Markers for dopaminergic neurotransmission in the cerebellum in normal individuals and patients with Parkinson's disease examined by RT-PCR
Abstract
The presence of neuronal elements that are indicative of dopaminergic neurotransmission in cerebellum suggest that this brain region may contribute to the motor symptoms or dyskinesia seen in Parkinson's disease. Reverse transcription polymerase chain reaction (RT-PCR) was used to examine the expression of markers for dopaminergic neurotransmission in the cerebellum from postmortem brain tissue obtained from normal subjects and patients dying with Parkinson's disease who were receiving treatment with dopaminergic drugs. Dopamine D1−3 receptors, tyrosine hydroxylase and dopamine transporter mRNA was detected in the uvula and nodulus (lobules 9 and 10, respectively) of the vermis of cerebellum from normal individuals. In Parkinson's disease, the level of dopamine D1 and D3 receptor mRNA was significantly reduced in lobule 9 and the level of tyrosine hydroxylase mRNA was significanty reduced in lobule 10. No alteration in the level of dopamine D2 receptor or dopamine transporter mRNA was found in either lobule in patients with Parkinson's disease. These results show that mRNA expression for the functional components of dopaminergic neurotransmission is present in human cerebellum. The discrete changes in the levels of dopamine D1 and D3 receptors and tyrosine hydroxylase mRNA in cerebellum from l-DOPA treated Parkinson's disease patients suggests that this brain area has a role in the symptoms of Parkinson's disease and/or the beneficial/side-effects of treatment.
Introduction
The major motor symptoms of Parkinson's disease (tremor, bradykinesia, akinesia, postural instability) are associated with decreased striatal dopamine levels that result from the degeneration of pigmented brain stem nuclei (Hornykiewicz, 1966). While these symptoms are adequately alleviated for several years by medication with l-DOPA or directly acting dopamine agonists, long-term treatment is complicated by continuing progression of the disease and the occurrence of severe side-effects of treatment such as dyskinesias, on/off periods and psychosis. The dopaminergic neurons of the substantia nigra pars compacta that project to the striatum are the most severely affected. Consequently, most studies have examined markers of dopaminergic neurotransmission in the striatum or other regions of the basal ganglia. However, areas outside of the basal ganglia may also play a role in the symptoms and/or treatment of Parkinson's disease. The cerebellum is potentially one such area as it is known to be involved in the coordination of movement, postural control and tremor (Ito, 1984). Furthermore, the cerebellum receives dopaminergic innervation from the substantia nigra (Simon et al., 1979; Björklund & Lindvall, 1984; Hökfelt et al., 1984; Panagopoulos et al., 1991; Ikai et al., 1992). Functional dopamine release occurs in rat cerebellum (Chrapusta et al., 1994) and lesions of the substantia nigra and ventral tegmental area decrease dopamine levels in the cerebellum (Kizer et al., 1976). A recent immunohistochemical study demonstrated the presence of tyrosine hydroxylase (TH) and the dopamine transporter (DAT) in macaque cerebellum (Melchitzky & Lewis, 2000). Radioligand binding studies have shown the five members of the dopamine receptor superfamily (D1−5) to be present in rodent (Boyson et al., 1986; Dubois et al., 1986; Camps et al., 1990; Bouthenet et al., 1991; Panagopoulos et al., 1991; Herroelen et al., 1994; Vessotskie et al., 1997) and human (Herroelen et al., 1994) cerebellum. In addition, immunoreactivity against dopamine receptor subtypes has been detected in both rat and human cerebellum (Khan et al., 1998, 2000; Barili et al., 2000) and the mRNAs for dopamine receptor subtypes are also present (Bouthenet et al., 1991; D'Souza et al., 1997).
