either the CVLM or the LPb, both sides of the spinal cord were analysed. Results from these experiments showed that most of the lamina I neurons that were retrogradely labelled on the ipsilateral side of the spinal cord were also labelled from the contralateral side (Table 2, Fig. 5G–I). For the CVLM injections between 81.0 and 100% (mean 92.1%) of labelled cells on the ipsilateral side were also labelled by the contralateral injection, while for the LPb injections the corresponding values were 82.9–91.3% (mean 87.5%) (Table 2).
In the L4 segment in experiments 1–3, the mean number of cells labelled from either CVLM, LPb or both sites was 12.33 per section, and of these 86.8% were labelled from the CVLM and 84.9% from the LPb (data from Table 2). To provide a more accurate value for the total number of cells labelled from either or both of these sites, the mean number of contralateral lamina I neurons per section (obtained from all appropriate experiments) that were labelled from the CVLM (9.51) or the LPb (9.9) can be divided by 86.8 or 84.9%, respectively. This gives an estimate of 11.31 cells per section that would be labelled from either the CVLM, the LPb, or both sites. Assuming a length of 2.5 mm for the L4 segment, this corresponds to 404 neurons throughout the whole of this segment.
Morphology and NK1 receptor expression
Between 105 and 151 retrogradely labelled lamina I neurons were analysed in the horizontal sections of L3 from experiments 4–9 (Table 3). Examples of retrogradely labelled lamina I neurons in horizontal section are shown in Fig. 6. The great majority of labelled lamina I neurons in each experiment (> 90%) could be assigned to one of the three morphological classes (fusiform, multipolar or pyramidal) (Fig. 7A–C). Cells in each morphological class were present in the populations of neurons retrogradely labelled from the CVLM, the LPb and the PAG (e.g. Fig. 6), and no significant relationship was found between the proportion of cells belonging to each morphological class and the projection target (Fig. 7; P > 0.05, Kruskall-Wallis anova).
| Experiment | CVLM + PAG | LPb + PAG | CVLM only | LPb only | PAG only | Total |
|---|---|---|---|---|---|---|
| 4 | 29 | – | 84 | – | 2 | 115 |
| 5 | 63 | – | 85 | – | 3 | 151 |
| 6 | 40 | – | 96 | – | 4 | 140 |
| 7 | – | 41 | – | 64 | 0 | 105 |
| 8 | – | 52 | – | 63 | 0 | 115 |
| 9 | – | 30 | – | 99 | 0 | 129 |
- The numbers of single-labelled and double-labelled lamina I neurons that were analysed in horizontal sections of L3 from experiments 4–9. From each experiment the horizontal section that contained most retrogradely labelled lamina I neurons was selected for analysis. Neurons were excluded from the sample if it was not possible to assign them to a morphological class because a significant part of the soma or proximal dendrites was not present on the section.
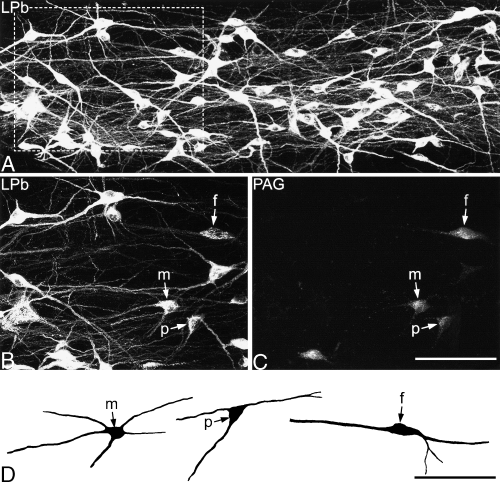
Retrogradely labelled lamina I neurons in the horizontal section of L3 that was analysed from experiment 7 (CTb injected into LPb, Fluorogold into PAG). (A) A confocal z-series of the CTb labelling in part of the section scanned through a 20× dry lens. Note the high density of labelled cells, and the extensive dendritic labelling. (B and C) Show the labelling with CTb and Fluorogold in the boxed area in A, scanned through a 40× oil-immersion lens. Several CTb-labelled neurons can be seen in B, and some are also Fluorogold-labelled. Three of these double-labelled neurons, a fusiform cell (f), a multipolar cell (m) and a pyramidal cell (p), are indicated with arrows. (D) Shows drawings of the three double-labelled neurons indicated with arrows in B and C. The drawings were made from the z-series of confocal images with Neurolucida for Confocal. Scale bars, 100 µm. Confocal images are projections of 36 (A) and 19 (B and C) optical sections at 1 µm z-spacing.
