Dopamine depresses cholinergic oscillatory network activity in rat hippocampus
Abstract
The dopaminergic neuronal system is implicated in cognitive processes in a variety of brain regions including the mesolimbic system. We have investigated whether dopamine also affects synchronized network activity in the hippocampus, which has been ascribed to play a pivotal role in memory formation. Gamma frequency (20–80 Hz) oscillations were induced by the cholinergic agonist carbachol. Oscillatory activity was examined in area CA3 of Wistar rat hippocampal slices, employing field potential and intracellular recordings. Application of carbachol initiated synchronized population activity in the gamma band at 40 Hz. Induced gamma activity persisted over hours and required GABAA receptors. Dopamine reversibly decreased the integrated gamma band power of the carbachol rhythm by 62%, while its frequency was not changed. By contrast, individual pyramidal cells recorded during carbachol-induced field gamma activity exhibited theta frequency (5–15 Hz) membrane potential oscillations that were not altered by dopamine. The dopamine effect on the field gamma activity was mimicked by the D1 receptor agonist SKF-383393 and partially antagonized by the D1 antagonist SCH-23390. Conversely, the D2 receptor agonist quinpirole failed to depress the oscillations, and the D2 antagonist sulpiride did not prevent the suppressive dopamine effect. The data indicate that dopamine strongly depresses cholinergic gamma oscillations in area CA3 of rat hippocampus by activation of D1-like dopamine receptors and that this effect is most likely mediated via impairment of interneurons involved in generation and maintenance of the carbachol-induced network rhythm.
Introduction
The hippocampal formation plays a central role in learning and memory in the mammalian brain (reviewed in Shen et al., 1994). Substantial progress in understanding the mechanisms underlying learning and memory was made by studying synaptic plasticity as a cellular model of memory (Bliss & Collingridge, 1993). Several lines of evidence indicate the importance of neuromodulators in synaptic plasticity within the hippocampus (Villani & Johnston, 1993; Auerbach & Segal, 1994; Maeda et al., 1994). Thus, recent work suggests that dopamine (DA) can enhance long-term potentiation (Frey et al., 1991; Yanagihashi & Ishikawa, 1992; Huang & Kandel, 1995; Otmakhova & Lisman, 1996) and inhibit depotentiation (Otmakhova & Lisman, 1998a) at hippocampal CA1 synapses. DA has also been demonstrated to be involved in facilitation of long-term depression in CA1 neurons (Chen et al., 1995). Finally, it has been shown that DA modulates transmitter release at cholinergic (Hersi et al., 2000) and glutamatergic (Otmakhova & Lisman, 1998b; Bouron & Reuter, 1999; Behr et al., 2000a,b; Yang, 2000) hippocampal synapses. The significance of DA in synaptic modulation within the hippocampus is supported by anatomical and biochemical studies indicating that the hippocampal formation receives dense mesencephalic dopaminergic input and expresses high levels of all five types of DA receptors (reviewed in Otmakhova & Lisman, 1996).
There is increasing experimental evidence that memory functions of the hippocampus strongly depend on network properties. Particularly, synchronization of neuronal activity within the hippocampal circuitry has been ascribed an important role in learning of sequences and the linkage of specific memories to context (reviewed in Lisman & Otmakhova, 2001). In hippocampus at least three different types of synchronous oscillatory network activity have been observed in vivo, which are expressed during distinct patterns of animal behaviour (Leung et al., 1982). Particularly, gamma activity at 20–80 Hz is suggested to be involved in setting the temporal relationships that may be crucial for various aspects of information processing, storage and retrieval (Singer, 1993; Gray, 1994; Ritz & Sejnowski, 1997). Hippocampal gamma oscillations can be obtained in vitro by tetanic stimulation (Whittington et al., 1997), by pharmacological activation of metabotropic glutamate receptors (Whittington et al., 1995), and via cholinergic excitation by the acetylcholine receptor agonist carbachol (CCh; Fisahn et al., 1998). In view of the proposed relationship between hippocampal gamma rhythms and higher cognitive functions on the one hand, and the important role of the dopaminergic hippocampal system in synaptic modulation on the other hand, it is surprising that so far little is known about the effects of DA on gamma activity.
In this study, we examined CCh-induced gamma oscillations in area CA3 of rat hippocampal slices, employing field potential and intracellular recordings. Three main issues were addressed. First, we have asked whether DA alters the properties of network gamma activity. Second, we have tried to verify the DA-receptor subtype mediating the DA effect. Finally, we have attempted to identify the cellular targets of the DA action onto field gamma oscillations.
Materials and methods
Slice preparation
Experiments were performed on hippocampal slices obtained from brains of 250 g weight adult Wistar rats of either sex. Animals were anaesthetized with ether, decapitated and their brains were quickly transferred to 4 °C cold aerated saline (ACSF, see below). After freehand razor cuts, the brains were placed on a vibroslicer (Campden, Loughborough, UK), and 400 µm thick horizontal slices were cut. The slices were transferred into an interface-type recording chamber continuously perfused at a constant flow rate of 2 mL/min with prewarmed (34 °C) oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (ACSF) containing (in mm) 124.0 NaCl, 1.25 Na2PO4, 26.0 NaHCO3, 3.0 KCl, 1.6 CaCl2, 1.8 MgSO4, and 10 glucose at pH 7.4. Subsequently, slices were allowed to recover for at least 1 h.