Thus, the cerebellum is a brain area not traditionally considered dopaminergic, but which contains neuronal elements indicative of dopaminergic neurotransmission. In support of a dopaminergic role for the cerebellum, chronic treatment of rats with haloperidol, sulpiride and clozapine caused a reduction in the BMAX for [3H]flunitrazepam binding to cerebellar membranes (Rupniak et al., 1987; Lloyd et al., 1997). More recently, chronic treatment with haloperidol and sulpiride was shown to down-regulate D1 receptor mRNA in cerebellum (D'Souza et al., 1997). Microinjection of D2 and D3 receptor agonists (apomorphine, quinelorane, 7-OH-DPAT) and antagonists (amisulpiride, nafadotride, haloperidol, raclopride) directly into lobules 9 and 10 of rat cerebellum were found to inhibit and stimulate locomotion, respectively (Barik & de Beaurepaire, 1996; Boulay et al., 2000; Kolasiewicz & Maj, 2001) thus showing functional involvement of dopaminergic systems in the cerebellum in motor activity.
The purpose of this study was to determine whether human cerebellum, like rodent cerebellum, contains the mRNA for dopamine D1−3 receptors, TH and DAT by RT-PCR and to determine whether alterations in the level of mRNA for these dopaminergic markers occur in the cerebellum after nigral degeneration in Parkinson's disease and chronic l-DOPA treatment to support a role for cerebellar involvement in the motor dysfunction that characterizes this disease.
Materials and methods
Human brain tissue
The study was performed on brain tissue from four patients who died with idiopathic Parkinson's disease and four control subjects who died with no known neurological or psychiatric disease. Details of each case are given in Table 1. The two groups were not significantly different for age at death (mean years ± SEM, control: 83.8 ± 2.3; Parkinson's disease, 80.5 ± 3.2; F = 0.682, P = 0.44) or the time elapsed between death and freezing of tissue (mean hours ± SEM, control: 6.3 ± 0.8; Parkinson's disease, 7.4 ± 2.6; F = 0.155, P = 0.71). The mean (± SEM) duration of illness was 13.3 ± 3.7 years. The diagnosis of Parkinson's disease was confirmed at postmortem by neuronal loss and the presence of extraneuronal melanin and Lewy bodies in the substantia nigra and locus coeruleus.
| Case | Age (Years) | Gender | Autopsy delay (h) | Duration of illness (Years) | Cause of death | Treatment |
|---|---|---|---|---|---|---|
| C1 | 89 | M | 5.25 | – | Stroke/pneumonia | None |
| C2 | 83 | F | 8.5 | – | Heart failure | None |
| C3 | 78 | M | 6.0 | – | Cardio-pulmonary collapse | None |
| C4 | 85 | F | 5.5 | – | Bladder cancer | None |
| Mean ± SEM | 83.8 ± 2.3 | – | 6.3 ± 0.8 | – | ||
| P1 | 71 | M | 5.0 | NA | Pneumonia | SIN, PER |
| P2 | 83 | F | 6.0 | 6 | Atherosclerosis | SIN, ELD |
| P3 | 83 | M | 15.0 | 18 | Pneumonia | SIN |
| P4 | 85 | M | 3.5 | 16 | Heart failure | SIN, ELD, PER,RIT |
| Mean ± SEM | 80.5 ± 3.2 | – | 7.4 ± 2.6 | 13.3 ± 3.7 |
- Drugs listed are those recorded as active perscriptions at time of death. Medication for peripheral complaints (e.g. heart disease) are not listed. Abbreviations: SIN, Sinemet (l-DOPA + carbidopa); ELD, Eldepryl (selegiline); PER , Permax (pergolide); RIT, Ritalin (methylphenidate); NA, not available; SD, standard deviation. All cases were Caucasian.
Brains were removed from the skull at autopsy, placed in sterile 0.9% saline solution on ice and then transported to the laboratory, where on arrival they were hemisected. Each hemisphere was then cut into coronal blocks, rapidly frozen in 2-methylbutane chilled to −30 °C and stored subsequently at −80 °C. Tissue punches (100–200 mg) were taken from the uvula (lobule 9) and the nodulus (lobule 10) of the vermis of human cerebellum and stored at −80 °C until used for RNA extraction. The use of human brain tissue was approved by the University of Miami School of Medicine ethics committee.