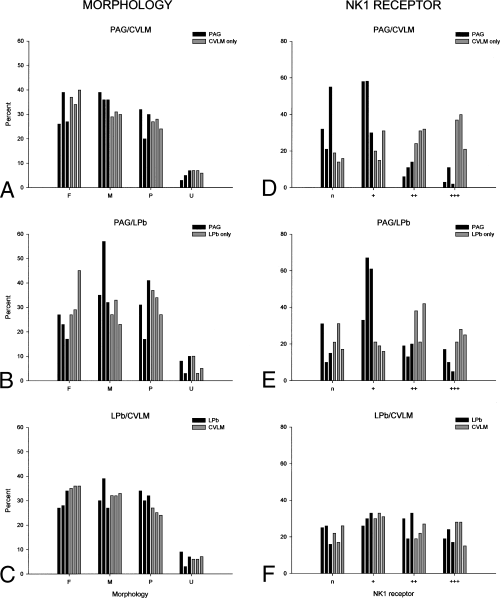
Histograms showing the morphology (A–C) and strength of NK1 receptor expression (D–F) in the retrogradely labelled lamina I neurons that were analysed in horizontal sections of L3 from experiments 4–9. Total numbers of retrogradely labelled neurons in each projection-population for these experiments are give in Table 3. (A) Shows the percentage of neurons belonging to each morphological class (F, fusiform; M, multipolar; P, pyramidal; U, unclassified) that were retrogradely labelled from the PAG (black bars) or that were labelled only from the CVLM (grey bars) in experiments 4–6. Percentages from each experiment are shown separately: in each cluster the left, middle and right bars (black or grey) represent experiment 4, 5 and 6, respectively. (B) Shows the equivalent morphological data for cells projecting to the PAG or that were only labelled from the LPb in experiments 7–9. In C the results for all cells labelled from the LPb (experiments 7–9, black bars) are compared with those for all cells labelled from the CVLM (experiments 4–6, grey bars). Again, each column shows results from a different experiment. The histograms in D–F show the percentages of retrogradely labelled neurons that were not NK1 receptor-immunoreactive (n), or that were weakly (+), moderately (++) or strongly (+++) NK1 receptor-immunoreactive. The arrangement of bars is the same as in A–C. Note that neurons with strong and moderate NK1 receptor-immunoreactivity are under-represented among the populations retrogradely labelled from the PAG in D and E.
NK1 receptor-immunoreactive profiles formed a dense plexus in lamina I, as described previously (e.g. Bleazard et al., 1994; Liu et al., 1994; Brown et al., 1995; Littlewood et al., 1995; Marshall et al., 1996; Todd et al., 2000). Immunostaining was present throughout the full thickness of the sections, indicating that both primary and secondary antibodies had penetrated through the tissue, however, the brightness of NK1 receptor-immunoreactivity was invariably stronger near the upper surface of the Vibratome sections and diminished progressively with increasing depth. At low magnification, strong NK1 receptor-immunostaining could be seen on the cell bodies and dendrites of certain retrogradely labelled neurons (Fig. 8). In sections scanned with an oil-immersion lens, NK1 receptor-immunostaining was seen on the plasma membrane surrounding the cell bodies and dendrites of most retrogradely labelled neurons (7, 9). In most cases the intensity of immunostaining on dendrites was very similar to that seen on the cell bodies from which they originated, however, in some cases NK1 receptor-immunoreactivity became stronger on dendrites, as reported previously (Marshall et al., 1996). The intensity of NK1-staining varied considerably among retrogradely labelled cells and each immunoreactive neuron was classified as strong (+++), moderate (++) or weak (+). Examples of cells with different intensities of NK1 receptor-immunostaining are shown in Fig. 9. The proportion of all retrogradely labelled neurons in each experiment that were NK1 receptor-immunoreactive varied from 72.1 to 83.8% (mean 77.4 ± 4.9 SD.). When cells retrogradely labelled from the PAG were compared with those that were labelled only from the CVLM or the LPb, there was no significant difference between the percentage that were NK1 receptor-immunoreactive in each population (P > 0.1, Kruskall-Wallis anova, Fig. 7D–F). However, we did observe that in all six experiments, neurons with strong (+++) or moderate (++) NK1 receptor-immunoreactivity appeared to be under-represented in the population that was retrogradely labelled from the PAG (7-9). Subsequent statistical analysis of NK1 receptor-immunostaining intensity confirmed that this differed significantly between these different projection populations (P < 0.001, General Linear Model). Tukey's test posthoc showed that cells that were retrogradely labelled from the PAG had significantly lower NK1 receptor-immunostaining intensity than those that were only labelled from the CVLM or LPb (P < 0.02).
