Decreased iNOS synthesis mediates dexamethasone-induced protection of neurons from inflammatory injury in vitro
Abstract
Brain inflammation is accompanied by transection of axons and death of neurons in the acute lesions of multiple sclerosis. We explored mechanisms of inflammatory damage to neurons in vitro using cocultures of rat embryonal cortical neurons with microglia activated by interferon-gamma (IFNγ) and lipopolysaccharide (LPS). Previously, we have demonstrated that microglia are highly toxic to neurons and that nitric oxide (NO) derived from inducible nitric oxide synthase (iNOS) is necessary and sufficient to mediate this toxicity. Here, we show that addition of dexamethasone (1 µM) to activated cocultures provides effective neuroprotection. We demonstrate that dexamethasone down-regulates NO production of primary microglia by ≈ 50% and reduces steady-state iNOS protein and mRNA expression by ≈ 70%. These changes were reversed by the glucocorticoid receptor blocker RU-486. Furthermore, we analysed the stability of iNOS protein and show that whilst inhibitors of the proteasome blocked iNOS degradation they did not reverse the dexamethasone effect. Our results indicate that the main mechanism of corticosteroid activity on iNOS is reduction in protein synthesis, not destabilization as previously suggested.
Introduction
Axonal loss is thought to be primarily responsible for neurological disability in the chronic progressive stage of multiple sclerosis (MS), an inflammatory demyelinating disease of the human central nervous system. The density of activated microglia surrounding injured axons (Trapp et al., 1998) is increased in active lesions (Banati et al., 2000) and correlates with the extent of axonal injury (Bitsch et al., 2000), suggesting that microglia contribute directly to neurodegeneration. Microglia have evolved to kill invading pathogens, for instance by synthesizing nitric oxide (NO) via inducible nitric oxide synthase (iNOS). However, NO can also kill neighbouring cells, for example through DNA damage, poly-ADP ribose polymerase (PARP) overactivation and adenosine triphosphate (ATP) depletion (Zhang et al., 1994), p53 accumulation (Forrester et al., 1996) or opening of the mitochondrial permeability transition pore (Brown & Borutaite, 2001). NO potently inhibits mitochondrial respiration, leading to ATP depletion in neurons, glutamate release and excitotoxicity (Bal-Price & Brown, 2001; Golde et al., 2002). Energy failure after NO exposure may similarly contribute to conduction block and axon degeneration in rat dorsal root preparations (Smith et al., 2001). iNOS expression and nitration of tyrosine residues have been demonstrated in the plaques of MS (Bagasra et al., 1995; De Groot et al., 1997; Cross et al., 1998), and nitrites and nitrates are increased in cerebrospinal fluid and serum from affected individuals (Johnson et al., 1995; Giovannoni et al., 1997; Yamashita et al., 1997). iNOS is up-regulated in peripheral blood monocytes, stimulated ex vivo, and glucocorticoid treatment lowers their NO production during acute relapses due to MS (Sarchielli et al., 1997).
We have previously modelled inflammatory injury of neurons in vitro by coculturing rat embryonic cortical neurons with microglia activated by interferon-gamma (IFNγ) + lipopolysaccharide (LPS) (Golde et al., 2002) and showed that iNOS-derived nitric oxide alone mediates neurotoxicity of activated microglia. Here, we extend those observations to demonstrate how microglial activation can be modified to protect neurons. Glucocorticoids have pleiotropic anti-inflammatory functions and also down-regulate NO production (Radomski et al., 1990) but the neuroprotective effect of these manipulations has not been defined. Furthermore, the mechanism whereby dexamethasone reduces NO production is not known. Studying IFNγ-stimulated RAW 264.7 macrophages, Walker et al. (1997) demonstrated that, while dexamethasone reduced iNOS mRNA by multiple mechanisms, calpain-inhibitor 1 (N-acetyl-L-leucinyl-L-leucinyl-L-norleucinal; ALLN) stabilized iNOS protein in dexamethasone-treated cells to a far greater extent than in -untreated cells, equalizing the amounts of iNOS protein in both samples, implying that dexamethasone reduces NO by destabilizing iNOS protein. Subsequently, Walker et al. (2001) reported that purified calpain degraded iNOS translated in vitro. However, ALLN is not specific for calpain but also potently inhibits the proteasome. iNOS was recently identified as a proteasome substrate, whereas it is readily degraded in the presence of calpastatin (Musial & Eissa, 2001). Therefore, rather than affecting calpain activity, glucocorticoids may regulate iNOS expression by accelerating proteasome-dependent degradation.
In this study, we show that dexamethasone protects neurons from microglial toxicity in vitro by down-regulating NO production. We investigate the relative contributions to this glucocorticoid effect of reduction in iNOS mRNA levels and modulation of the proteasome-dependent degradation of iNOS protein.
Materials and methods
Materials
(Z)-1-[2-(2-Aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate] (DETA-NoNoate) was purchased from Alexis Biochemicals, San Diego, CA, USA; IFNβ and IFNγ were from Serotec Ltd., Oxford, UK; lactacystin, epoxomycin and ALLN were purchased from Affiniti Research Products Ltd., Matford Court, Exeter, UK; dexamethasone, mifepristone (RU-486), minocycline and LPS, and all other chemicals unless specifically indicated, were from Sigma.
Cultures of cortical neurons
Highly purified neuronal cultures (≥ 98% β-tubulin III-positive) were grown as previously described (Golde et al., 2002). Briefly, after removal of meninges from brains of embryonic day 16 Sprague-Dawley rat embryos (Charles River Laboratories, UK), cerebral cortices were dissected, dissociated and tissue pieces incubated in trypsin (0.1% in HBSS without calcium and magnesium; Gibco, Invitrogen Ltd., Renfrew, UK) for 20 min at 37 °C, washed in DNase (0.001% in HBSS) and manipulated in triturating solution (1 g Albumax, Gibco; 50 mg trypsin inhibitor; and 1 mg DNase per 100 mL HBSS) using flame-polished Pasteur pipettes. Cells were resuspended in serum-free defined culture medium [Dulbecco's modified Eagle's medium (DMEM)]; 1% penicilline–streptomycine–fungizone (PSF); and 2% B27 supplement; all from Gibco). Viable cells, demonstrated by Trypan-blue exclusion, were plated on poly-l-lysine (0.01% in distilled water)-coated 24-multiwell plates (Nunclon, Life Technologies, Paisley, UK) at a density of 2.26 × 105 cells/cm2, or onto poly-l-lysine-coated 13-mm-diameter glass coverslips at a density of 1.13 × 105 cells/cm2 for immunocytochemistry, and grown for 12 days before coculture. Cultures were maintained in DMEM–B27–PSF at 37 °C in a 5% CO2 humidified atmosphere.
