Effect of NT-4 and BDNF delivery to damaged sciatic nerves on phenotypic recovery of fast and slow muscles fibres
Abstract
We investigated whether neurotrophin-4 (NT-4) and brain-derived neurotrophic factor (BDNF) affected the reinnervation of slow and fast motor units. Neurotrophin-impregnated or plain fibronectin (FN) conduits were inserted into a sciatic nerve gap. Fast extensor digitorum longus (EDL) and slow soleus muscles were collected 4 months postsurgery. Muscles were weighed and fibre type proportion and mean fibre diameters were derived from muscle cross-sections. All fibre types in muscles from FN animals were severely atrophied and this correlated well with type 1 fibre loss and atrophy in soleus and type 2b loss and atrophy in EDL. Treatment with NT-4 reversed soleus but not EDL mass loss above the FN group by significantly restoring type 1 muscle fibre proportion and diameters towards those of normal unoperated animals. BDNF did not increase muscle mass but did have minor effects on fibre type and diameter. Thus, NT-4 significantly improved slow motor unit recovery, and provides a basis for therapies intended to aid the functional recovery of muscles after denervating injury.
Introduction
Our general objective is to reduce the time that target tissues are without mature and relevant nerve support after nerve injuries and hence to reduce muscle atrophy and functional deficit. We showed previously that delivery of neurotrophin-3 (NT-3) to proximal stumps of cut sciatic nerves improved reinnervation of fast motor units by fast fatiguable motoneurons (Sterne et al., 1997a). Neurotrophin-3 applied directly to proximal stumps of cut sciatic nerves in rat thighs caused specific post-reinnervation recovery of type 2B muscle fibres in terms of normalizing their proportion and diameter (Sterne et al., 1997b). There was no such recovery of any other muscle fibre type. This improvement was not a result of increased speed of reinnervation per se but rather was because of a more rapid recovery of the maturation of neuromuscular junctions (Simon et al., 2000) and that this specific effect appeared to correlate with differential distribution of tyrosine kinase (trk)C receptors on motoneurons (Simon et al., 2002). Thus, our hypothesis was that NT-3 has specific and direct effects on a subpopulation of fast-fatiguable motoneurons and that this effect was long lasting (at least 240 days) even though the NT-3 applied to the nerve was turned over within 7 days (Sterne et al., 1997a). The implication was therefore that other types of motoneuron would be similarly supported by other neurotrophic factors.
Here, we investigated whether two other trophic factors neurotrophin-4 (NT-4) and brain-derived neurotrophic factor (BDNF) had selective effects on motoneurons. Support for a functional role for NT-4 on motoneuron repair was suggested by the observation that during muscle exercise, increased NT-4 expression by skeletal muscle induced sprouting in adult motoneurons (Funakoshi et al., 1995). Moreover, NT-4 as well as BDNF appear to be involved in maintaining slow motoneuron phenotype (Gonzalez & Collins, 1997; Munson et al., 1997a). Both these neurotrophins are expressed by adult motoneurons (Buck et al., 2000) and muscles (Funakoshi et al., 1995; Sakuma et al., 2001). They both share the tyrosine kinase B (trkB) high affinity receptor (Lindsay, 1996) that is expressed in motoneurons (Friedman et al., 1995a). However, their regulation appears to be different in that, on the one hand, after axotomy BDNF mRNA is upregulated in motoneurons and muscles and trkB mRNA is upregulated in motoneurons (Griesbeck et al., 1995; Kobayashi et al., 1996), whilst on the other hand, NT-4 transcription is downregulated. Interestingly, treatment with BDNF and with NT-4 reverses the downregulation of choline acetyltransferase expression in axotomised motoneurons and increases the expression of p75 receptors (Friedman et al., 1995a). Moreover, it was shown that NT-4 reverses the downregulation of conduction velocity of axotomised motor axons (Munson et al., 1997b) and BDNF facilitates motor axon outgrowth of avulsed motoneurons (Kishino et al., 1997; Novikov et al., 1997).