Electrophysiological recordings
Field activity was recorded using a SEC 10L amplifier (npi Instruments, Tamm, Germany). Bridge mode recordings were performed with low resistance, ACSF-filled micropipettes with an open diameter of approximately 5 µm pulled from borosilicate glass tubing (1.2 mm OD) on a Brown-Flaming-puller (P87, Sutter Instruments, Novato, CA, USA). Slices were considered acceptable if electrical stimulation evoked population spikes ≥3 mV in amplitude within the pyramidal cell layer. Oscillatory network activity was examined in area CA3, employing extracellular recordings in stratum pyramidale. Extracellular voltage signals were acquired with an IBM-compatible PC and an ITC−16 interface (Instrutech, Great Neck, NY, USA) controlled by TIDA data acquisition software (HEKA, Lambrecht/Pfalz, Germany). Signals were digitized at 2 kHz and low-pass filtered at 300 Hz. Intracellular recordings from hippocampal neurons were performed in the pyramidal cell layer. Sharp microelectrodes were filled with 2.5 m K-acetate and had resistances varying from 50 to 80 MΩ. Pyramidal cells were identified by distinct electrophysiological characteristics including resting membrane potentials at −60 to −70 mV, overshooting long-duration action potentials (amplitude >60 mV, duration at half-spike amplitude >1 ms), and spike accommodation in response to depolarizing intracellular current pulses with subsequent afterhyperpolarization (AHP). For intracellular stimulation, short constant current pulses (duration: 100–200 ms, amplitude: 0.3–1.5 nA) were injected into the recorded neurons to determine changes in input resistance, spike firing and AHP. Intracellular potentials and current signals were digitized at 10 kHz, filtered at 3 kHz and subsequently stored on a computer disk.
Data analysis was performed off-line using Spike2 (Cambridge Electronic Design, Cambridge, UK) and Origin 6.0 (Microcal Software, Inc., Northampton, MA, USA) software. Field potential oscillations were characterized by calculating power spectra employing a fast Fourier transform algorithm (Spike2 software) over 10 s recording epochs. Gamma band power was computed by integrating the 20–80 Hz segments of the power spectra. Main oscillation frequency was taken as the peak frequency determined from the respective power spectra. The same procedure was applied for analysing intracellular membrane oscillations with theta band power being computed by integrating the 3–15 Hz segment of the spectra.
All numerical data are expressed as mean ± SEM. Statistical evaluation was performed applying a Student's t-test for paired data (Origin 6.0). P-values of less than 0.05 were considered to indicate a significant difference between means.
Drugs
The following drugs were used: carbamoylcholine chloride (carbachol, CCh), 20 µm; (−)-bicuculline methiodide, 5 µm; dopamine hydrochloride (dopamine, DA), 200 µm; 4-(4-[p-chlorophenyl]-4-hydroxypiperidino)-4′-fluorobutyrophenone (haloperidol), 20–100 µm; trans-(−)-(4aR)-4,4a,5,6,7,8,8a,9-octahydro-5-propyl-1H-pyrazolo[3,4-g]quinoline [(−)-quinpirole hydrochloride], 25–100 µm; R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine [R(+)-SCH-23390 hydrochloride], 20–100 µm; (+/−)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol [(+/−)-SKF-38393 hydrochloride], 20 µm; (+/−)-5-(aminosulphonyl)-N-[(1-ethyl-2-pyrrolidinyl)methyl]-2-methoxybenzamide [(+/−)-sulpiride], 20 µm. Drugs were purchased from CN Biosciences (Nottingham, UK; carbachol) and Sigma–Aldrich (Taufkirchen, Germany; all other compounds). To prevent rapid oxidation of dissolved DA in warm saline we generally added 1 mm ascorbic acid (Sigma-Aldrich) to the ACSF. All drug-containing solutions were freshly prepared prior to the experiments. With the exception of haloperidol and (+/−)-sulpiride, the drugs were diluted in ACSF from stock solutions dissolved in distilled water. Haloperidol and sulpiride were made up in dimethyl sulfoxide before being dissolved in ACSF to the desired concentration.
Results
Carbachol induces gamma frequency network oscillations in rat hippocampal area CA3
To investigate the effects of DA on synchronized network activity in the hippocampus, we employed the ‘carbachol model’. This model is based on recent experimental findings demonstrating that the acetylcholine receptor agonist CCh induces persistent gamma frequency (20–80 Hz) oscillations in area CA3 in vitro (Fisahn et al., 1998; Stenkamp et al., 2001).