Cultures of primary microglia
Microglia were isolated from mixed glial cell cultures as previously described (Golde et al., 2002). Briefly, cells dissociated from neonatal rat (Sprague-Dawley) cerebral hemispheres were plated in 75-cm2 poly-l-lysine-coated tissue culture flasks (Orange Scientific, Triple Red, Thame, UK) at a density of two brains per flask in culture medium consisting of DMEM supplemented with 10% fetal calf serum. Culture medium was changed after 24 h and then twice per week. After 2–3 weeks, the loosely adherent microglia on top of a confluent glial cell layer were harvested using a rotary shaker (Lukham R300) at 70% for 20 min. After centrifugation (180 g for 5 min), cells were resuspended in serum-free defined culture medium (DMEM with 1% PSF and 2% B27 supplements). Cell viability was determined with Trypan-blue exclusion, and viable cells were plated at a ratio of 1 : 2 onto 12-day-old neuronal cultures, or at the equivalent density of 1.12 × 105 cells/cm2 directly onto 24-multiwell plates (Nunclon). For immunostaining, microglia were plated at 5.6 × 105 cells/cm2 onto neurons grown on glass coverslips. After 20 min, cultures were washed once with HBSS to remove nonadherent contaminating glia and incubated in serum-free defined medium for 3 days with or without microglial activators: LPS (1 µg/mL) and IFNγ (100 U/mL, Serotec). These concentrations of LPS and IFNγ were used in all experiments involving microglia.
Cultures of N9 microglial cells
The N9 murine microglial cell line was kindly given to us by Thomas Horn, Institute for Medical Neurobiology, Otto-von Guericke-Universitaet, Magdeburg, Germany. N9 cells were grown in Iscove's Modified Dulbecco's Medium (IMDM; ICN Biomedicals Inc., Aurora, Ohio, USA) with 2 mm l-glutamine, 5% fetal calf serum, 1% penicillin–streptomycin and 50 µmβ-mercaptoethanol as described previously (Corradin et al., 1993). Cells were plated at 2 × 105 per well for metabolic labelling and acid precipitation of lysates.
NO2− determination
NO2− levels, measured with the Griess reagent, were taken as an estimate of NO production. Griess reagent (equal volumes of 0.1% N-1-naphthylethylenediamine dichloride in water and 1% sulphanilamide plus 5% H3PO4 in water) was added to an equal volume of cell culture supernatant (100 µL) and incubated for 20 min at room temperature. The optical density was measured at 570 nm and a standard curve established using NO2− at a range of 1–100 µm.
MTT assay
The viability of cell cultures was estimated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Cells were incubated with MTT (0.5 mg/mL in culture medium) for 1 h at 37 °C and then washed in PBS before adding 10% Triton X-100 in 0.1 m HCl and shaking for at least 20 min at room temperature to dissolve the formazane crystals before the optical density of the mixture was measured at 570 nm.
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde for 10 min at room temperature followed by permeabilization with methanol for 10 min at −20 °C and blocking with DMEM with 10% fetal calf serum. Incubation with primary antibodies was carried out overnight at 4 °C or for 1 h at 37 °C. Monoclonal antibodies against β-tubulin, isotype III (Chemicon International, Temecula, CA, USA and Sigma) were used at 1 : 200 dilution; hybridoma supernatants against A2B5 (clone 105, ECACC) and GalC (clone IC-07, ECACC) were used at 1 : 5 and 1 : 10, respectively. Rabbit anti-GFAP (Chemicon) was used at 1 : 500. Appropriate conjugated secondary antibodies were purchased from Harlan-Sera-Laboratory, Belton, Loughborough, UK, Vector Laboratories, Peterborough, UK, Chemicon and Serotec, and used 1 : 200 for 1 h at room temperature. Microglia were labelled with FITC-conjugated B4-isolectin (0.04 mg/mL).
Preparation of cell extracts, SDS-PAGE and Western blotting
Microglia were plated at 4 × 105 cells/cm2 and grown for 24 h in the presence or absence of IFNγ + LPS, dexamethasone (1 µm) and RU-486 (10 µm), then washed with PBS and lysed for 30 min at 107 cells/mL in NP40 lysis buffer [1% (v/v) NP40, 50 mm Tris, pH 7.5, 150 mm NaCl, 1 mm MgCl2, 1 mm CaCl2, Complete™ Mini, and EDTA-free protease inhibitor cocktail tablets (Hofmann-La Roche Ltd, Basel, Switzerland)]. Lysates were cleared of debris by centrifugation (15500 g for 15 min) and supernatants stored at −20 °C. The protein concentration of each lysate was determined using the BCA Protein Assay (Pierce, Rockford, IL, USA). Cell lysates were mixed with one quarter volume of 4 × gel loading buffer [200 mm TrisCl (pH 6.8), 8% SDS, 0.4% bromophenol blue and 40% glycerol] boiled for 5 min at 95 °C, resolved on 8% polyacrylamide–SDS gels and electroblotted onto PVDF membranes (Immobilon™-P, Millipore, Bedford, MA, USA). The membranes were blocked for 1 h at room temperature in 5% milk and 0.05% Tween 20 in PBS, then incubated with mouse monoclonal anti-iNOS (Transduction Laboratories, BD Biosciences, Bedford, MA, USA; 1 : 10.000 in blocking buffer) overnight at 4 °C followed by rabbit anti-mouse Ig (1 µg/mL; Dako, Ely, UK) in blocking buffer for 1 h at room temperature. Subsequently, blots were incubated with 125I-labelled protein A (ICN Biomedicals, Irvine, CA, USA) at a final concentration of 0.5 µCi/mL in 0.5% bovine serum albumin for 1 h at room temperature. The blots were washed four times for 10 min in PBS, air dried, and then exposed to film (Kodak Biomax™ MS) and viewed using a Hewlett Packard 3300C scanner. Densitometric quantification was performed with the scion image software (Scion corporation, Frederick, MD, USA; http://www.scioncorp.com).