When skeletal muscles are denervated muscles rapidly lose mass because of muscle fibre atrophy (Pellegrino & Franzini, 1963) If reinnervation occurs there is gradual recovery in mass because of recovery of muscle fibre size, though this is highly variable depending upon the extent of successful reinnervation (Bertelli & Mira, 1995) but does correlate with functional recovery in maximum force of contraction (Gillespie et al., 1987). Normal adult muscles have characteristic proportions of the various fibre types with slow type 1 fibres dominating in tonically contracting postural muscles such as soleus and fast 2a, 2x/d and 2b fibres dominating in muscle performing powerful phasic contractions (Bar & Pette, 1988). The fibres within an individual muscle vary and the proportion of the various fibre types also varies between muscle types and this correlates with their physiological characteristics (Burke et al., 1971; Peter et al., 1972). Muscle phenotype is largely a result of the type of motoneuron innervation it receives and changes in neuromuscular interaction alter muscle fibre type (Romanul & Van der Meulen, 1966; Fex & Sonneson, 1970; Salmons & Sreter, 1976; Mendell et al., 1994). Thus, after denervation in the rat the majority of fibres, including those that were slow type 1 become fast (Fields & Ellisman, 1986). This indicates functional innervation is required to induce the expression of slow myosin heavy chain that characterizes type 1 muscle fibres (Bar & Pette, 1988) and that conversely the reacquisition of type 1 muscle fibre type after denervation is an indicator of functional reinnervation. Therefore, the reacquisition of muscle fibre typing and recovery of muscle fibre diameter are indicative of reinnervation and phenotypic recovery. In this study, we tested the hypothesis that NT-4 and/or BDNF applied to cut nerves have differential effects on the biochemical and morphological recovery of muscle fibres.
Materials and methods
Animals and surgical procedure
All procedures were carried out in accordance with the requirements of the UK Animals (Scientific Procedures) Act 1986.
Eight-week-old-inbred male Lewis rats were anaesthetized using 0.3 mL/kg intramuscular Hypnorm (0.315 mg/mL fentanyl citrate and 10 mg/mL fluanisone; Janssen Pharmaceuticals Ltd) and 2.5 mg/kg intraperitoneal diazepam (Phoenix Pharmaceuticals Ltd.).
A 5-mm length of sciatic nerve of was removed to produce a 10-mm gap after retraction of nerve ends. Gaps were bridged using fibronectin conduits (Sterne et al., 1997a, b; Simon et al., 2000) impregnated with either BDNF (1000 ng/mL, BDNF group, Autogen Bioclear, Wilts, UK), NT-4 (1000 ng/mL, NT-4 group, Autogen Bioclear) or NT-3 (500 ng/mL, NT-3 group, Regeneron Pharmaceutical Inc., NY, USA). Plain fibronectin conduits were inserted in a fourth group of rats (FN group) and a further unoperated group was used as controls (U group). A total of 5–6 animals from each group were killed by cervical dislocation at 120 days postsurgery. Previous studies showed that this was sufficient time for reinnervation of muscles to occur even in fibronectin-only treated animals and for the identification of any effects of neurotrophic factors on muscle fibre phenotype (Simon et al., 2000; Sterne et al., 1997b). We also showed previously that NT-3 and nerve growth factor were turned over within approximately 7 days (Sterne et al., 1997a). During this period no appreciable reinnervation of muscle occurred. Fast EDL and slow soleus muscles were dissected at autopsy and muscle weights measured. A transverse disk corresponding to the proximal part of the distal third of each muscle was processed for cryosectioning and immunochemistry. Muscle fibre phenotype was assessed by immunostaining of myosin heavy chain (MHC) 1 (BA-F8 monoclonal antibodies, 1 : 50 dilution), 2a (SC-71 monoclonal antibodies, 1 : 50 dilution) and 2b (BF-F3 monoclonal antibodies, 1 : 10 dilution; Schiaffino et al., 2000). Serial 10-µm transverse sections collected on Vectabond-coated slides (Vector, UK) were air dried, washed in phosphate-buffered saline (PBS; 5 min) and incubated in PBS containing 5% normal goat serum, 5% normal swine serum, 2% bovine serum albumin and 0.1% triton X-100 (1 h). Sections were incubated with the primary antibodies against MHCs and laminin (polyclonal rabbit anti-laminin, Sigma, UK; 1 : 200 in PBS containing 2% bovine serum albumin and 0.1% triton X-100, for 3 h at room temperature). Sections were washed in 3 × 15 min changes of PBS, and incubated with cyanin-3-conjugated goat anti-mouse serum (1 : 200 in PBS; Affinity, UK) and fluorescein isothiocyanate-conjugated swine anti-rabbit polyclonal secondary antibodies (1 : 200 in PBS; Affinity) before a final rinse in PBS (3 × 10 min). Sections were mounted in Vectashield (Vector).