Bath application of CCh (20 µm) induced prominent field potential oscillations in stratum pyramidale of area CA3 (1-3; top traces). Oscillatory activity appeared spontaneously within 15–30 min, and once established persisted over hours. In power spectra (1-3; controls), the CCh-induced oscillations gave rise to distinct narrow peaks in the gamma band between 25 and 55 Hz with an average frequency value of 40 ± 1 Hz (n = 53). Maintenance of the oscillations required GABAA receptors. Thus, addition of the GABAA antagonist bicuculline (5 µm) reversibly abolished the field gamma activity (n = 5, data not shown).
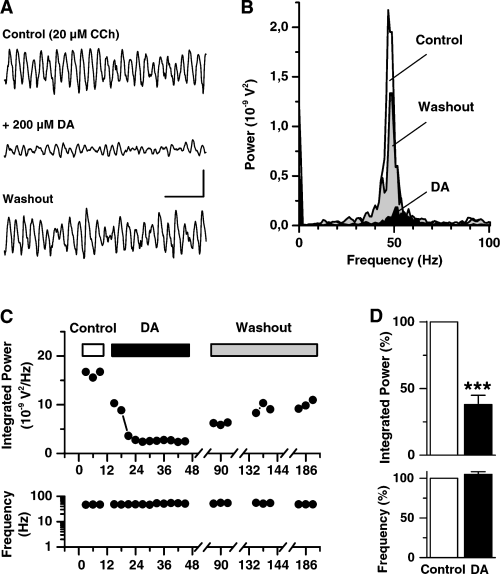
Dopamine (DA) suppresses cholinergic induced gamma frequency oscillatory activity in rat hippocampal slices. (A) Representative field potential recordings from stratum pyramidale in area CA3 under control conditions (CCh-ACSF, top trace), 30 min after bath application of DA (middle trace), and following 1 h washout (bottom trace). (B) Power spectra obtained for the recordings shown in A. Distinct peaks in the gamma frequency range around 47 Hz are evident during control and washout. (C) Time-course of the integrated gamma band (20–80 Hz) power (upper plot) and the corresponding oscillation frequency (lower plot) during the experiment given in A. (D) Summary of seven recordings (in seven slices) showing integrated gamma band power (upper bar graphs) and oscillation frequency (lower bar graphs) normalized in controls and in the presence of DA. Asterisks in D denote a significance level of P ≤ 0.0002. Scale bars, 300 µV, 100 ms (A).
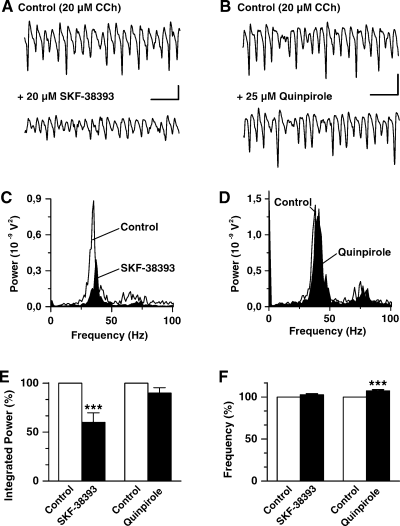
Dopaminergic suppression of CCh-induced gamma oscillations in rat hippocampal area CA3 is mimicked by a D1-like DA receptor agonist but not by D2 agonists. (A) Representative field potential recordings from stratum pyramidale in a slice under control conditions (CCh-ACSF; top trace) and 30 min following bath application of the D1/D5 agonist SKF-38393 (bottom trace). (B) Field potential recordings from another slice in control solution (top trace) and 30 min following application of the D2 agonist quinpirole (bottom trace). (C and D) Power spectra showing the responses with CCh (control; white) and during agonist's treatment (black) for the recordings illustrated in A and B, respectively. (E and F) Bar graphs summarizing the results for the effects of SKF-38393 (n = 7) and quinpirole (n = 6) on integrated gamma band (20–80 Hz) power (E) and oscillation frequency (F). Asterisks denote a significance level of P ≤ 0.007. Scale bars, 300 µV, 100 ms (A and B).
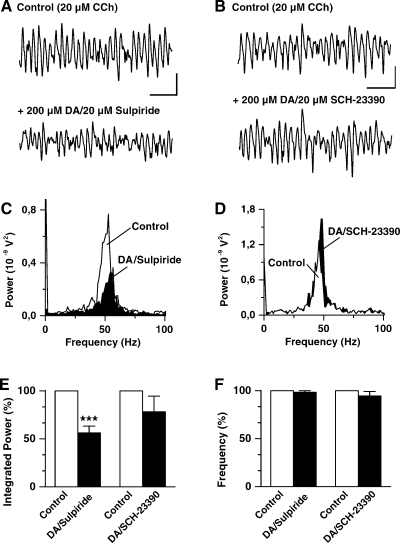
The DA-mediated suppression of CCh-induced gamma frequency oscillatory activity is not altered by D2-like DA receptor antagonists but counteracted by a D1/D5 receptor antagonist. (A) Representative field potential recordings from stratum pyramidale in area CA3 of a slice in control solution (CCh-ACSF; top trace) and in the presence of ACSF additionally containing DA and the D2 antagonist sulpiride (bottom trace). (B) Field potential recordings from another slice under control conditions (top trace) and 30 min following application of ACSF containing DA and the D1/D5 antagonist SCH-23390 (bottom trace). (C and D) Power spectra showing the responses with CCh (control; white) and during coapplication of DA with the antagonists (black) for the recordings shown in A and B, respectively. (E and F) Bar graphs summarizing the results for the effects of sulpiride (n = 8) and SCH-23390 (n = 6) on integrated gamma band (20–80 Hz) power (E) and oscillation frequency (F). Asterisks denote a significance level of P ≤ 0.005. Scale bars, 200 µV, 100 ms (A); 300 µV, 100 ms (B).