Real-time quantitative RT-PCR
Microglia (3.75 × 106) were grown (i) for 24 h in the presence or absence of IFNγ + LPS, dexamethasone (1 µm) and RU-486 (10 µm), or (ii) for 6 h with IFNγ + LPS with or without dexamethasone (1 µm) for an additional 2 h. Cultures were then washed with PBS and total RNA prepared using the SV Total RNA isolation system including DNase treatment (Promega, Madison, WI, USA). Reverse transcription was performed using the proStar kit with random hexamers (Stratagene, Cedar Creek, TX, USA). From a total volume of 50 µL per cDNA, 2.5 µL were used in the PCR reactions. Real-time quantification was performed using gene-specific fluorogenic probes and the MasterMix (Oswell, University of Southampton, UK) in a final volume of 25 µL. The reaction mixture contained all primers at 300 nm and the probe at 100 nm. The enzyme was heat-activated for 10 min at 95 °C. A two-step PCR procedure of 15 s at 95 °C and 60 s at 60 °C was applied for 50 cycles. PCR and TaqManTM. analysis were performed using the ABI/PRISM 7700 sequence detector system (PE Applied Biosystems, Warrington, UK). PCR reactions were performed in singleplex using the following primers and probes: FAM-labelled rat iNOS probe 5′-CCTGCCCCTTCAATGGTTGGTACATG-3′, forward primer 5′-GGTGGGTGGCCTCGAGTT-3′, reverse primer 5′-CAGAAGTCTCGGACTCCAATCTC-3′; JOE-labelled β-actin probe: 5′-TTTGAGACCTTCAACACCCCAGCCA-3′, forward primer 5′-AGGCCCCTCTGAACCCTAAG-3′ and reverse primer 5′-CCAGAGGCATACAGGGACAAC-3′. Standard curves for each primer–probe triplet were generated from the cDNAs from (i) unstimulated (β-actin), IFNγ + LPS, dexamethasone and RU-486-treated sample (iNOS), and (ii) from IFNγ- + LPS-stimulated sample (β-actin and iNOS) and were used to calculate cycle threshold, i.e. cycle at which sample crosses a set threshold of reporter signal (CT) and hence the relative amount of each product for each sample, run in triplicate in each 96-well plate. Results are expressed as the mean iNOS/β-actin ± SEM for each triplicate.
Metabolic labelling and immunoprecipitation
For pulse-chase analyses, 2 × 106 microglia were plated into each 25-cm2 tissue culture flask (Orange Scientific) and grown over night in DMEM–B27–PSF. Microglia were then stimulated with IFNγ (100 U/mL) + LPS (1 µg/mL) for either 24 or 6 h as indicated. Dexamethasone was either added at the same time as IFNγ and LPS for 24 h or after 6 h with a 1 h stimulation period as shown. Thereafter, the medium was aspirated, cultures were washed once with PBS and the cells were incubated for 1 h in starving medium [RPMI 1640 without methionine, cysteine or glutamine, supplemented with 2 mm glutamine, 10 mm HEPES, 1 mm pyruvate (all Gibco), PSF and B27 plus IFNγ, LPS and dexamethasone, where indicated] to deplete intracellular stores of methionine. Subsequently, [35S]in vitro cell-labelling mix (Promix™, Amersham Biosciences UK Limited, Little Chalfont, UK) at a final concentration of 100 µCi/mL and protease inhibitors (lactacystin, epoxomycin or ALLN) were added, as shown. Cells were pulsed for 2 h and then washed with PBS. Cultures were treated in NP40 lysis buffer at 0, 2 or 4 h after the pulse for 30 min at 4 °C, and then centrifuged at 13358 g for 15 min to separate nuclei and cellular debris. Supernatants were stored at −20 °C. For immunoprecipitation, lysates were precleared in the presence of 5 µL normal rabbit serum three times on 100 µL Staph. A. (Calbiochem, La Jolla, CA, USA) and once on 25 µL of a 50% solution of protein A-sepharose CL-4B, then immunoprecipitated with 2.25 µg of purified mouse IgG1 (clone MOPC 21), 2.25 µg of rabbit anti-mouse immunoglobulin (Dako) to mediate binding of the IgG1 primary antibody to protein A and 25 µL of 50% protein A-sepharose. Supernatants were cleared of remaining antibodies on 100 µL Staph. A and immunoprecipitated with 2.25 µg of monoclonal anti-iNOS (Transduction Laboratories) plus 2.25 µg of rabbit anti-mouse immunoglobulin. All immunoprecipitates were washed five times in low-detergent buffer (0.05% NP40; 150 mm NaCl; 50 mm Tris, pH 7.4; and 5 mm EDTA) and proteins were solubilized from beads by heating for 5 min at 95 °C in gel loading buffer (once in NP40 lysis buffer) and separated by SDS gel electrophoresis. Gels were then fixed for 30 min in (isopropanol : acetic acid : water, 25 : 10 : 65), soaked for 30 min in Amplify™ (Amersham), dried and exposed to film (Biomax™ MR 1, Kodak Ltd, Hemel Hempstead, UK).
Acid precipitation of [35S]-labelled protein
After a 2-h pulse with [35S]-methionine and lysis of the cells, 25 µL of lysate and 25 µL of 2% bovine serum albumin were spotted in quadruplicate onto Whatman filter paper. One set of two samples was directly measured in the scintillation counter (total intracellular [35S]-methionine), the second set was covered by 10% ice-cold trichloracetic acid (TCA) for 10 min, then washed for 10 min with 5% ice-cold TCA, followed by 10 min in cold acetone. After acid precipitation the samples were also counted in the scintillation counter ([35S]-methionine incorporated into protein).
Results
Dexamethasone protected neurons by lowering microglial NO production
Cortical neurons from E16 rat embryos matured in culture for 12 days were cocultured with microglia from primary rat mixed glial cultures for 3 days in the presence or absence of the microglial activators IFNγ (100 U/mL) and LPS (1 µg/mL). Viability of cocultures, established by MTT metabolism, decreased by 66% in activated cocultures compared to nonactivated controls, representing neuronal death as previously described (Golde et al., 2002). There was a dose-dependent increase in MTT metabolism when dexamethasone (1 µm−1 nm) was added to activated cocultures of microglia and cortical neurons. MTT rose from 34% of controls in activated cocultures without glucocorticoid to 71% and 64% in the presence of 100 nm and 1 µm dexamethasone, respectively. This increase was significantly different for the 100 nm dose (Fig. 1A). Dexamethasone had no effect on the MTT metabolism of nonactivated cocultures or neuronal and microglial control cultures (data not shown). Microglial NO–nitrite secretion must reach a high threshold (≈ 60 µm nitrite at 3 days) to cause toxicity of these cortical neurons (Golde et al., 2002). As shown in Fig. 1B, dexamethasone (1 µm−1 nm) dose-dependently lowered, but did not abolish, microglial NO–nitrite production. Neuroprotective concentrations of the glucocorticoid (100 nm and 1 µm) decreased NO–nitrite to just below the neurotoxic threshold. The loss of neurons following microglial activation was confirmed by counting β-tubulin-positive cells (Fig. 1C). The decrease in NO–nitrite production and the neuroprotection observed using dexamethasone were also associated with an increase in β-tubulin-positive neurons. These effects were both reversed when the glucocorticoid receptor antagonist RU-486 (10 µm) was added to activated cocultures at the same time as dexamethasone (1 µm) (Fig. 1C and D). To test whether susceptibility of neurons to NO was affected by dexamethasone, neurons were treated with different concentrations of the NO donor DETA-NoNoate. While accumulated nitrite concentrations of up to ≈ 40 µm did not cause much toxicity to the neurons, there was a steep decline in MTT metabolism at higher concentrations with maximal toxicity at accumulated nitrite concentrations of 100 µm (Fig. 2). Dexamethasone treatment did not change this toxicity of the NO donor.