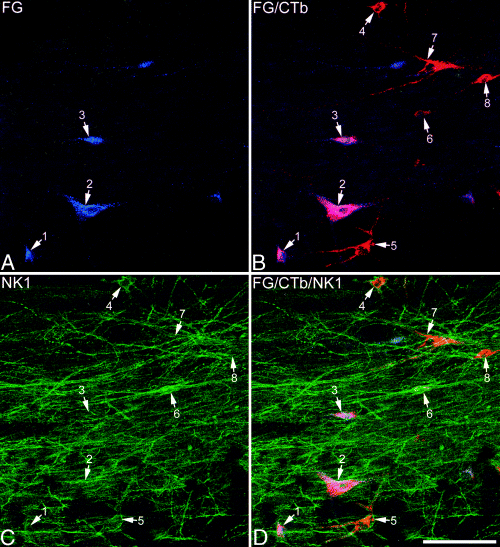
Part of the horizontal section of L3 that was analysed from experiment 4 (CTb injected into CVLM, Fluorogold into PAG). The section was scanned with a 20× dry lens to reveal Fluorogold (blue), CTb (red) and NK1 receptor (green). Each image shows a different antigen or antigen combination. (A and B) The field contains cell bodies of eight retrogradely labelled lamina I neurons (numbered arrows). Three of these (1–3) were labelled from both the PAG (Fluorogold) and CVLM (CTb), and appear pink in B, while the remaining cells (4–8) were labelled from CVLM but not PAG, and appear red in B. (C and D) In many cases it is difficult to resolve NK1 receptor-immunostaining on individual neurons in projected images at this magnification, but it is possible to see that cells 4, 5, 6 and 7 are outlined by NK1 receptor-immunoreactivity. Images through each of these cells scanned with an oil-immersion lens are shown in Fig. 9. Scale bar, 100 µm. These confocal images are projections of 16 optical sections at 1 µm z-separation.
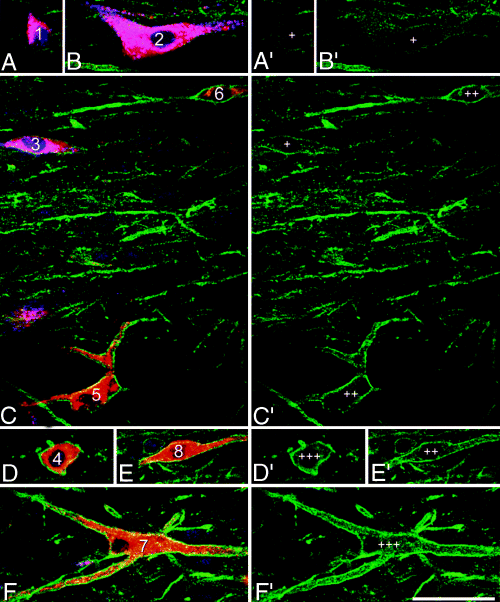
Single optical sections scanned through the cell bodies of the eight retrogradely labelled neurons shown in Fig. 8. All images were scanned with a 40× oil-immersion lens. In each case, the image on the left (A–F) shows Fluorogold (blue), CTb (red) and NK1 receptor (green), while that on the right (A′–F′) shows only NK1 receptor. Cells are numbered as in Fig. 8. Cells 1–3 contain both Fluorogold (transported from the PAG) and CTb (from the CVLM), and therefore appear pink, while cells 4–8 are only labelled with CTb, and appear red. Although all of the cells show NK1 receptor-immunoreactivity on their plasma membrane, the intensity of staining varies considerably. Cells 1–3 were scored as weakly NK1 receptor-immunoreactive (+), cells 5, 6 and 8 were classed as moderately immunoreactive (++), and cells 4 and 7 as strongly immunoreactive (+++). Scale bar, 50 µm.