Dopamine suppresses carbachol-induced gamma oscillations
To investigate, whether synchronized network activity in rat hippocampus underlies a dopaminergic modulation, we tested the effects of DA on CCh gamma oscillations. Bath application of DA (200 µm) for 10–30 min markedly suppressed field gamma oscillations (Fig. 1A, middle trace). The DA-mediated suppression was partially reversible with oscillatory activity recovering slowly upon washout within 30 min to 1.5 h. The quantification of the observed effect for the experiment illustrated in Fig. 1, revealed a decrease in oscillation gamma band power from 16.3 × 10−9 V2/Hz under control conditions to 2.4 × 10−9 V2/Hz with DA (Fig. 1C). The depression in oscillation power was associated with a small increase in oscillation frequency from 48 Hz in control to 51 Hz with DA (Fig. 1B and C). Similar results were obtained in another six preparations. On average, DA decreased the power of CCh-induced gamma oscillations to 38 ± 7% of control (P ≤ 0.0002, n = 7, upper graph in Fig. 1D), while oscillation frequency was slightly increased but not significantly changed (105 ± 3% of control, P = 0.175, n = 7, lower graph in Fig. 1D).
Depression of carbachol-induced gamma oscillations is mediated via D1-like DA receptors
To elucidate the DA receptor subtype involved in the depression of cholinergic gamma activity, first we examined the effects of D1 and D2 receptor agonists. As illustrated in Fig. 2A, bath application of SKF-38393 (20 µm) suppressed oscillatory gamma activity. Likewise DA, the D1 agonist decreased oscillation power without changing its frequency significantly (Fig. 2C). Similar results were obtained in another six slices. On average, gamma band power with SKF-38393 amounted to 60 ± 9% of control (P ≤ 0.007, n = 7, Fig. 2E). Although the DA-mediated reduction in oscillation power was stronger (to approximately 40%), the two effects were not significantly different (P = 0.198). Oscillation frequency was slightly but not significantly increased with SKF-38393 to 103 ± 1% of control (P = 0.079, n = 7, Fig. 2F). As observed for DA, the SKF-38393 effect was partially reversible upon prolonged washout (data not shown). By contrast, the D2 agonist quinpirole (25 µm) failed to suppress CCh-induced field gamma activity (Fig. 2B and D). Thus, integrated gamma band power with the D2 agonist, estimated from six different slices, amounted to 90 ± 5% of control (P = 0.126; Fig. 2E). Here, however, the slight increase in oscillation frequency to 107 ± 1% of control observed during quinpirole treatment was significant (P ≤ 0.004, n = 6, Fig. 2F). Similar results were observed even when the D2 agonist was applied at a concentration of 100 µm (n = 2, data not shown).
Secondly, we investigated the effects of D1 and D2 receptor subtype specific antagonists on the DA-mediated suppression of cholinergic gamma oscillations employing sulpiride and haloperidol (D2 antagonists), and SCH-23390 (D1 antagonist). Pretreatment (30 min) of slices with the antagonists alone had no detectable effects on the field gamma activity (n = 3, data not shown). As illustrated in Fig. 3A and C, the D2 antagonist sulpiride (20 µm) did not prevent the DA effect on CCh gamma. On average, the reduction in integrated gamma band power after coapplication of DA and sulpiride amounted to 56 ± 7% of control (P ≤ 0.0005, n = 8; Fig. 3E), which is not different (P = 0.122) from the value observed with DA alone (approximately 40%). Oscillation frequency was not changed significantly upon simultaneous treatment with DA and sulpiride (98 ± 2% of control, P = 0.351, n = 8, Fig. 3F). Similar results were obtained with the D2 antagonist haloperidol (20–100 µm, n = 4, data not shown). On the other hand, the D1 antagonist SCH-23390 counteracted the depressant action of DA. As illustrated in Fig. 3B, coapplication of DA with SCH-23390 (20 µm) prevented a suppression of the field gamma oscillation. For the example shown, it is obvious from the respective power spectrum (Fig. 3D) that here SCH-23390 blocked the DA effect completely with oscillation power being actually slightly increased. A similar effect was observed in another two slices. In three slices, however, the D1 antagonist did not completely block the DA effect. On average, integrated gamma band power after coapplication of DA with SCH-23390 amounted to 78 ± 16% of control (P = 0.807, n = 6; Fig. 3E), which is significantly different (P ≤ 0.02) from the power value observed with DA alone (38 ± 7% of control). Incomplete blockage of the DA effect on oscillation power by SCH-23390 was observed even when the antagonist was applied at higher concentrations (up to 100 µm, n = 3; data not shown). Oscillation frequency was slightly decreased to 95 ± 5% of control in slices simultaneously treated with DA and SCH-23390 (n = 6, Fig. 3F). However, this change was not significant (P = 0.816) and did not differ (P = 0.143) from that obtained with DA alone (105 ± 3% of control).