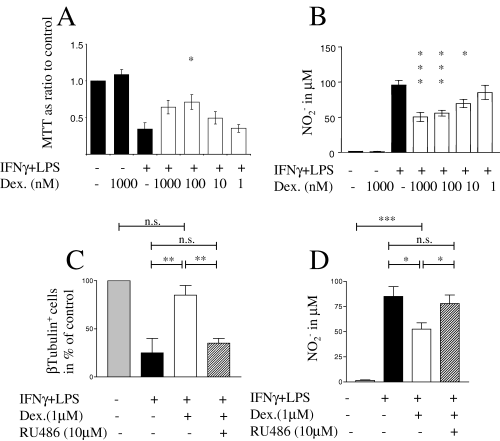
Neuroprotection by dexamethasone was due to attenuation of NO secretion and was mediated by the glucocorticoid receptor. Microglia and cortical neurons were cocultured for 3 days in the presence or absence of IFNγ + LPS and dexamethasone as indicated. (A) MTT metabolism: cytotoxicity in activated cocultures was attenuated dose-dependently by dexamethasone. (B) Nitrite measurements: dexamethasone reduced NO production of activated cocultures in a similar dose-dependent manner. (C) Immunocytochemistry: the neuronal marker β-tubulin III shown in 3-day-old cocultures in the presence or absence of IFNγ + LPS and dexamethasone demonstrates that neuronal loss in activated cocultures was prevented by dexamethasone. The glucocorticoid receptor antagonist RU-486 blocked neuroprotection by corticosteroids. (D) NO production correlated with neurotoxicity in cocultures and this effect was reversed by RU-486. Values represent the mean ± SEM of at least three independent experiments, each performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001 (one-way anova and Newman–Keuls post hoc test).
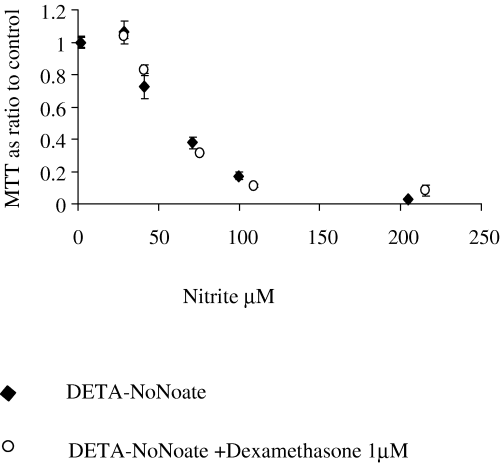
Dexamethasone did not affect susceptibility of neurons to NO. Neurons were treated with DETA-NoNoate at different concentrations (500, 250, 125, 62.5, 31.25 and 0 µm), and dexamethasone (1 µm) was added to half the samples. Neurons were then grown for 3 days, after which NO release was measured by the Griess reaction and viability assessed with the MTT assay. MTT metabolism is plotted as a ratio of nitrite concentrations in samples and controls. The experiment was performed in quadruplicate and confirmed in two independent experiments. Shown are means ± SEM. Statistical analysis was carried out using Student's t-test with the Bonferoni correction for multiple testing. There was no statistical difference in MTT values between dexamethasone-treated and -untreated samples when pairs of samples treated with the same amount of DETA-NoNoate were compared.
We reported previously that the cytokine IFNβ inhibits IFNγ-dependent up-regulation of MHC class II expression in vitro (Hall et al., 1997); down-regulation of iNOS by IFNβ has also been reported (Stewart et al., 1997; Lopez-Collazo et al., 1998; Gao et al., 2000). The tetracycline derivative, minocycline, blocks microglial activation in vitro (Amin et al., 1996; Tikka et al., 2001) and inhibits experimental allergic encepholymyelitis (Popovic et al., 2002). We incubated neurons with IFNβ (200 and 1000 U/mL, given 1 h before IFNγ + LPS) and minocycline (20–0.02 µg/mL, given 1 h before IFNγ + LPS) but did not find any reduction in neurotoxicity (data not shown). Similarly, NO production was not affected by either agent (Table 1).
| Treatment | Pretreatment* | Nitrite (µm) |
|---|---|---|
| IFNγ + LPS | – | 81.68 ± 1.52 |
| IFNγ + LPS | Minocycline (20 µg/mL) | 98.84 ± 2.93 |
| IFNγ + LPS | Minocycline (2 µg/mL) | 96.16 ± 5.91 |
| IFNγ + LPS | Minocycline (0.2 µg/mL) | 82.95 ± 5.82 |
| IFNγ + LPS | Minocycline (0.02 µg/mL) | 95.1 ± 1.15 |
| IFNγ + LPS | – | 81.68 ± 1.52† |
| IFNγ + LPS | IFNβ (200 U/mL) | 93.41 ± 3.1† |
| IFNγ + LPS | – | 74.42 ± 0.16‡ |
| IFNγ + LPS | IFNβ (1000 U/mL) | 72.38 ± 1.02‡ |
- * Microglia–neuronal cocultures were treated with minocycline or IFNβ at the concentrations indicated 1 h before activation with IFNγ+ LPS. Nitrite (±SEM) was determined in the culture supernatants after 3 days. Neither minocycline nor IFNβ could reduce NO production in the activated cocultures. (P > 0.05 vs. IFNγ+ LPS alone, one-way anova and Newman–Keuls post hoc test except †Student's t-test, ‡Student's t-test, separate experiment). Shown is a representative experiment which was repeated three times.
Dexamethasone reduced NO production at multiple timepoints
In order to assess how the timing of exposure to dexamethasone relative to microglial induction affects the down-regulation of NO–nitrite production, microglial cultures were treated with dexamethasone (1 µm) before, during or 6 and 24 h after activation. Dexamethasone effectively down-regulated microglial NO–nitrite production under each of these conditions, but to different extents (Fig. 3). NO–nitrite production was most reduced by 24-h pretreatment with dexamethasone (64% reduction after 3 days). When dexamethasone was washed out before addition of IFNγ + LPS, reduction was still 40% after 3 days, indicating that this glucocorticoid achieves long-lasting block of iNOS-induction. However, an effect was still present after the onset of microglial activation, with reduction of NO–nitrite production at 35 and 20% when glucocorticoid was added 6 and 24 h after IFNγ + LPS, respectively, compared to a 41% reduction when dexamethasone was added at the same time as iNOS inducers.