We investigated how NK1 receptor-immunoreactivity varied with morphology by comparing the proportion of NK1 receptor-immunoreactive lamina I projection neurons in each morphological class for all of the retrogradely labelled lamina I neurons pooled from all six experiments (n = 755; Table 4). This analysis showed that the proportion of NK1-positive cells differed according to shape (P < 0.05, Kruskall-Wallis anova). The Mann–Whitney U-test posthoc showed that significantly fewer unclassified cells were NK1 receptor-immunoreactive (61% ± 13 SD) compared to multipolar (80% ± 6) and pyramidal neurons (81% ± 10) (P < 0.05). When intensity of NK1 receptor immunostaining was compared to morphology, we found that pyramidal neurons had significantly stronger immunoreactivity than both fusiform and unclassified cells (General Linear Model, P < 0.01; Tukey's test posthoc, P < 0.05).
| Morphology | NK1 receptor immunostaining intensity | Total | |||
|---|---|---|---|---|---|
| n (%) | + (%) | ++ (%) | +++ (%) | ||
| Fusiform | 62 (25) | 69 (28) | 76 (31) | 40 (16) | 247 |
| Multipolar | 47 (19) | 87 (36) | 52 (21) | 58 (24) | 244 |
| Pyramidal | 44 (20) | 61 (28) | 56 (26) | 57 (26) | 218 |
| Unclassified | 18 (39) | 16 (35) | 2 (4) | 10 (22) | 46 |
- The table shows data pooled from all of the cells analysed in the L3 horizontal sections from experiments 4–9 (n = 755). Columns 2–5 show the numbers of neurons that were not NK1 receptor-immunoreactive (n), or that were weakly (+), moderately (++) or strongly (+++) NK1 receptor-immunoreactive. The figures in brackets show these numbers expressed as percentages of the total population of cells in that morphological class.
Estimate of the total number of lamina I neurons
Counts of lamina I neurons in an 11-µm thick depth of tissue obtained by using the modified disector method gave a mean value of 36.6 (± 7.4 SD) per side, corresponding to 3.33 neurons per µm. Assuming that the length of the L4 segment is 2.5 mm, this would give a value of 8318 lamina I neurons on each side in this segment.
Discussion
The major findings of this study were: (i) that injections into either the CVLM or the LPb labelled similar numbers of lamina I neurons on the contralateral side of the dorsal horn (approximately 10 cells per 70 µm-thick transverse section of L4), and that over 80% of the cells labelled from either of these targets were also labelled from the other; (ii) that while fewer lamina I neurons were labelled by injections into the contralateral PAG (approximately three cells per section), over 90% of these were also labelled from either LPb or CVLM; (iii) that most of the retrogradely labelled neurons that were seen in lamina I on the ipsilateral side following injections into CVLM or LPb were also labelled from the contralateral side, which indicates that they project bilaterally; (iv) that the morphology of lamina I projection neurons on the contralateral side of the spinal cord did not differ significantly for different projection targets, and (v) that although the proportion of NK1 receptor-immunoreactive contralateral lamina I projection neurons did not differ between the different projection populations, neurons labelled from the PAG displayed a significantly lower level of NK1 receptor-immunostaining than those that were only labelled from the CVLM or LPb.