Intracellular recordings from pyramidal cells during carbachol-induced field gamma activity in area CA3
To evaluate the cellular targets involved in the DA action onto cholinergic gamma oscillations, we combined extracellular field potential measurements with intracellular recordings of activity from hippocampal cells. In all, 21 hippocampal neurons (10 from area CA3, 11 from area CA1) were recorded that exhibited pyramidal cell characteristics (cf. Materials and methods).
In six CA3 neurons, alterations of cell behaviour were observed before and during application of CCh. Under control conditions the mean resting membrane potential of the cells amounted to −62 ± 2 mV (n = 6). Their input resistances varied between 16 and 54 MΩ with a mean value of 36 ± 6 MΩ (n = 5). At resting membrane level, the cells showed small subthreshold low-frequency membrane potential oscillations in the theta range between 3 and 11 Hz (mean: 6 ± 1 Hz, n = 6, Fig. 4C) with intracellular voltage fluctuations varying from 0.2 to 2 mV (spectrum inset Fig. 4C). Half of the cells displayed spontaneous spiking activity at 0.1–2.3 Hz (mean frequency: 0.9 ± 0.7 Hz, n = 3). Bath application of CCh (20 µm) produced a sustained membrane depolarization of about 7 ± 2 mV (range: 2–13 mV, n = 6, Fig. 4) in the cells. Membrane depolarization was accompanied by a slight but not significant decrease in input resistance to 89 ± 5% of control (P = 0.076, n = 6, asterisks in Fig. 4A and B). In the case of spontaneous spiking neurons, the basal firing rate increased to 5.6 ± 3.8 Hz (range: 0.7–13 Hz, n = 3) after application of CCh (data not shown). Under control conditions, intracellular injection of short suprathreshold depolarizing current pulses into CA3 cells evoked trains of accommodating action potentials followed by a pronounced AHP (Fig. 4A). CCh treatment resulted in both a reduction of the AHP and a suppression of spike accommodation (Fig. 4B). Similar results were observed in altogether six neurons recorded before and after exposure to CCh. Another four cells were impaled after at least 1 h continuous CCh application. Also these neurons showed depolarized membrane potentials at values between −57 and −54 mV, presented slight or no AHP, and did not express spike accommodation. With respect to subthreshold intracellular membrane oscillations (Fig. 4C and D), we noted that upon CCh application, oscillation frequency was slightly increased to 8 ± 2 Hz (range: 5–17 Hz, n = 6) compared to 6 ± 1 Hz (range: 3–11 Hz) under control conditions but not significantly changed (P = 0.076). The amplitude of the intracellular potential fluctuations, however, was notably increased to values in the range of 0.5–5 mV (cf. 0.2–2 mV in controls, spectra insets in Fig. 4C and D). The latter is also reflected in the corresponding power spectra. For the example shown in Fig. 4C and D the integrated theta band (3–15 Hz) power of the intracellular oscillation with CCh was increased to 123% of control. Similar results were observed in another five cells (in five slices).
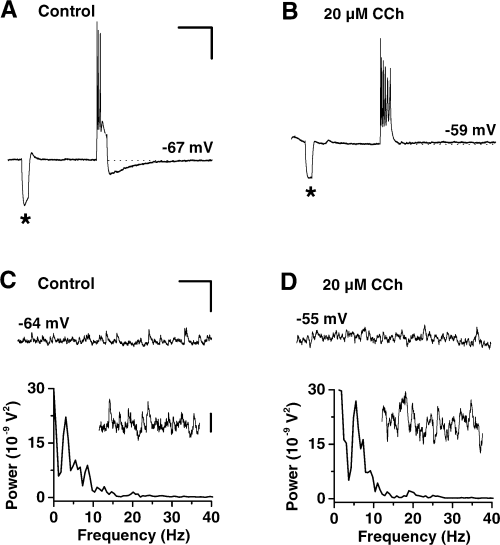
Multiple effects of CCh on membrane parameters and intrinsic properties of individual CA3 pyramidal neurons. (A and B) Intracellular recordings of the responses of a CA3 cell to depolarizing current injection (pulse duration: 150 ms, pulse amplitude: 1.2 nA) before (A) and after (B) 45 min perfusion with CCh. In the presence of the cholinergic agonist spike accommodation and AHP were suppressed. Changes in response behaviour were accompanied by a sustained membrane depolarization of 8 mV. Asterisks denote membrane potential responses to hyperpolarizing current pulses (100 ms, −0.5 nA) injected for monitoring membrane input resistance. (C and D) Intracellular recordings (upper panels) from another CA3 pyramidal cell under control conditions (C) and during prolonged CCh application (D) showing low (theta)-frequency subthreshold membrane potential oscillations. Following CCh treatment, the intracellular theta rhythm of the depolarized cell was augmented. The power spectra (lower panels) indicate a slight shift in oscillation frequency and an increase in theta band (3–12 Hz) power with the cholinergic agonist. Spectra insets: detailed views of the intracellular records at a higher magnification. Scale bars, 15 mV, 0.5 s (A and B); 5 mV, 1 s (C and D); 1 mV (inset in C).