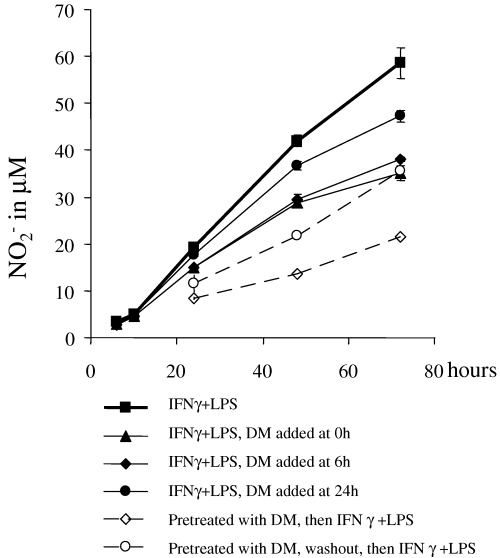
Dexamethasone reduced NO when given before, during or after iNOS-induction. Microglia were activated with IFNγ + LPS for 3 days. Dexamethasone (1 µm) was either given 24 h before stimulation, at the same time, or 6 or 24 h afterwards. Nitrite concentrations were determined from culture supernatants at the indicated time points. Statistical analysis was carried out using one-way anova and the Newman–Keuls post hoc test. At 72 h, all dexamethasone-treated samples had nitrite values significantly different from the untreated sample (all P-values < 0.001). At 24 and 48 h, all nitrite values apart from the sample treated with dexamethasone after 24 h were significantly different from the dexamethasone-untreated sample (all P-values < 0.01). Means ± SEM of a representative experiment are shown but results were reproduced in three independent experiments. DM, dexamethasone.
Dexamethasone reduced steady-state iNOS protein levels and mRNA to a similar extent
To determine whether the dexamethasone-induced suppression of NO/nitrite production in microglia is due to reduced levels of iNOS protein, microglial cultures were stimulated for 24 h with IFNγ + LPS, alone or in the presence of dexamethasone (1 µm) or the combination of dexamethasone (1 µm) and RU-486 (10 µm). At this time point the rate of NO production was maximal and stable over the next 24 h (Fig. 3). Because NO production by iNOS is mainly dependent on protein expression, iNOS concentrations at 24 h should represent the steady-state situation. Western blot analysis showed that iNOS was undetectable in unstimulated microglia and strongly induced by IFNγ + LPS (Fig. 4). Dexamethasone reduced the iNOS protein to 33% of the level seen in IFNγ- + LPS-treated cells. Simultaneous incubation with RU-486 abolished the effect of dexamethasone, with iNOS levels equalling those seen in IFNγ- + LPS-treated cells (Fig. 4A, lanes 2 and 4). We next investigated whether the dexamethasone-induced reduction in iNOS protein depends on reduction in iNOS mRNA. Microglia were treated as described above. After 24 h, total RNA was isolated and steady-state levels of iNOS mRNA quantified against β-actin mRNA as control using real-time RT-PCR. As shown in a representative experiment (Fig. 5A), very low levels of iNOS mRNA were detected in unstimulated microglia and IFNγ + LPS caused a 25-fold induction. Dexamethasone consistently reduced iNOS mRNA to ≈ 33% of the levels found in IFNγ- + LPS-treated cultures. These results match reductions observed at the protein level. Unexpectedly, treatment with RU-486 not only reversed the dexamethasone-effect but led to a 5- (shown here) to 28-fold increase in iNOS mRNA compared to IFNγ- + LPS-treated cells in repeat experiments. Dexamethasone can affect iNOS mRNA concentrations quite rapidly, as addition for only 2 h to microglial cultures (which were stimulated with IFNγ + LPS for 6 h) reduced iNOS mRNA to 60% (Fig. 5B).
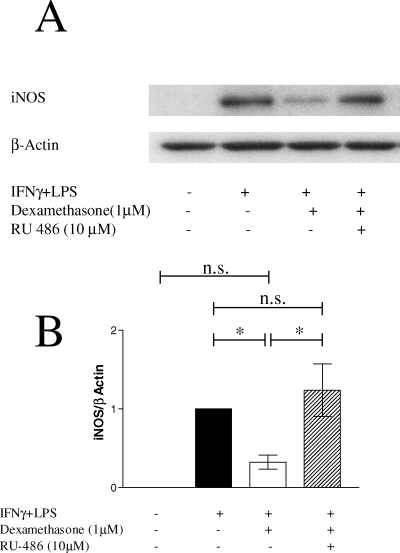
Dexamethasone down-regulated total iNOS protein in IFNγ- + LPS-stimulated microglia. Microglia were grown in the presence or absence of IFNγ + LPS for 24 h. Dexamethasone (1 µm) and RU-486 (10 µm) were added with activators as indicated. Cells were lysed, separated by SDS-PAGE and transferred to PVDF membrane. Immunoblots were developed using monoclonal anti-iNOS antibody and 125I-labelled protein A. (A) A representative example of autoradiography from three similar experiments is shown. (B) Total amounts of iNOS protein analysed densitometrically from three independent experiments are shown (mean ± SEM). *P < 0.05 (one-way anova and Newman–Keuls post hoc test).
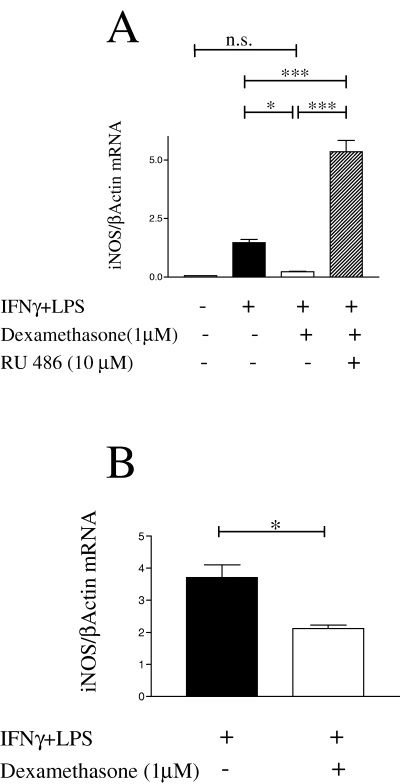
Microglia steady-state iNOS mRNA levels were reduced by dexamethasone. (A) Total RNA was isolated from microglia grown for 24 h in the presence or absence of IFNγ + LPS; dexamethasone (1 µm) and RU-486 (10 µm) were added with these activators as shown. iNOS and β-actin mRNA levels were analysed by real-time quantitative RT-PCR. The ratio of iNOS : β-actin concentration is shown for a representative experiment performed in triplicate (mean ± SEM). iNOS mRNA was rapidly reduced by dexamethasone. (B) Microglia were stimulated with IFNγ + LPS for 6 h, dexamethasone (1 µm) was then added for 2 h where indicated. After a total of 8 h, cells were harvested for isolation of total RNA and real-time RT-PCR. The ratio of iNOS : actin concentration is shown (experiment performed in triplicate, mean ± SEM). *P < 0.05, **P < 0.01, ***P < 0.001 (one-way anova and Newman–Keuls post hoc test).