Quantification and morphometric analysis
Five fields (objective × 10 for U and NT-4 groups, × 20 for FN and BDNF groups) were chosen at random for each muscle and images for each MHC isoform-immunolocalization plus that for laminin were captured using appropriate emission filters, and were combined to provide dual-labelled images of laminin/MHC. Image Pro-Plus software (Media Cybernetics) was used to measure the diameters of and count different MHC-labelled muscle fibres.
Statistical analyses
Statview was use to calculate one-way analysis of variance (anova) and a Krushkal–Wallis one-way anova on rank to analyse the results of muscle fibre percentages and diameters. Differences between individual groups were subsequently identified by the Student t-test.
Results
Effect of NT-4, BDNF and NT-3 on muscle mass recovery
Figure 1 shows that at 120 days postsurgery, the masses of both EDL and soleus muscles in the FN group were significantly smaller than muscles from the control unoperated group (U group). Incorporation of NT-4 in fibronectin mats (NT-4 group) caused a significant recovery of soleus muscle mass (NT-4 vs. FN group, P < 0.0003; NT-4 vs. U group, P < 0.02; Fig. 1) but had no effect on EDL. BDNF, however, did not produce an increase in the mass of either soleus or EDL over the FN group. As we found previously, NT-3 specifically increases EDL muscle mass (NT-3 vs. FN group, P < 0.004; Fig. 1; Sterne et al., 1997b) and this correlated with preferential reinnervation by fast motoneurons after NT-3 treatment (Simon et al., 2000). NT-3 had no effect on soleus muscle mass.
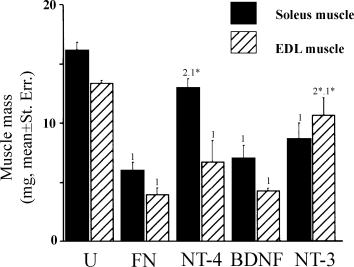
Differential effects of NT-4, BDNF and NT-3 on muscle mass (mean ± standard error) recovery of reinnervated fast EDL and slow soleus muscles at 120 days after sciatic nerve axotomy and repair. One-way anova, P < 0.001. Student's t-test or Fisher's PLSD test, FN : NT-4 : BDNF : NT-3 vs. U ‘1’ = P < 0.001, ‘1*’ = P < 0.05. NT-4 : NT-3 vs. FN ‘2’ = P < 0.0005, ‘2*’ = P < 0.005.
Effect of neurotrophin treatment on EDL and soleus muscle fibre type proportion
It should be noted that at the time of this study antibodies specific for MHC2x/d were not available and so the percentages presented reflect the proportions of Type 1, 2a and 2b muscle fibres. The remainder may be representative of muscle fibres that were potentially of type 2x/d.
In unoperated control rats (U), soleus muscles comprised a large majority of slow muscle fibres (94%) and few fast 2a muscle fibres (3%) whereas EDL muscles contained only a few slow muscle fibres (3%) scattered among fast muscle fibres that were mainly of fast 2b type (66%) 2-4).