Retrograde tracers and injection sites
We used CTb and Fluorogold as retrograde tracers as both can give extensive filling of labelled neurons and both can be revealed with confocal microscopy. All of the PAG injections involved Fluorogold and in most cases the CVLM was injected with CTb, however, for the LPb there were five injections with each tracer (Table 1) and it is therefore possible to compare the numbers of labelled cells resulting from injections with each tracer. In the LPb experiments, the mean number of retrogradely labelled contralateral lamina I neurons per section in L4 was 10 for Fluorogold injections and 9.8 for those involving CTb (calculated from data in Table 2). This suggests that both tracers had a similar efficiency of labelling, and makes it unlikely that the choice of tracer influenced the numbers of cells labelled from each target.
Both retrograde and anterograde tracing studies have shown that the PAG, LPb and CVLM are major targets of lamina I neurons in rat, cat and monkey (Menétrey et al., 1982, 1983; Cechetto et al., 1985; Hylden et al., 1989; Lima & Coimbra, 1989; Lima et al., 1991; Slugg & Light, 1994; Craig, 1995; Feil & Herbert, 1995; Villanueva & Bernard, 1999). The main termination zones of spinal afferents in the PAG are the lateral and ventrolateral columns, and the spinal input is largely restricted to its caudal part (Lima & Coimbra, 1989; Bernard et al., 1995; Craig, 1995; Keay et al., 1997; Mouton & Holstege, 1998). This area was included in all of the PAG injection sites (1, 2). Spinal projections to the LPb are arranged somatotopically (e.g. Feil & Herbert, 1995). Those from the lumbar spinal cord arborize profusely in the lateral part of the LPb (dorsal lateral and central lateral nuclei and the lateral crescent), with some extension into adjacent areas (external lateral, internal lateral and Kölliker-Fuse nuclei) (Slugg & Light, 1994; Feil & Herbert, 1995). In all of our experiments that involved LPb injections, the spread of tracer at the rostrocaudal level of the injection included the main termination zone of axons from the lumbar spinal cord 1-3). Hylden et al. (1989) counted the number of spinoparabrachial neurons in lumbar spinal cord sections following injections of tracer into the LPb. They reported a mean value of 10.8 labelled contralateral lamina I cells per 50 µm section counted in three rats, which is approximately 50% higher than our estimate (9.9 cells per 70 µm section). However, at least part of the difference between these estimates may be due to technical reasons, as Hylden et al. (1989) did not apparently correct for the presence of cells that were transected at the section surfaces, and do not state whether cells were only included if the nucleus was at least partly contained within the section. In the cat and monkey the CVLM is known to be a major target for axons of lamina I neurons, which terminate extensively in the area between the lateral reticular and spinal trigeminal nuclei (Craig, 1995). Lima et al. (1991) identified this as a region from which large numbers of lamina I cells could be retrogradely labelled in the rat. However, many axons ascending from the spinal cord lie in the white matter just ventral to the lateral reticular nucleus (Mehler, 1969; Zemlan et al., 1978), and with the relatively large CVLM injections used in these experiments, it is possible that some of the retrograde labelling that we observed resulted from uptake by axons that passed through the injection site but did not terminate within it.
Lamina I neurons labelled from more than one injection site
Our finding that virtually all of the lamina I neurons labelled following injections into the PAG were also labelled from the LPb suggests that the cells which project to the PAG are a subset of lamina I spinoparabrachial neurons. To our knowledge, this has not been demonstrated before with anatomical methods, but it is compatible with the report by McMahon & Wall (1985) who identified the PAG and an area including the LPb as major sites of termination for axons of cells recorded in lamina I in the rat, and reported that some of these cells had axons that sent collaterals to both regions.
The substantial overlap of the populations labelled from CVLM and LPb (experiments 1–3) or CVLM and PAG (experiments 4–6) is more difficult to interpret, as the CTb labelling in these cases may have resulted from uptake of tracer by axons passing through the injection site as well as by collaterals terminating within the CVLM (see above). However, the results of these experiments demonstrate that the populations of lamina I neurons labelled from the CVLM and LPb overlap substantially, and also suggest that injection of tracer in either site will label the great majority of lamina I projection neurons (see below).