RT-PCR
RNA was prepared, reverse transcribed and the cDNA amplified as described previously (Hurley et al., 2001), with the following modifications. A ‘touchdown’ method of amplification (i.e. a sequential lowering of the annealing temperature with each cycle) was used for some primers sets in order to obtain single bands, this was not necessary when amplifying cDNA made from other brain areas (data not shown) and may reflect the high levels or RNA found in the cerebellum or the presence of unknown similar sequences. Details of the primers are given in Table 2. The specificity of the primers was determined using the blast algorithm (Altschul et al., 1990) accessed via the Internet (http://www.ncbi.nih.nlm.gov/BLAST). All primers were intron spanning, except for the D1 primers, because the D1 receptor gene lacks introns. Consequently, to control for any residual contaminating genomic DNA, an equivalent amount of total RNA to that amplified from the RT reaction was amplified with the D1 primers in a separate reaction. Any signal detected from these reactions was subtracted from that of the D1 amplification of the cDNA from the RT reactions. The identity of the PCR products was confirmed by restriction enzyme digestion. Reaction parameters varied for each primer set (see Table 3) but for all amplifications there was an initial denaturation at 94 °C for 3 min and a 30-s denaturation per cycle. Extension was 72 °C for 60 s. When all cycles were completed there was a final 10 min incubation at 72 °C. Separate PCR reactions were run from aliquots of a 1 : 5 dilution of cDNA (RT reaction product) for each primer set. Following PCR, 10 µL aliquots of the reactions were electrophoresed through 5% nondenaturing polyacrylamide gels. Each gel was run in duplicate. The gels were then fixed and silver stained using a DNA silver staining kit (AmershamPharmacia Biotech.). Stained gels were then dried using an air gel drying system (BIORAD). Dried gels were digitized and then quantified using nih image (v. 1.62). Variation between samples was reduced by expressing the level of PCR products for each sample as a percentage of the level of β-actin PCR product from sister reactions. Representative polyacrylamide gels are shown in Fig. 1.
| Sequence | Primer sequences | PCR product length (bp) | Restriction enzyme/site | GenBank accession number |
|---|---|---|---|---|
| D1 receptor | 5′-ggtccaaggtgaccaacttc-3′5′-gtcgagtcgaccgtgttccgt-3′ | 331 | Eco0109I/115 | X58987 |
| D2 receptor | 5′-gtcctgtccttcaccatctc-3′5′-aactctaggtctggtacggg-3′ | 560 | HincII/189 | M29066 |
| D3 receptor | 5′-gccatcagcatagacaggta-3′5′-cggaagtaacagacgaccga-3′ | 658 | BamHI/299 | U32499 |
| Dopamine Transporter | 5′-tccggcttcgtcgtcttctc-3′5′-ccgtcagtcgctgctgtag-3′ | 447 | SacI/263 | M95167 |
| Tyrosine hydroxylase | 5′-cctgagccggactgctgcca-3′5′-tgcggttcctgttcgagtcc-3′ | 365 | SacI 227 | X05290 |
- Restriction enzyme/site refers to the enzyme which cuts the PCR product once asymmetrically and the position of the cut within the PCR product sequence.
| 1 Touchdown cycles (n) | 2 Annealing temp (°C) | 3 Cycles (n) | 4 [Mg++](mM) | |
|---|---|---|---|---|
| D1 receptor | 10 | 60 | 20 | 3.0 |
| D2 receptor | 10 | 60 | 30 | 1.5 |
| D3 receptor | 10 | 60 | 30 | 3.5 |
| DAT | 6 | 64 | 30 | 1.5 |
| TH | 0 | 58 | 30 | 1.5 |
| β-actin | 10 | 60 | 15 | 1.5 |
- All reactions had an initial denaturation for 3 min. Then each cycle consisted of a 30 s denaturation at 94 °C, 30 s annealing and 60 s extension at 72 °C. Annealing temperatures are listed below. For touchdown reactions, the annealing temperature started at 70 °C and decreased by 1 °C per cycle (first column), following the touchdown cycles, the annealing temperature remained at the temperature shown in the second column for the remaining number of cycles (third column). The optimized [Mg++] concentration for the reactions is shown in fourth column. Reactions were nonradioactive and used platinum (hotstart) PCR supermix (Life Tech. Inc.).
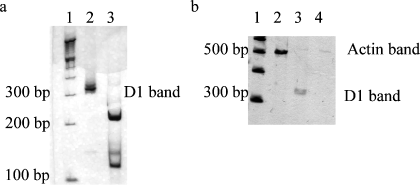
Representative silver stained polyacrylamide gels. Panel (a) shows the 331 base pair D1 receptor band (lane 2) and the restriction digestion products of the D1 receptor PCR product (lane 3). Panel (b) shows the 518 base pair actin PCR product (lane 2) and lanes 3 and 4 show the PCR products produced by amplification of the RT reaction and RNA samples, respectively, using dopamine D1 receptor specific primers. Lane 1 (both panels) shows 100 bp ladder (Life Tech. Inc.).
Statistics
To test whether the levels of mRNA measured for each dopaminergic marker in control and parkinsonian tissue differed from each other, data were analysed by one-way anova followed, where necessary, by post hoc Fisher's protected least significant difference test, using superanova software (Abacus Concepts Inc., San Diego, CA, USA).
Results
PCR with each primer set produced products of 331 bp (D1), 560 bp (D2), 658 bp (D3), 447 bp (DAT) and 365 bp (TH). These PCR products were of the predicted size for each gene under investigation and restriction digestion of them yielded fragments of the expected size. The relative amounts of mRNA for the dopaminergic markers were calculated for each sample by expressing the level of each dopaminergic marker PCR band as a percentage of the β-actin PCR band. Comparison of the levels of β-actin between control and parkinsonian brain tissue were not statistically different (F = 0.019, P= 0.89), indicating that Parkinson's disease does not affect the expression of this reference gene. Hence, comparing the normalized (to β-actin) values of control and parkinsonian tissue for each dopaminergic marker provides a measure of the relative amounts of mRNA in the tissue samples.
Dopamine D1−3 receptor, TH and DAT mRNA was detected in lobules 9 and 10 of the vermis of human cerebellum (Fig. 2). Dopamine D1, D2 and D3 receptor, TH and DAT mRNA represented 63%, 118%, 115%, 106% and 35% of the β–actin value in lobule 9, respectively. In lobule 10, dopamine D1, D2 and D3 receptor, TH and DAT mRNA represented 70%, 119%, 93%, 138% and 59% of the β–actin value, respectively.
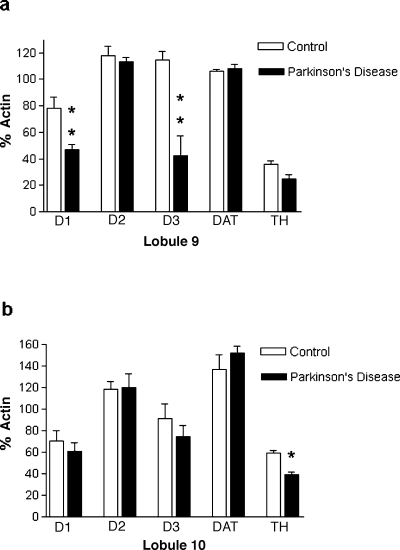
Comparison of the relative levels of dopaminergic neurotransmission markers in (a) lobule 9 and (b) lobule 10 of vermis from human cerebellum from control and parkinsonian brain. The level of PCR products were normalized for each sample by expressing them as a percentage of the level of the β-actin PCR product for each sample. Data represent mean ± SEM for 4 cases. *P < 0.05, **P < 0.01.
In cerebellum from patients dying with Parkinson's disease, a significant reduction in the level of dopamine D1 (40% reduction, F = 12.13, P = 0.01) and D3 (63% reduction, F = 19.93, P= 0.004) receptor mRNA was found in lobule 9 but not lobule 10 (Fig. 2). TH mRNA was significantly reduced in lobule 10 of the parkinsonian cerebellum (35% reduction, F = 34.24, P = 0.001) and there was a trend for a reduction in TH in lobule 9 (F = 5.53, P= 0.057) (Fig. 2). No differences were observed for dopamine D2 receptor or DAT mRNA levels between cerebellar tissue from normal and parkinsonian individuals (Fig. 2).
Discussion
This study has shown that mRNA for TH, DAT and dopamine D1−3 receptors is present in lobules 9 and 10 of the vermis of human cerebellum. In addition, the present investigation has demonstrated that some changes in the level of expression of D1 and D3 dopamine receptor and TH mRNA occur in cerebellum from patients dying with Parkinson's disease who were treated with l-DOPA. It should be noted that direct comparisons between the calculated levels of the relative abundance of each dopaminergic marker (e.g. D1 vs. D2) should not be made, as the PCR products were of differing lengths and the numbers of reaction cycles varied between primer sets. In addition, for the same reasons, the percentage β–actin value does not represent the absolute percentage value (of β–actin) for each dopaminergic marker in the cerebellum.
These data provide further evidence that markers of a functional dopaminergic innervation occur in human cerebellum as found in rodents and nonhuman primates (Boyson et al., 1986; Dubois et al., 1986; Camps et al., 1990; Bouthenet et al., 1991; Panagopoulos et al., 1991; Herroelen et al., 1994; D'Souza et al., 1997; Vessotskie et al., 1997; Khan et al., 1998, 2000; Barili et al., 2000; Melchitzky & Lewis, 2000). The presence of mRNA for dopaminergic markers in the cerebellum indicate that the gene products are present in neurons intrinsic to this brain area or in efferent neurons, as mRNA is only associated with cell bodies and dendrites (Steward & Banker, 1992). Further in situ hybridization studies at the cellular level are required to determine precisely those cell populations which contain dopaminergic elements. From these data it is not possible to conclude whether the alterations in TH and dopamine D1 and D3 receptor mRNA were due to the disease process or caused by the drug treatment of the Parkinson's disease patients.
Indirect evidence that the cerebellum contains dopaminergic neurons comes from studies using the dopaminergic neurotoxin MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine). Takada et al. (1993) reported that MPTP administration to mice damaged Purkinje cells, while Chen et al. (2001) showed that MPTP induced c-fos, c-jun and bax mRNA in the cerebellum. MPTP is converted to MPP+ (1-methyl-4-phenylpyridine) in brain and is then selectively taken up into dopaminergic neurons via the dopamine transporter. Thus, the ability of MPTP to exert toxicity in the cerebellum and induce mRNA for immediate early genes, provides evidence that dopaminergic perikarya are present. Consequently, some of the behavioural effects of MPTP seen in animal models of Parkinson's disease may result from a toxic effect on cerebellar cells, although this remains controversial.
Further evidence that dopaminergic systems operate in the cerebellum come from two recent functional studies. Feigin et al. (2001) showed that metabolic activity was reduced in putamen, thalamus and cerebellum of parkinsonian patients following l-DOPA treatment. While Timmermann et al. (2003) used magnetoencephalography to provide evidence that the cerebellum was included in motor circuits involved in Parkinson's disease tremor.
Changes in dopaminergic markers in the human cerebellum may also result from pathological damage in Parkinson's disease and might explain why some patients show poor motor co-ordination and balance, although these impairments are generally not responsive to dopaminergic drugs. This view is supported by the recent findings of Piao et al. (2003) who found α-synuclein positive inclusions in Bergmann glia of cerebellum from parkinsonian patients. Conversely, the ability of dopamine antagonists to decrease dopamine receptor expression in the cerebellum (see Introduction) and the reduction of cerebellar dopamine receptor mRNA found in this study supports the concept that the changes observed are a drug treatment effect, but this appears less likely in view of the decrease in TH mRNA in lobule 10. The apparent contradiction whereby dopamine antagonist and l-DOPA or dopamine agonist treatment are able to both cause a reduction in cerebellar dopamine receptor expression can be explained by the use of normal animals for the antagonist treatment studies compared to parkinsonian drug-treated human brain used in this study.
It is possible that the TH measured in this study was from noradrenergic, rather than dopaminergic, neurons and the data from this study does not permit exclusion of noradrenergic neurons as the source of TH. However, the presence of DAT, which is a specific marker for dopaminergic neurons (Ciliax et al., 1995), in the same samples implies the presence of neurons with dopaminergic components. This is supported by the findings of Melchitzky & Lewis (2000) who demonstrated that where TH expression coincides with DAT in macaque cerebellum, there is a low level of dopamine-β-hydroxylase. In contrast, areas lacking DAT immunoreactivity but which contained TH immunoreactivity, had higher levels of dopamine-β-hydroxylase. Whether the reduction in TH was due to a loss of dopaminergic neurons within the cerebellum or due to a down regulation of TH within the neurons requires morphological analysis. The absence of a change in DAT mRNA levels supports an adaptive change, as a similar reduction in DAT would be expected if there was neuronal loss. The concomitant reduction in D1 and D3 receptor mRNA, but not D2 receptor mRNA, provides indirect evidence that the D1 and D3 receptors function in the cerebellum in a similar fashion to that found in ventral striatum, where they have been shown coexpressed on the same cells and have opposing effects to D2 receptors (Diaz et al., 1994, 1995; Le Moine & Bloch, 1996). Alternatively, the absence of a decrease in TH could be because the projections originate from non-nigral (e.g. ventral tegmental area) dopaminergic cell groups.
The contribution of these alterations in TH and D1 and D3 receptor mRNA expression to the symptoms of Parkinson's disease or to the side-effects of treatment remain unknown. It is becoming increasingly apparent, however, that dopaminergic systems within the cerebellum have a significant role in the normal control of movement and the pathophysiology of movement disorders like Parkinson's disease (Stein & Aziz, 1999). Recently, projections from the cerebellum to the frontal cortex have been demonstrated in primate brain (Middleton & Strick, 1997). Thus, in addition to a role in movement, the cerebellum could also be involved in the cognitive deficits that occur in Parkinson's disease, or the psychosis often observed following chronic treatment with l-DOPA or dopamine agonists.
In summary, this study has shown that dopamine D1−3 receptor, TH and DAT mRNA is expressed in lobules 9 and 10 of the of human cerebellum and that alterations in D1, D3 and TH occur in l-DOPA-treated Parkinson's disease. These findings suggest that cerebellar dopamine systems have a role in the symptoms of Parkinson's disease or contribute to the beneficial and/or side-effects produced by dopamine replacement therapy.
Acknowledgements
This work was supported by the National Parkinson Foundation Inc. Miami. Human brain tissue was provided by the National Parkinson Foundation/University of Miami Brain Endowment Bank. Expert technical assistance with brain tissue handling was provided by Margaret Basile M.S.
Abbreviations
-
- DAT
-
- dopamine transporter
-
- l-DOPA
-
- l-3,4-dihydroxyphenylalanine
-
- MPTP
-
- 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
-
- MPP+
-
- 1-methyl-4-phenylpyridine
-
- PCR
-
- polymerase chain reaction
-
- RT
-
- reverse transcription
-
- TH
-
- tyrosine hydroxylase.