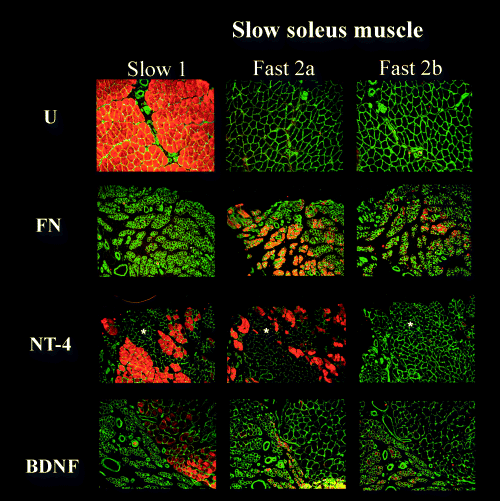
Transverse soleus muscle sections immunostained for MHC 1, 2a and 2b isoforms, from fibronectin-mat (FN), BDNF-treated (BDNF) and NT-4-treated groups (NT-4) at 4 months after nerve repair compared with unoperated controls (U). Stars indicate examples of areas of unclassified muscle fibres. Scale bar, 100 µm (objective × 10).
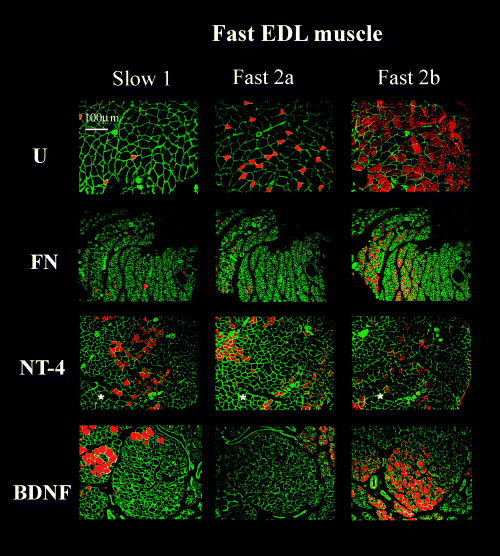
Transverse EDL muscle sections immunostained for MHC1, 2a and 2b isoforms from fibronectin-mat (FN) BDNF-treated (BDNF) and NT-4-treated groups (NT-4) at 4 months after nerve repair compared with unoperated controls (U). Stars indicate examples of areas of unclassified muscle fibres. Scale bar, 100 µm (objective × 10).
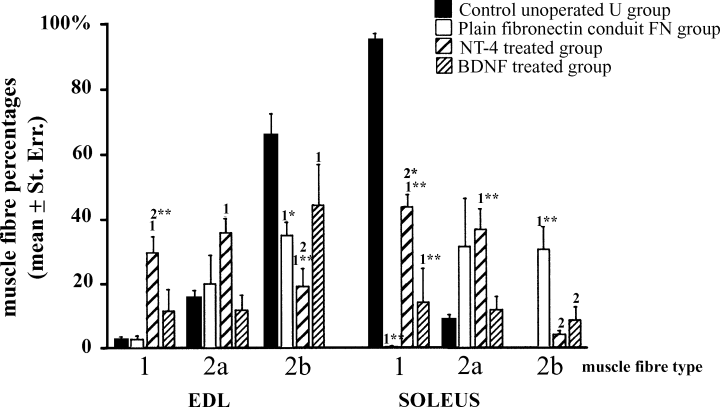
Effects of NT-4 and BDNF on muscle fibre percentages (mean ± standard error) of reinnervated fast EDL and slow soleus muscles at 120 days after sciatic nerve axotomy and repair. One-way anova, P < 0.001. Student's t-test or Fisher's PLSD test, FN : BDNF : NT-4 vs. U ‘1’ = P < 0.05; ‘1*’ = P < 0.005; ‘1**’P < 0.001. NT-4 : BDNF vs. FN ‘2’ = P < 0.05; ‘2*’ = P < 0.001; ‘2**’ = P < 0.0001.