Simultaneous extracellular and intracellular recordings in stratum pyramidale of area CA3 revealed a striking difference. As illustrated in Fig. 5A continuous bath application of CCh induced stable field gamma oscillations at 53 Hz. However, the cell recorded close to the field pipette at the same time displayed a sturdy subthreshold membrane oscillation in the theta frequency band at 11 Hz (Fig. 5C). Similar results were obtained in another three slices. As demonstrated above, DA markedly suppressed the power of the CCh field gamma oscillation (Fig. 5A and B). It had, however, no detectable effects on the intracellular theta rhythm of the CA3 neurons (Fig. 5C and D). Although the analysis for the cell recorded in Fig. 5 revealed a small decline in oscillation frequency from 11.6 Hz in control to 7.9 Hz during DA treatment as well as a decrease in the integrated theta band power from 48 × 10−9 V2/Hz to 33 × 10−9 V2/Hz, on average both parameters were not significantly altered. Thus, with DA oscillation frequency amounted to 84 ± 9% of control (P = 0.157, n = 4) and oscillation power reached 98.7 ± 15.4% of control (P = 0.941, n = 4).
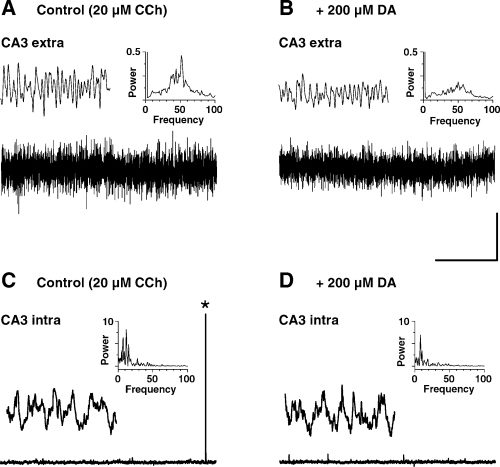
Effects of DA on CCh-induced network gamma and intracellular theta oscillation. (A and B) Representative field potential recordings from stratum pyramidale in area CA3 during prolonged CCh perfusion before (A) and 30 min after (B) application of DA. Insets, left: expanded views of the extracellular oscillatory activity; right: power spectra obtained from the field recordings shown in A and B, respectively. (C and D) Simultaneous intracellular recordings of the activity of a pyramidal cell measured in close vicinity to the field pipette before (C) and 30 min after (D) the DA treatment. Insets, left: expanded sections from the intracellular records at a higher magnification; right: power spectra obtained from the intracellular recordings shown in C and D, respectively. Asterisk in C denotes a spontaneous action potential. Scale bars, 300 µV, 3 s (applies for A and B); 20 mV, 3 s (applies for C and D); 300 µV, 300 ms (applies for insets in A and B); 1 mV, 300 ms (applies for insets in C and D).
The pyramidal cells recorded within stratum pyramidale in area CA1 (n = 11) responded similarly to CCh as CA3 neurons. Thus, upon CCh application the cells showed sustained membrane depolarization, reduced spike frequency accommodation, and blockade of AHP. Also these neurons did not exhibit any gamma frequency membrane oscillations during CCh-induced field gamma activity but expressed distinct intracellular theta oscillations. Finally, as observed for CA3 cells, DA had no effect on the intracellular theta rhythm of the CA1 cells (data not shown).
Discussion
The main finding of this paper is that DA strongly depresses CCh-induced gamma oscillations in hippocampal slices without any effect on the frequency of these oscillations. This effect is apparently mediated through activation of D1-like DA receptors. Besides, intracellular recordings from CA1 and CA3 cells demonstrate that pyramidal cells do not necessarily participate in the evoked gamma oscillations and that the observed CCh-mediated theta frequency membrane oscillations in principal cells, which perhaps might drive inhibitory interneurons, are not affected by DA.
The hippocampus receives dopaminergic input particularly from the ventral tegmental area, expresses all types of DA receptors, and exhibits much of the enzymatic machinery associated with dopaminergic target cells (reviewed in Otmakhova & Lisman, 1996). A functional role of the hippocampal dopaminergic system has been indicated by behavioural studies, demonstrating an enhancement of positive reinforcement learning, visual discrimination and passive avoidance behaviour after intrahippocampal injections of DA agonists, as well as an impairment of spatial navigation after depletion of hippocampal DA (reviewed in Lisman & Otmakhova, 2001). Besides, DA has been shown to powerfully alter synaptic plasticity in rat hippocampus (for LTP, see Frey et al., 1991; Huang & Kandel, 1995; Otmakhova & Lisman, 1996; for depotentiation, see Otmakhova & Lisman, 1998a; for LTD, see Chen et al., 1995).