iNOS was degraded by the proteasome independently of dexamethasone treatment
Previously it has been suggested that the major mechanism of glucocorticoid-induced iNOS suppression in RAW 264.7 cells is acceleration of iNOS degradation. Therefore we investigated iNOS stability in primary microglial cultures under dexamethasone treatment. In particular, we wanted to know whether dexamethasone could accelerate proteasome-dependent degradation of iNOS. We chose first to study the steady-state situation for which we had already analysed total iNOS protein and mRNA levels (see above). Microglia were stimulated for 21 h with IFNγ + LPS in the presence or absence of dexamethasone (1 µm), and then metabolically labelled in either the presence or absence of lactacystin (10 µm) which, at this concentration, specifically inhibits proteasome-dependent degradation. Resolution of the iNOS immunoprecipitates revealed specific iNOS bands at 130 kDa with others running at regular intervals a few kDa higher than iNOS (Fig. 6); these may be polyubiquitinated forms of iNOS (Kolodziejski et al., 2002). Figure 6 shows that lactacystin clearly stabilized iNOS in dexamethasone-treated (compare lanes 2 with 4, 6 with 8 and 10 with 12) as well as untreated cells (compare lanes 1 with 3, 5 with 7 and 9 with 11) during the 4-h chase period. Bands running at regular intervals above iNOS are also increased by lactacystin, supporting the conclusion that they represent polyubiquitinated iNOS. Despite the inhibition of iNOS degradation by lactacystin during this pulse, very little iNOS protein could be recovered at t0, indicating that iNOS synthesis is substantially decreased in dexamethasone-treated samples. This correlates with the glucocorticoid suppression of iNOS mRNA (Fig. 5A).
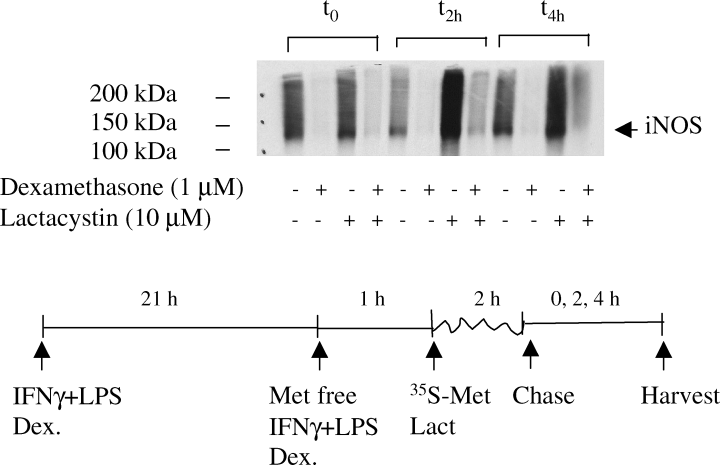
Proteasome inhibition stabilized iNOS but did not modulate the effect of dexamethasone. Microglia were stimulated for 21 h with IFNγ + LPS in the presence or absence of dexamethasone (1 µm). Cells were starved of l-methionine for 1 h and subsequently pulsed with l-[35S]-methionine for 2 h in the presence or absence of lactacystin (10 µm). Chase medium containing an excess of unlabelled l-methionine was added for 0, 2 and 4 h; cells were then lysed and sequentially immunoprecipitated using purified mouse immunoglobulins, IgG1 isotype, as control followed by monoclonal anti-iNOS. No signal was obtained from control IgG1 immunoprecipitates (not shown), even after 1 week of exposure. Signals after iNOS immunoprecipitation following <24 h exposure are shown; no signal was obtained from unstimulated controls. Autoradiographs show specific bands at 130 kDa, the expected molecular weight for iNOS (marked with arrow), but also many regularly spaced bands at higher molecular weights up to the end of the running gel.
Short-term effects of dexamethasone on iNOS protein levels were also regulated at the mRNA level
In order to establish whether immediate effects of dexamethasone on iNOS suppression involve regulation of iNOS mRNA levels or protein degradation, we stimulated microglial cultures with IFNγ + LPS for 6 h and then added dexamethasone (1 µm) to half these cultures for 2 h. As shown above, iNOS mRNA was already reduced to 60% after this short dexamethasone treatment (Fig. 5B). For pulse-chase analysis of iNOS protein, microglia were cultured for 6 h in the presence of IFNγ + LPS before adding dexamethasone to half the cultures for 1 h as well as during the starving (1 h) and labelling (2 h) periods. The proteasome inhibitors lactacystin (10 µm) and epoxomycin (1 µm), and the proteasome and calpain inhibitor ALLN (100 µM), previously implicated in dexamethasone regulation of iNOS degradation (Walker et al., 1997), were added during the pulse period (Fig. 7). As expected from the 40% reduction in iNOS mRNA seen at the beginning of the pulse period (Fig. 5B) the amount of newly synthesized iNOS protein was reduced by dexamethasone, although not to the extent seen in the steady state (Fig. 7A and B). Lactacystin, epoxomycin and ALLN all stabilized iNOS protein, but neither protease inhibitor attenuated the glucocorticoid effect. In fact, differences between dexamethasone-treated and untreated samples were more apparent at both the 0- and 2-h time points (Fig. 7A). To control for any effects dexamethasone may have on total protein synthesis, N9 microglial cells were pulsed with 35S-methionine and treated with dexamethasone and lactacystin as shown (Fig. 7C). Dexamethasone reduced neither uptake of labelled methionine into the cells nor its incorporation into newly synthesized protein.