At 120 days after nerve injury in the FN group there was almost complete loss of type 1 muscle fibres from the soleus (<1%; 2-4). This loss was prevented substantially by NT-4 (42% Type 1) and also to a lesser but significant extent by BDNF (16% Type 1). Type 1 fibre loss in the FN group correlated with a significant increase in the proportion of both type 2a (31%) and the appearance of a significant proportion (30%) of type 2b muscle fibres. This was striking, as type 2b muscle fibres were not present in unoperated control muscles. NT-4 had no effect on the type 2a proportion but reduced the proportion of type 2b to only 4%. BDNF reduced the proportion of type 2a (12%) and 2b (8%) fibres in soleus over that seen in the FN group. As the total percentage of the three stainable fibres types in the BDNF group came to approximately (35%), it suggests that the balance could be of type 2x/d.
In the EDL the proportion of type 1 and 2a fibres in the FN group was similar to controls (Type 1 U and FN approx. 3%; Type 2a U, 15 vs. FN, 20%). However there was a significant reduction in the proportion of 2b muscle fibres (U, 66% vs. FN, 35%), again perhaps suggesting an increase in the proportion of type2x/d fibres. NT-4 treatment significantly increased the proportion of type 1 fibres (30%) and type 2a (36%) and further reduced the proportion of 2b fibres (19%) over that seen in the FN group. BDNF did not significantly increase the proportion of either fibre type.
Effect of neurotrophin treatment on EDL and soleus muscle fibre diameter
The biggest muscle fibres in control soleus (U) muscle were slow type 1 (ratio of muscle fibre diameters type1 : type 2a = 2.4; Fig. 5) whereas in EDL muscle the biggest were the fast 2b muscle fibres (ratio 2b : 2a = 1.8 and 2b : 1 = 1.9). Type 1 muscle fibres were bigger in soleus than type 1 fibres in the EDL (ratio EDL : soleus = 1.6) whereas type 2a muscle fibres were bigger in EDL than in soleus (2a muscle fibre diameter ratio soleus : EDL = 1.6). Interestingly, the mean diameters of 2b muscle fibres (44 µm) in EDL muscles were similar to that of type 1 muscle fibres in soleus muscles (38 µm).
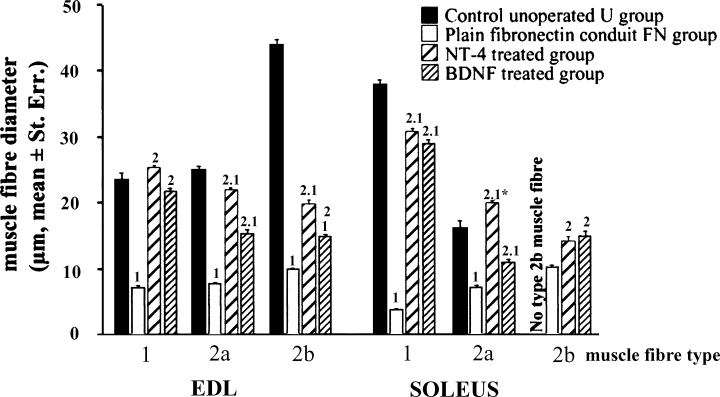
Effects of NT-4 and BDNF on muscle fibre diameter (mean ± standard error) of reinnervated fast EDL and slow soleus muscles at 120 days after sciatic nerve axotomy and repair. One-way anova, P < 0.001. Student's t-test or Fisher's PLSD test, FN : BDNF : NT-4 vs. U ‘1’ = P < 0.001; ‘1*’ = P < 0.005; NT-4 : BDNF vs. FN ‘2’P < 0.001.
In the soleus of the FN group both type1 and type 2a muscle fibres are dramatically atrophied compared with controls (ratio FN : U, type 1 = 0.1; type 2a = 0.45; Fig. 5). Treatment by NT-4 restored type 1 and type 2a muscle fibre mean diameters almost to normal (NT-4 : U, type 1 = 0.81; type 2a = 1.2). BDNF also caused almost complete recovery of type 1 fibre diameter (BDNF : U type 1 = 0.76) but whereas BDNF also improved type 2a fibres, this was less impressive (BDNF : U type 2a = 0.68). As no type 2b fibres were detected in control soleus muscles direct comparisons with those that appeared in FN, NT-4 and BDNF treated animals were not possible. Type 2b fibres in FN, NT-4 and BDNF treated animals were significantly smaller than type 1 fibres in controls. Nevertheless, type 2b fibres in NT-4- and BDNF-treated animals had diameters similar to those of 2a fibres in controls but were, however, smaller than type 2b fibres in control EDL muscles.
In the EDLs from the FN group there was atrophy of all fibre types (ratio FN : U type 1 = 0.30; type 2a = 0.31; type 2b = 0.23). NT-4 restored type 1 and type 2a muscle fibre diameters to normal (ratio type1 NT-4 : U = 1.1; type 2a NT-4 : U = 0.88) and BDNF treatment also showed significant effects on size recovery (BDNF : U type 1 = 0.93; type 2a = 0.6). The 2b fibres in EDL remained atrophied after NT-4 or BDNF treatment (ratio NT-4 : U type 2b = 0.45; BDNF : U type 2b = 0.34).
Discussion
The hypothesis that NT-4 and/or BDNF applied to cut nerves differentially effect biochemical and morphological recovery of muscle fibres following reinnervation is supported by the data obtained in this study.
Soleus muscles in the FN group were atrophied, had lost virtually all their type 1 fibres and were comprised of type 2a and 2b. In addition, all muscle fibres were significantly smaller in diameter than those of controls. By 120 days the proportion of 2b fibres in EDL had fallen but the proportion of 2a fibres was unchanged from controls. Therefore, in both muscles the impression is one of a move towards a ‘middle ground’ phenotype with loss of the slowest and fastest fibres and increases of type 2a fibres and presumptive 2x/d fibres.
To a large extent NT-4 prevented reduction in soleus muscle mass 120 days after axotomy but it had no effect on EDL muscle mass. The beneficial effect of NT-4 on slow soleus muscle seems to be the prevention of axotomy-induced loss of large diameter slow type 1 muscle fibres and prevention of the atrophy of 2a muscle fibres. However, NT4 did not prevent the loss of large type 2b fast muscle fibres from EDL muscles 120 days after axotomy. Whereas BDNF did cause a partial recovery in the proportion of type 1 fibres in the soleus and these were of approaching normal diameter, the effect was only one-third as large as that seen for NT4. In addition, although 2a fibres also recovered in number, these were only half the diameter of the NT4 group. The result was no detectable recovery of EDL muscle mass. As we have shown in previous studies, NT3 caused specific mass recovery of the EDL muscle (Simon et al., 2000).
In contrast to the FN group where there was substantial loss of type 2b muscle fibres without change in type 1 or 2a proportion in highly atrophied EDL muscles, NT-4 treatment further decreased the proportion of type 2b fibres and substantially increased the proportion of slow type 1 and fast type 2a muscle fibres, both of which were of normal diameter. The impression is therefore in EDL that NT4 causes a shift to a slower phenotype than either the U or FN groups but that muscle masses do not alter because some fibre types increase in proportion and size but others decrease.
Taken together these data show that NT-4 treatment of damaged sciatic nerves generated very significant recovery of the slow phenotype of the soleus muscle and its potential contractile function, as represented by recovery of mean muscle fibre size. NT-4 also imposes a slower phenotype upon the normally fast EDL but although it can increase the diameter of type 1 and 2a fibres it has no effect on 2b.
The observed effects of neurotrophins on muscle fibre type proportions and diameters must be indirect as their administration within the FN mats was at the level of the sciatic notch close to the spinal cord, a considerable distance from the analysed target soleus and EDL muscles. It should also be noted that our previous studies showed that the neurotrophins are completely turned over within a week of implantation (Sterne et al., 1997a). Thus the effects of neurotrophins administered in this fashion are transient and act directly on damaged nerves.