Cloning of DA receptors has revealed at least five distinct metabotropic receptor subtypes, which on the basis of biochemical and pharmacological properties, have been grouped into the D1-like (D1, D5) and the D2-like (D2, D3, D4) families (reviewed in Missale et al., 1998). The available physiological data indicate the significance of D1-like DA receptors in dopaminergic synaptic modification within rat hippocampus. Thus, in the majority of investigations the obtained DA-mediated effects were not only mimicked by selective D1/D5 receptor agonists but also specifically prevented by D1 antagonists (Chen et al., 1995; Otmakhova & Lisman, 1996, 1998a; Bouron & Reuter, 1999; Behr et al., 2000a,b; Hersi et al., 2000; Yang, 2000). So far, there are only two reports providing experimental evidence for the involvement of D2-like receptors in dopaminergic hippocampal modulation (Hsu, 1996; Stenkamp et al., 1998).
Supporting a particular role of the D1-receptor family, our pharmacological data suggest that the DA-induced depression of cholinergic field gamma activity is most likely mediated by activation of D1-like receptors. Thus, the D1/D5 receptor agonist SKF-38393, but not the D2 agonist quinpirole, mimicked the suppressive DA effect. Consistently, both the D2 antagonists, sulpiride and haloperidol, failed to prevent the action of DA on CCh-induced gamma oscillation, while the D1/D5 receptor antagonist SCH-23390 partially antagonized DA. However, the SCH-23390 actions were inconsistent in our preparation. Irregular blocking of the DA effect was obtained even when the SCH-23390 concentration was increased from 20 up to 100 µm. At those concentrations of the antagonist, the binding of DA to D1 receptor sites should be more than 95% inhibited (Andersen & Jansen, 1990). Difficulties in mimicking or antagonizing DA effects are reported by others (Pralong & Jones, 1993; O'Donnell & Grace, 1994; Nicola et al., 1996; Otmakhova & Lisman, 1998b; Behr et al., 2000a) and are interpreted in terms of either a decreased sensitivity of D1-like receptors to SCH-23390 or a subset of atypical DA receptors that do not specifically interact with classic D1/D5 ligands. As the D1 agonist SKF-38393 was less effective than DA in suppressing gamma oscillation power, the latter most likely could explain our results. On the other hand, the DA/SKF-38393 action might be mediated via other types of monoamine receptors. Recent work indicates that, like DA, noradrenaline and serotonin suppress perforant path transmission in rat hippocampus and that this inhibition is blocked by the nonselective DA antagonist clozapine (Otmakhova & Lisman, 2000). In agreement with those results, preliminary data of our group revealed that fenfluramine, a substance known to evoke presynaptic serotonin release, depresses cholinergic gamma oscillation alike as demonstrated for DA in this study (Heinemann, personal communication). Besides, a DA-mediated activation of nondopamine receptors has been demonstrated in hippocampal pyramidal cells (Malenka & Nicoll, 1986) and sympathetic neurons (Aguayo & Grossie, 1994). However, the employed DA concentrations in those experiments were reported to be at least five-fold higher than those we employed (Haas & Konnerth, 1983).
The DA concentration used in this study must be related to the experimental conditions. Thus, full equilibrium of applied drugs within slices takes at least 1 h (Müller et al., 1988). The demonstrated DA effects, however, were recorded after 10–30 min. Rapid oxidation (Sutor & ten Bruggencate, 1990) and DA uptake will additionally reduce the final DA concentration. Hence, the applied DA concentration (200 µm) was undoubtedly much higher than that actually inducing the observed effects. Similar DA concentrations were also reported to be necessary to observe synaptic effects in hippocampus in vitro (Gribkoff & Ashe, 1984; Behr et al., 2000a,b).