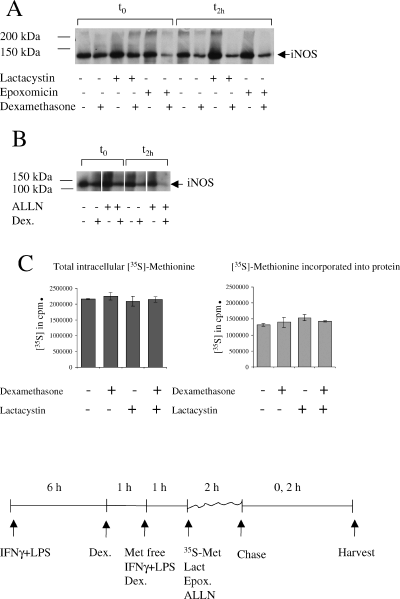
Reduced iNOS levels following short dexamethasone treatment were due to attenuation of protein synthesis, not acceleration of iNOS degradation. Microglia were stimulated with IFNγ + LPS for 6 h. Dexamethasone (1 µm) was then added for 1 h where indicated. Cells were starved of l-methionine for 1 h and subsequently pulsed with l-[35S]-methionine for 2 h in the presence or absence of lactacystin (10 µm), epoxomycin (1 µm) and ALLN (100 µm). Chase medium was added for 0 and 2 h; cells were then lysed and sequentially immunoprecipitated, as described above. No signal was obtained from control IgG1 immunoprecipitates (not shown). Autoradiographs show specific bands at 130 kDa, the expected molecular weight for iNOS (marked with arrow), and higher molecular weight bands up to the end of the running gel can also be seen. (A) Representative experiment using the proteasome inhibitors lactacystin and epoxomycin. (B) Immunoprecipitates from ALLN-treated cells and controls in a separate experiment. (C) N9 microglial cells were stimulated and treated with or without dexamethasone and lactacystin as described for the primary microglia. Radioactivity in lysates of the pulsed cells was counted before (total intracellular [35S]) and after acid precipitation ([35S] incorporated into protein). Shown are the means ± SEM of triplicate samples.
Discussion
Studying rat primary neurons in culture, we have shown that dexamethasone protects neurons from toxicity associated with exposure to activated microglia by down-regulating NO production. By interacting with the glucocorticoid receptor, dexamethasone rapidly reduced iNOS mRNA and, thereby, protein synthesis even when iNOS induction was already established. We confirm that iNOS degradation in primary microglial cultures is proteasome-dependent. However, changes in iNOS degradation play no role in the glucocorticoid-induced suppression of microglial NO production.
Steroid hormones inhibit proliferation, NO production, cytokine secretion and glutamate release of cultured microglial cells (Chao et al., 1992; Ganter et al., 1992; Jun et al., 1994; Piani & Fontana, 1994). Pre-treatment of microglial cells with dexamethasone suppresses the release of excitotoxins and prevents toxicity of microglia-conditioned medium to neurons (Flavin et al., 1997). Conversely, glucocorticoids can be neurotoxic. They have been shown to enhance hippocampal neurodegeneration (Sapolsky et al., 1986; Kerr et al., 1991) and increase the vulnerability of neurons to excitotoxic insults, hypoglycaemia and ischaemia, possibly by compromising energy production (Chan et al., 1996) or exacerbating lipid peroxidation (Brooke et al., 1997; Howard et al., 1999).
We demonstrated previously that microglia-derived NO induces excitotoxicity in mature cortical neurons (Golde et al., 2002), presumably through NO-induced inhibition of respiration, ATP decrease and neuronal glutamate release (Bal-Price & Brown, 2001). Here, we consider whether dexamethasone protects neurons by attenuating microglial activation or, paradoxically, damages neurons perhaps by worsening the impairment of energy production and weakening the antioxidative defence. We found that dexamethasone (up to 1 µm) almost completely protected cortical neurons from injury by IFNγ- + LPS-activated microglia. This neuroprotection correlates well with the ability of dexamethasone to down-regulate NO production in our primary microglial cells. We showed previously that inhibition of microglial NO production with the iNOS-specific inhibitors abolished microglial neurotoxicity in this coculture system. Conversely, high concentrations of NO, resulting in the accumulation of >60 µm nitrite over 3 days, were necessary and sufficient for neurotoxicity (Golde et al., 2002). We confirm here that high threshold levels of NO (≈ 80 µm nitrite/3 days) are required to kill most of the neurons; levels resulting in the accumulation of ≤ 40 µm nitrite do not appear to be toxic. Although dexamethasone did not abolish NO production in IFNγ- + LPS-induced microglia, it lowered NO release below the toxic threshold, thus explaining its neuroprotective effect. The glucocorticoid did not alter the neurotoxicity of the NO donor DETA-NoNoate. Therefore, the protective effect in cocultures cannot be explained by scavenging NO, as described for nonsteroidal anti-inflammatory drugs (Asanuma et al., 2001), or by changing neuronal susceptibility to NO.
Most effects of glucocorticoids are mediated by binding to the intracellular receptor and adhering to glucocorticoid response elements flanking target genes (Barnes & Adcock, 1993; Beato et al., 1996), or modulating the activation of transcription factors NFκB or AP-1 (Jonat et al., 1990; Mukaida et al., 1994). Microglia express glucocorticoid receptors (Tanaka et al., 1997). The glucocorticoid receptor antagonist RU-486 blocked both the reduction of NO production and the neuroprotection of dexamethasone, indicating that these effects are fully dependent on signalling through the glucocorticoid receptor.
Interestingly, dexamethasone reduced NO production when added before, with or up to 24 h after microglial activators, although it was most effective when given before activation. In contrast to IFNβ, which may reduce NO production only when administered in a narrow time window not covered in the experiments presented here (Gao et al., 2000), glucocorticoids might be useful for the protection of neurons in a clinical setting where inflammation is usually already ongoing when treatment commences.
Inhibition of NO production following iNOS induction with LPS or cytokines was first demonstrated by Radomski et al. (1990) and has subsequently been shown in a variety of cell types and in vivo (Knowles et al., 1990; Geller et al., 1993; Walker et al., 1997; Drew & Chavis, 2000; Matsumura et al., 2001). However, the mechanisms of this glucocorticoid effect are less clear. Dexamethasone can modulate iNOS activity by limiting cofactors such as terahydrobiopterin or the substrate arginine (Simmons et al., 1996). However, iNOS is a strongly inducible enzyme and expression of this protein is a major factor regulating high level NO output (MacMicking et al., 1997). We show here that iNOS protein concentration is reduced by dexamethasone. The effect is prevented by the glucocorticoid receptor blocker RU-486. In similar rat primary microglial cultures, Minghetti et al. (1999) demonstrated that dexamethasone, as well as a peptide derived from the N-terminus of lipocortin 1, reduced nitrite production and iNOS protein after stimulation with LPS. A lipocortin 1-neutralizing antiserum partially reduced the effect of dexamethasone on iNOS. Although these results should be treated with caution because the investigators report nonspecific effects of the same serum, lipocortins are important mediators for anti-inflammatory effects of glucocorticoids. Lipocortin 1 released by dexamethasone-treated J774 macrophages lowered iNOS mRNA and NO production after LPS stimulation (D'Acquisto et al., 1997). Conversely, in synovial macrophages it mediated the reduction in NO release but not the suppression of iNOS expression by dexamethasone (Yang et al., 1998). Although lipocortin 1 may be one intracellular mediator of glucocorticoid effects, it is not known how it modulates iNOS concentration or NO release.
In principle, reduction in iNOS protein concentration can result either from reduction of transcription, destabilization of mRNA, reduced translation or increased protein degradation. Walker et al. (1997), using the IFNγ-stimulated murine macrophage line RAW 264.7, demonstrated that dexamethasone can modulate iNOS at each of these levels. However, these complex effects appeared to be of little biological significance as the glucocorticoid mediated its effect through the acceleration of iNOS protein degradation (Walker et al., 1997).
We show here that dexamethasone, given at the same time as IFNγ and LPS, reduced both iNOS protein and steady-state mRNA levels in primary microglia by ≈ 66% at 24 h. Similar to the effect on NO production, the glucocorticoid receptor antagonist RU-486 prevented dexamethasone-related suppression of iNOS protein expression. Unexpectedly, RU-486 consistently caused a large increase in iNOS mRNA expression, greater even than that seen in IFNγ- + LPS-stimulated dexamethasone-untreated cells. This indicates that the iNOS gene in activated microglia is already subjected to some negative control by steroids, either through internal steroid-receptor ligands or by cortisol contained within the B27 medium supplement (Brewer & Cotman, 1989). As these high mRNA levels are not associated with increased iNOS protein, post-transcriptional mechanisms must be part of the machinery controlling iNOS levels in microglia.
Suppression of iNOS expression by reduction of iNOS mRNA may be effective during long-term exposure to steroids. It is intriguing to speculate, however, that a quick way to remove iNOS protein during ongoing inflammation may be the acceleration of its degradation, as suggested by the work of Walker et al. (1997). iNOS degradation in macrophages is proteasome-dependent (Musial & Eissa, 2001), although under in vitro conditions it can also be digested by calpain (Walker et al., 2001). Ubiquitination is needed for the degradation of iNOS, underlining the fact that the proteasome is the major degradation machinery for iNOS in cells (Kolodziejski et al., 2002). Using pulse-chase analysis, we showed that long- (24 h treatment before pulse) and short-term (2 h treatment before pulse) exposure to dexamethasone drastically reduced iNOS protein synthesis without affecting total protein synthesis. We confirmed that inhibition of the proteasome with specific inhibitors lactacystin and epoxomycin as well as with the nonspecific peptide ALLN (Musial & Eissa, 2001; Yedidia et al., 2001) stabilizes iNOS. However, stabilization occurred in both dexamethasone-treated and untreated cells and did not reverse any long- or short-term effects of dexamethasone on iNOS protein.
Conversely, inhibition of proteasome activity exposes the glucocorticoid effect. Recent reports indicate that a large percentage (≈ 30–60%) of newly synthesized proteins are rapidly degraded by the proteasome after translation (Schubert et al., 2000). This means that protein synthesized at a high rate will show considerably more stabilization by proteasome inhibitors. Our observation of enhanced iNOS stabilization in dexamethasone-untreated cells indicates that the dexamethasone effect is evidently due to reduced protein synthesis, in contrast to the findings of Walker et al. (1997) using a similar experimental design based on IFNγ-stimulated RAW 264.7 cells.
Importantly, reduced iNOS synthesis after only 2 h of dexamethasone treatment was associated with a 40% reduction in iNOS mRNA. iNOS mRNA half-life was determined to be ≈ 6–12 h (Walker et al., 1997; Korhonen et al., 2002). Therefore, a 40% decrease within 2 h cannot be achieved by inhibition of transcription alone. Destabilization of iNOS mRNA by dexamethasone has recently been shown in LPS-treated macrophages. By analogy with the dexamethasone-mediated effects on COX-2 mRNA (Lasa et al., 2001), destabilization of iNOS mRNA may be associated with activation of p38-MAPKinase and represent a mechanism for rapid down-regulation of iNOS protein and NO production during ongoing inflammation.
Our results clearly show that reduction in protein synthesis, resulting from reduced mRNA levels, is the key step in regulation of iNOS expression by dexamethasone, while accelerated degradation of the protein plays no role in primary rat microglia.
Clinically, glucocorticoids are used to treat a wide variety of autoimmune and chronic inflammatory conditions. Specifically, high doses of methylprednisolone are given to shorten MS relapses on the basis that these reduce oedema and accelerate recovery of function dependent on conduction through myelinated axons. However, the debate is ongoing whether treatment of inflammation also reduces neurodegeneration. In a placebo-controlled trial, regular pulsed therapy with intravenous methylprednisolone for 5 years did not alter relapse rate in patients with MS but did reduce brain atrophy, suggesting that corticosteroids protect from the effects of inflammation (Zivadinov et al., 2001). Glucocorticoids may be neuroprotective when given early during acute inflammation by reducing the period of exposure to NO and other inflammatory mediators of acute axonal injury.
Acknowledments
This work was supported by a Marie-Curie Individual Fellowship of the European Commission (S.G.), the Raymond and Beverley Sackler Foundation (S.G.), the Medical Research Council (S.G. and A. Compston) and the Wellcome Trust (A. Coles and A. Compston).
Abbreviations
-
- ALLN
-
- N-acetyl-L-leucinyl-L-leucinyl-L-norleucinal
-
- ATP
-
- adenosine triphosphate
-
- DETA-NoNoate
-
- (Z)-1-[2-(2-Aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate]
-
- DMEM
-
- Dulbecco's modified Eagle's medium
-
- HBSS
-
- Hank's buffered salts solution
-
- IFN
-
- interferon
-
- Ig
-
- immunoglobulin
-
- iNOS
-
- inducible nitric oxide synthase
-
- LPS
-
- lipopolysaccharide
-
- MS
-
- multiple sclerosis
-
- MTT
-
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
-
- NO
-
- nitric oxide
-
- PARP
-
- poly-ADP ribose polymerase
-
- PSF
-
- penicillin–streptomycin–fungizone
-
- RU-486
-
- mifepristone
-
- TCA
-
- trichloroacetic acid.