Following injections into the LPb or CVLM, most of the retrogradely labelled lamina I neurons are contralateral to the injection site, however, a few are present on the ipsilateral side. The results from experiments 10–13 suggest that the ipsilateral cells project to both sides of the brain, and that there are relatively few lamina I neurons with exclusively ipsilateral projections. Yamada & Kitamura (1992) injected different fluorescent tracers into the right and left LPb and found some double-labelled cells in lamina I, however, fewer than half of the neurons on the ipsilateral side were double-labelled in their experiments. The difference between their results and ours is probably due to the much more restricted injection sites that they used, which involved only the medial half of the LPb, an area that receives relatively sparse input from lamina I neurons in the lumbar spinal cord (Slugg & Light, 1994; Feil & Herbert, 1995).
Morphology of lamina I projection neurons
Lima & Coimbra (1989) and Lima et al. (1991) reported substantial differences in the proportions of neurons belonging to each morphological class that were labelled from different brainstem targets in the rat. They concluded that fusiform neurons projected mainly to the CVLM or LPb, and pyramidal neurons to the PAG. Following injections into the CVLM, Lima et al. (1991) stated that 80% of the labelled neurons in lamina I were fusiform. In contrast, we found that cells in each morphological class were approximately equally represented among the populations of lamina I neurons labelled from each target (Fig. 7A–C), and that there were no significant differences between these populations in terms of morphology. In addition, we observed that only 35–36% of neurons labelled from the CVLM were fusiform (Fig. 7C). It is difficult to explain the differences between our findings and those of Lima and colleagues. In our study, the morphological analysis was carried out on a single horizontal section from each animal, and neurons in the lateral third of the dorsal horn were therefore not examined. However, between 105 and 148 neurons labelled from CVLM or LPb were included in the sample analysed from each experiment (Table 3), and assuming that the size of the populations in L3 and L4 are similar, this represents at least 30% of the total population that would have been labelled from each of these targets (see Table 2). Although there may be some differences in the morphology of projection neurons in the lateral third of the dorsal horn, it is unlikely that this could account for the dramatic differences between our results and those of Lima et al. (1991). Our findings are more in line with those of Andrew et al. (2003), who reported that approximately 40% of the lamina I neurons labelled with CTb from the ventrolateral medulla in the cat were fusiform cells.
A further difference between our results and those of Lima et al. (1991) is that they observed more retrogradely labelled lamina I neurons on the ipsilateral side of the spinal cord than on the contralateral side following CVLM injections, whereas we have consistently seen many more cells on the contralateral side (Table 2; Todd et al., 2000). The pattern that we have observed is consistent with the report by McMahon & Wall (1983) that most neurons recorded in lamina I of the rat lumbar spinal cord have axons projecting through the contralateral white matter.
NK1 receptor expression by lamina I projection neurons
NK1 receptor-immunoreactivity has been found on lamina I neurons projecting to several sites, including the thalamus, PAG, LPb and CVLM (Ding et al., 1995; Li et al., 1996, 1998; Marshall et al., 1996; Todd et al., 2000, 2002). In a previous quantitative study of different populations of lamina I projection neurons, we estimated that NK1 receptor-immunoreactivity was present on 74–82% of those labelled from the LPb and 78–85% of those labelled from the CVLM (Todd et al., 2000). The sample of neurons that projected to the PAG in that study was too small to allow accurate quantification, but the great majority of these cells (29 of 36, data pooled from three animals) were NK1 receptor-immunoreactive. In the present study, NK1 receptor-immunoreactivity was analysed in the horizontal sections from three experiments each involving LPb and CVLM injections, and from six experiments with PAG injections (experiments 4–9). The proportions of projection neurons in each population that were NK1 receptor-immunoreactive were 74–84% for cells labelled from the LPb, 74–83% for those labelled from the CVLM and 45–90% (median 74%) for cells labelled from the PAG (Fig. 7D–F). These results are consistent with the percentages that we reported previously (Todd et al., 2000).
The strength of NK1 receptor-immunoreactivity varies considerably between neurons in lamina I, and we therefore investigated whether immunostaining intensity was related to projection target or morphology. Although pixel luminance values can be used to quantify immunofluorescence intensity and compare different structures in a single optical plane, this approach was not feasible in the present study. Retrogradely labelled cells that were included in the analysis were located at varying depths within the 70-µm thick Vibratome sections, and as the intensity of fluorescence corresponding to NK1 receptor-immunostaining intensity was always greatest near the upper surface of the section and progressively diminished at increasing depths (due to absorption of light from the laser by fluorochromes in tissue above the optical plane), cells near the upper surface tended to appear brighter than those deep in the section, making it impossible to compare cells at different depths in each section. We therefore used a scoring system to rate neurons as strongly (+++), moderately (++) or weakly (+) immunoreactive, based on a comparison with other immunoreactive structures in the same optical section. Although not quantitative, this approach allowed us to compensate for variation in the apparent staining intensity at different depths in the section. An additional benefit was that staining over both the cell body and dendrites (which were often followed through several optical sections) could be taken into account when allocating a score for each cell.
We found that although many lamina I neurons with strong or moderate NK1 receptor-immunoreactivity were retrogradely labelled from CVLM or LPb, they were seldom labelled by PAG injections, and this result indicates that the cells which project to the PAG constitute a specific subset of lamina I projection neurons. Intrathecal injection of substance P conjugated to the ribosome-inactivating protein saporin selectively destroys NK1 receptor-expressing neurons in the dorsal horn, and has a significant antihyperalgesic effect in rats with either inflammatory or neuropathic pain states (Mantyh et al., 1997; Nichols et al., 1999). Interestingly, doses of substance P-saporin that reduce hyperalgesia do not completely destroy NK1 receptor-immunoreactive lamina I neurons (Mantyh et al., 1997; Nichols et al., 1999), and as the majority of cells that project to the PAG express a low level of the receptor, it is possible that these neurons are less severely affected following substance P-saporin treatment.
Yu et al. (1999) found that only 6% of NK1 receptor-immunoreactive neurons in lamina I of the monkey spinal cord were pyramidal cells, while 75% of pyramidal cells that projected to the thalamus lacked the receptor. They therefore concluded that pyramidal cells were considerably under-represented among lamina I neurons with the NK1 receptor. In contrast, we found in a previous study that at least 80% of the pyramidal neurons in lamina I of the rat spinal cord that were labelled from the CVLM were NK1 receptor-immunoreactive (Todd et al., 2002). In the present study, we have extended this observation by showing that when results from the L3 segments of experiments 4–9 were pooled, 80% of all projection neurons identified as pyramidal cells were NK1 receptor-immunoreactive, and that for many of these cells (52%) immunoreactivity was classed as strong or moderate. It is likely that the discrepancy between our findings and those of Yu et al. (1999) is due to a species difference between rat and monkey. A possible explanation is that the lack of NK1 receptor on most pyramidal neurons in the monkey is a feature of the spinothalamic projection, which makes up only a very small component of the lamina I projection in the rat lumbar cord (see below).
The number of lamina I projection neurons in mid-lumbar spinal cord
We have estimated that the total number of lamina I neurons on one side of the L4 segment that would be labelled by injections of tracer into both the CVLM and the LPb on the contralateral side is approximately 400 (see above). In order to determine the total number of lamina I neurons with axons that project to the brain, it is necessary to consider whether other tracts make a significant additional contribution, i.e. whether cells projecting to other sites in the brain would not have been labelled from the contralateral CVLM or LPb. We found that 94–97% of the lamina I neurons that project to the contralateral PAG were also labelled from the CVLM or LPb, and therefore all (or virtually all) of these will be included in the contralateral CVLM and/or LPb populations. Similarly, the great majority of lamina I neurons on the ipsilateral side labelled from the CVLM (92%) or the LPb (88%) project bilaterally, and will also be included in this population.
The number of spinothalamic tract neurons in lamina I of the rat lumbar spinal cord appears to be very small compared to the numbers that can be labelled from the CVLM or LPb. Burstein et al. (1990a) estimated that there were 25 contralateral lamina I spinothalamic neurons in the L4 and L5 segments combined, while Lima & Coimbra (1988) obtained a mean value of 37.5 of these cells in the L3 and L4 segments (without using stereological correction). In a previous study of rats with thalamic injections, we found on average 0.3 labelled contralateral lamina I cells per 60-µm section in the lumbar cord, which corresponds to 14 cells in a 2.5-mm spinal segment (Marshall et al., 1996). As Hylden et al. (1989) reported that 80% of lamina I spinothalamic neurons in rat lumbar cord were also labelled by injection of tracer into the LPb, it is likely that the spinothalamic tract will not add significantly to the number of lamina I projection neurons estimated above. A spinohypothalamic tract has been reported arising partly from lamina I cells in the rat (Burstein et al., 1990b); however, axons belonging to these cells are thought to pass through the posterior nucleus of the thalamus (Kostarczyk et al., 1997), and are therefore likely to have been included in the spinothalamic population examined by Hylden et al. (1989).
Lamina I neurons also project to various other sites, including the nucleus of the solitary tract and the dorsal reticular nucleus (e.g. Menétrey & Basbaum, 1987; Lima, 1990; Villanueva & Bernard, 1999). We found that injections into an area that included both of these nuclei resulted in labelling of approximately two contralateral lamina I neurons per 70-µm section in rat lumbar spinal cord (Todd et al., 2000). Although there are apparently no retrograde tracing studies to indicate whether these cells are included in populations labelled from other sites, McMahon & Wall (1985) reported that axons of units recorded in the superficial dorsal horn and with collaterals that entered the dorsal part of the medullary reticular formation invariably projected to the PAG. Therefore at least the majority of lamina I cells labelled from dorsal reticular or solitary tract nuclei are likely to be spinomesencephalic neurons.
It is therefore likely that injections of tracers into both CVLM and LPb will label virtually the entire population of lamina I neurons with supraspinal projections, and that approximately 400 such cells are present on each side in the L4 segment (equivalent to 1.6 cells per 10 µm length of spinal cord). Bice & Beal (1997b) estimated the total number of lamina I projection neurons in the L1 segment of the rat, by making large tracer injections into the thalamus, midbrain and medulla, in an attempt to label the entire population of projection cells. They concluded that there were 1.22 lamina I projection neurons per 10-µm section; however, they assumed that cells labelled on the ipsilateral side did not project bilaterally, and therefore (according to our findings) they would have overestimated the size of the population by around 25%. The difference between our estimate and the value obtained by Bice and Beal can be accounted for by the smaller cross-sectional area of lamina I in the L1 segment.
Overall, our results suggest that the L4 segment of the rat contains approximately 400 neurons that project to the brain. The great majority of these (approximately 85%) send collaterals to the contralateral LPb (and in many cases terminate within it), while approximately 30% reach the caudal PAG, and fewer than 5% project as far as the diencephalon. At least 85% of lamina I projection neurons can also be labelled from the CVLM, either via collaterals that terminate there or else through uptake of tracer into the parent axons. Although most lamina I cells have axons that decussate and remain exclusively on the contralateral side of the brain, a significant proportion (approximately 25%) project bilaterally in the rat. As we estimate that the L4 segment of the rat contains approximately 8300 lamina I neurons, we conclude that projection neurons make up around 5% of the neuronal population in this lamina.
Acknowledgements
We thank Dr E. Polgár for helpful discussion, Mrs M. M. McGill, Mrs C. Watt and Mr R. Kerr for expert technical assistance, and the Wellcome Trust for financial support.
Abbreviations
-
- CTb
-
- cholera toxin B subunit
-
- CVLM
-
- caudal ventrolateral medulla
-
- DAPI
-
- 4′,6-diamidino-2-phenylindole
-
- LPb
-
- lateral parabrachial area
-
- NK1
-
- neurokinin 1
-
- PAG
-
- periaqueductal grey matter.