Our new hypothesis is that NT-4 affects all motoneurons and transforms them by imposing a slower phenotype that is reflected in increased proportions of type 1 and 2a muscle fibres in reinnervated muscles. This is supported by the observation that NT-4 selectively improved the reinnervation of slow motor units in soleus muscles as well as in EDL muscles. It is also supported by evidence showing that NT-4 seems to be involved in maintenance of slow motoneuron phenotype (Gonzalez & Collins, 1997; Munson et al., 1997a). Moreover, the selective localization of NT-4 in slow muscle fibres (Funakoshi et al., 1995) suggests that it could act as a retrograde factor inducing a slow phenotype for innervating motoneurons (Mendell et al., 1994). However, NT-4 might also preferentially improve regeneration of motoneurons innervating slow motor units. In fact, it has been shown that trkB protein was observed more frequently at neuromuscular junctions of slow soleus muscles compared with fast gastrocnemius muscles (Sakuma et al., 2001). This result suggests that slow motor units might be more responsive to trkB ligands. In both cases, 2a muscle fibres probably represent the population of atrophied 2b muscle fibres that are reinnervated by slow motoneurons and that undergo phenotypic change (Bacou et al., 1996). This might explain why in soleus muscles type 2a muscle fibres in the NT-4 group are bigger than in the U group, their size being intermediate between muscle fibres of type 1 and 2a. It is also intriguing that not only does NT4 induce a shift towards a slow muscle phenotype but it also resulted in recovery in the diameters of all fibre types in both soleus and EDL over the FN group. This was even true for type 2b fibres in both muscles.
Although, BDNF and NT-4 share the same trkB high affinity receptor (Lindsay, 1996), BDNF had no significant beneficial effect on soleus reinnervation compared to NT-4. The only positive effect that BDNF induced was the size recovery of a few slow and fast 2a muscle fibres, but these healthy fibres were present within a majority of unclassifiable and atrophied muscle fibres. This result was surprising for two reasons: first BDNF had no real beneficial effect on muscle reinnervation; and second, BDNF and NT-4 had differing effects.
In fact, it has been shown previously that BDNF can improve neurite outgrowth of avulsed motoneurons (Kishino et al., 1997; Novikov et al., 1997), increase expression of cholinergic markers (Yan et al., 1994; Friedman et al., 1995b) and prevent soma atrophy of axotomised motoneurons (Li et al., 1995); all these results suggested that BDNF should improve muscle reinnervation. However, other studies reported totally opposite results (Clatterbuck et al., 1994; Henderson et al., 1994). The numerous discrepancies concerning the biological effects of BDNF could be because of the different models and concentrations of BDNF that have been used.
Differing effects of BDNF and NT-4 on the same neurons have been observed previously. It has been shown for example that NT-4 can decrease the sensitivity of some motoneurons to glutamate or malonate toxicity whereas BDNF does not (Corse et al., 1999; Van Westerlaak et al., 2001). In explants of avian occulomotor and trochlear nuclei, BDNF enhanced neurite outgrowth whereas NT-4 had no effect (Steljes et al., 1999).
Both the discrepancies concerning the biological effects of BDNF and the differing effects of BDNF and NT-4 on motoneurons could depend on one major factor. One has to take into account that unlike normal motoneurons, injured motoneurons express the low affinity p75 receptor as well as trkB receptors (Friedman et al., 1995a). Thus, the resulting effect of trkB ligands on motor axonal regeneration may depend on the delicate balance between positive effects via trkB and negative influences via p75 receptors (Boyd & Gordon 2001). We are inclined to believe that this balance would be different for each trkB ligand and for each model employed.
Altogether, our results show that NT-4 preferentially supports and improves the functional reinnervation of slow motor units. BDNF, under the conditions of our experiment, had no significant overall beneficial effect on muscle reinnervation.
Acknowledgements
The authors would like to thank Action Research for the funding of this project.
Abbreviations
-
- anova
-
- analysis of variance
-
- BDNF
-
- brain-derived neurotrophic factor
-
- EDL
-
- extensor digitorum longus
-
- FN
-
- fibronectin
-
- MHC
-
- myosin jeavy chain
-
- NT
-
- neurotrophin
-
- PBS
-
- phosphate-buffered saline
-
- trk
-
- tyrosine kinase
-
- U
-
- unoperated.