Previous modelling work and studies on gamma oscillations in hippocampus suggest that reciprocal connections between GABAergic interneurons and glutamatergic pyramidal cells with cholinergic synaptic activation of interneurons and synaptic inhibition between interneurons and onto pyramidal cells participate in the generation of network oscillations and provide a precise temporal pattern in the gamma band (Bragin et al., 1995; Soltesz & Deschenes, 1993; Whittington et al., 1995; Traub et al., 1996, 2000; Fisahn et al., 1998; Penttonen et al., 1998; Fellous & Sejnowski, 2000). To elucidate cellular mechanisms accounting for the decrease in gamma oscillation power, we recorded intracellularly from pyramidal neurons during cholinergic field gamma activity. Generally, as has been shown in a variety of studies on hippocampus (Benardo & Prince, 1982; Dodd et al., 1981; Haas, 1982; Segal, 1982; Cole & Nicoll, 1983; Müller & Misgeld, 1986; Müller et al., 1988), CCh induced multiple effects on membrane parameters and response properties of CA1/CA3 pyramidal cells, as sustained membrane depolarization, suppression of spike frequency accommodation and reduction of the AHP following a train of action potentials. With respect to rhythmic activity, we found that although stable field gamma oscillations had been established, CCh did not elicit gamma activity in pyramidal neurons but instead established slow subthreshold membrane potential fluctuations in the theta frequency range. Likewise, the activity of spiking cells was invariably limited to theta frequencies and hence clearly below that of the field gamma rhythm. This compares well to studies in the entorhinal cortex (EC) where CCh induced theta oscillations when directly applied to stellate cells (Dickson & Alonso, 1997; Klink & Alonso, 1997) and deep layer projection cells (Gloveli et al., 1999). Cholinergic theta oscillations were also reported for hippocampal pyramidal cells (Bland et al., 1988; MacVicar & Tse, 1989; Leung & Yim, 1991; Williams & Kauer, 1997; McMahon et al., 1998). It might be noted that in the EC membrane fluctuations induced in superficial projection cells, which were evoked by CCh application to deep layer cells, exhibited theta oscillations with superimposed gamma components (Gloveli et al., 1999). Thus, it seems feasible that theta oscillations in pyramidal cells can be converted through an interneuronal network into gamma activity. Such a scenario could explain the sensitivity of CCh-induced oscillations to AMPA/kainate receptor antagonists (Williams & Kauer, 1997; Fisahn et al., 1998; Dickson et al., 2000; Traub et al., 2000) reflecting the dependence of gamma activity onto glutamatergic excitation. The depressant effect of DA might therefore relate to a reduced release of glutamate from presynaptic terminals of pyramidal cells tonically entraining an interneuron population that is responsible for the gamma network rhythm. In terms of such DA action, it has been reported that DA and SKF-38393 decrease transmitter release at glutamatergic hippocampal synapses while GABAergic synaptic transmission is unaffected (Behr et al., 2000a,b).
On the other hand, the fact that DA did not block theta oscillations in pyramidal cells in our preparation would suggest that the generation of the gamma oscillations does not necessarily depend on the interaction between interneurons and pyramidal cells, as is the case with gamma activity induced by tetanic stimulation or direct activation of metabotropic glutamate receptors (Whittington et al., 1995, 1997; Traub et al., 1996, 2000; Buhl et al., 1998; Fisahn et al., 1998; Penttonen et al., 1998). Independent synchronization of inhibitory interneuronal networks has been suggested to be sufficient in sustaining gamma oscillations (Traub et al., 1996; Whittington et al., 1995; Wang & Buzsaki, 1996). Gamma oscillations in networks of interneurons have been shown to be mediated via GABAergic synaptic transmission between interneurons (Traub et al., 1996; Whittington et al., 1995; Wang & Buzsaki, 1996). Consistent with these findings, the GABAA receptor antagonist bicuculline abolished the field gamma activity in our preparation. Several lines of evidence indicate that DA is involved in inhibition of GABAA receptor-mediated synaptic activity (Cooper & Stanford, 2001; Floran et al., 1997; Delgado et al., 2000; Shen & Johnson, 2000). Concerning possible mechanisms underlying such depressant DA actions, Liu et al. (2000) recently reported a direct protein–protein coupling between D5 and GABAA receptors that enables inhibitory interactions between the two receptor systems. Finally, as gap junction conductances have been shown to be crucial to synchronization within networks of interneurons (Galarreta & Hestrin, 1999; Gibson et al., 1999; Fukuda, 2000; Tamas et al., 2000), DA might also alter interneuronal coupling and thereby suppress gamma activity.
The large diversity of hippocampal interneurons (reviewed in Freund & Buzsaki, 1996) poses the question, as to which distinct subset of them might be involved in the generation and maintenance of CCh-induced gamma oscillations and thus represent the target for the DA action. Identification of the various types of interneurons is an absolute prerequisite for studying their significance in network gamma activity as well as their individual impairment by DA. In spite of the open question as to which type of cells is predominantly responsible for the DA-mediated depression of the cholinergic gamma frequency network oscillation, our findings suggest that there might be different subroutine-like roles of specific interneuron subsets in the hippocampus. In fact, there is first experimental evidence for cell-type-specific recruitment of hippocampal interneurons in different states of synchronization in vivo (Klausberger et al., 2003) supporting the hypothesis that interneurons may subserve different tasks in binding hippocampal activity under different behavioural conditions and hence far exceed the classical functions of inhibitory neurons.
Acknowledgements
We thank J. Breustedt for technical help at various stages of the study and D. Manahan-Vaughan for helpful criticism on earlier versions of the manuscript.
This work was supported by grants of the Deutsche Forschungsgemeinschaft (SFB 515 TP/B 8, He 1128/11–2 and Ve 187/1–3).
Abbreviations
-
- ACSF
-
- artificial cerebrospinal fluid
-
- AHP
-
- afterhyperpolarization
-
- CCh
-
- carbamoylcholine chloride (carbachol)
-
- DA
-
- dopamine
-
- EC
-
- entorhinal cortex
-
- GABA
-
- gamma aminobutyric acid
-
- SCH-23390
-
- R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine
-
- SKF-38393
-
- (+/−)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol.