The D2 receptor is critical in mediating opiate motivation only in opiate-dependent and withdrawn mice
Abstract
According to the dual systems model for opiate reward, dopamine mediates opiate motivation when an animal is in a deprived motivational state (i.e. opiate-dependent and in withdrawal) and not when an animal is in a nondeprived state (i.e. previously drug-naive). To determine the role of the D2 dopamine receptor subtype in mediating opiate motivation, we examined the behaviour of N5 congenic D2 receptor knockout mice and their wild-type siblings in opiate-naive and opiate-dependent and withdrawn place conditioning paradigms. Opiate-naive D2 receptor knockout mice demonstrated acquisition of morphine-conditioned place preference but failed to acquire place preference when conditioned in the deprived state. We propose that D2 receptor function is critical in mediating the motivational effects of opiates only when the animal is in an opiate-dependent and withdrawn motivational state. These findings also underscore the important influence of the genetic background to a given phenotype, as evidenced by the observation that increasing the allelic contribution from the 129/SvJ strain abolishes morphine place preference in C57BL/6 wild-type mice.
Introduction
Major drugs of abuse are thought to function by increasing the concentrations of extracellular dopamine in the mesolimbic system and it has been suggested that changes in dopamine levels are both necessary and sufficient in mediating the motivational effects of all stimuli (Wise, 1982). However, dopamine-independent mechanisms of motivation exist (Dworkin et al., 1988; Berridge et al., 1989; Bechara et al., 1992; Cunningham et al., 1992; Bechara et al., 1995; Nader & van der Kooy, 1997). Even advocates of the original anhedonia hypothesis have revised the theory, demonstrating that at least some drugs mediate their motivational effects through dopamine-independent mechanisms (Rompre & Wise, 1989; Carlezon, & Wise, 1996). This work has led to the development of a ‘dual systems’ hypothesis to explain the role of dopamine in motivation. The hypothesis purports the existence of two motivational systems in the brain and that the contribution of each system depends upon the deprivation state of the animal. According to this model, dopamine mediates the motivational properties of stimuli only when an individual is in a deprived motivational state such as opiate-dependent and in withdrawal or food-deprived, and not when the animal is in a nondeprived state such as when an animal is drug-naive or food-sated (Nader et al., 1997).
Recent research has taken advantage of the specificity of gene knockout technology to examine the role of specific dopamine receptors in motivation. It has been reported that previously drug-naive dopamine D2 receptor knockout mice [D2R (–/–)] do not exhibit opiate reward as measured in a morphine-conditioned place preference paradigm (Maldonado et al., 1997). These results clearly contradict what would be predicted on the basis of the nondeprived/deprived model of motivation (Nader et al., 1997). We therefore chose to examine the nature of opiate motivation in an independently generated strain of D2R (–/–) mice and their D2R (+/+) (wild-type) siblings.
Historically, viable knockout mutations have been produced primarily using the embryonic stem cells from the 129 mouse strain (Melton, 1994; Simpson et al., 1997). Unfortunately, the 129 strains perform poorly in a plethora of behavioural tasks (Crawley, 1996; Gerlai, 1996) and, as the C57BL/6 performs well on a large variety of behavioural tasks (Crawley, 1996; Crawley et al., 1997) this strain is commonly used as the parental background for the mutation. The genetic contribution of a given 129 strain to the resulting hybrid genome can be diminished by repeated backcrossing to wild-type inbred mice (i.e. C57BL/6). Extensive backcrossing can dilute the contribution of genes linked to the targeted mutation and produces mice with a very small region (10–20 cM) around the targeted locus differing between the three genotypes (wild-type, +/+ heterozygote, +/–; homozygote, –/–). Consequently, a lack of backcrossing increases the chance of expression, and possible interaction, of polymorphic alleles contributed by the 129 mouse strain (Crusio, 1996; Gerlai, 1996; Lathe, 1996). To dilute the contribution of such confounding genes, repeated backcrossing to the parental strain, for a minimum of five generations, has been recommended [Gerlai, 1996; Silva et al., 1997(Banbury Conference)].
One possible explanation for the contradiction between the dual systems hypothesis and these D2R (–/–) results involves the fact that the mice tested were genetic F2 hybrids. In other words, it is possible that the deficit observed in the D2R (–/–) mice was due to the expression, and possible interaction, of polymorphic alleles linked to the targeted mutation, and not the mutation itself.
In the present study we characterized the role of D2 receptors in opiate motivation by examining D2R (+/+) and (–/–) mice in opiate place conditioning procedures. Furthermore, we subsequently investigated the effects of genetic background in the assessment of knockout mice by examining mice, 129/SvJ mice, and F1 offspring of C57BL/6 × 129/SvJ matings in opiate place conditioning procedures.
Materials and methods
Subjects
All animals used in these experiments were young adult (25–35 g) male and female mice. Five times backcrossed D2R mice breeders were obtained by heterozygotic breeding (Kelly et al., 1997). All offspring were propagated at the University of Toronto. A total of 65 D2R(+/+) and 60 D2R(–/–) mice were used. C57BL/6 controls (n = 26) and 129/SvJ (n = 16) controls came from Charles River (Montreal, Canada) and The Jackson Laboratory (Bar Harbor, USA), respectively. Offspring from the C57BL/6 × 129/SvJ cross (n = 29) were propagated at the University of Toronto. Subjects were housed by gender, in groups of four, in plastic mouse cages in a sound-attenuated room at a temperature of 22 °C with lights on from 0700–1900 h. Access to food and water was ad libitum throughout the experiments. All procedures were carried out in accordance with the ethical standards set by both the University of Toronto Animal Care Committee and the Faculty Advisory Committee on Animal Services, Toronto, Canada.
Surgery
Mice trained in opiate-dependence and withdrawal procedures were anaesthetized under light Halothane anaesthesia (Halocarbon Laboratories, NJ, USA). Mice were then made opiate-dependent by the implantation of subcutaneous (s.c.) morphine microosmotic pumps (Alzet Model 1007D, Alza Corp, CA, USA) that administered a constant rate of morphine over the duration of the conditioning period (approximately 5 mg/kg/hour). Surgeries were performed 24 h before the initial conditioning trial. At the end of the conditioning trials, the mice were re-anaesthetized and pumps were removed. The mice were allowed to recover for one week before the test period.
Place conditioning apparatus
The place conditioning apparatus consisted of two environments that differed in colour and texture, each measuring 15 × 15 × 15 cm. One environment consisted of a black box with a smooth, black Plexiglas floor and the other environment was a white box with a white, wire mesh floor. The two boxes were separated by a removable metal wall, each side painted with the corresponding colour. The ceiling of the boxes was made of removable, clear Plexiglas. Time and activity levels were recorded with a computerized place conditioning program (Phoenix ROM BIOS Ver. 2.27, Phoenix Technologies Ltd., Phoenix, USA) using three opposing pairs of photobeams set 4 cm apart in each box.
Conditioned place preference procedure
The animals were subjected to unbiased place conditioning procedures using a standard place conditioning procedure (Mucha & Walker, 1987). The procedure was unbiased as C57BL/6 and 129/SvJ control mice expressed no baseline biases in preferences for the two conditioning environments (15 minute trial: black environment mean ± SEM = 439.075 ± 15.03; white environment mean ± SEM = 460.7 ± 14.97; t1,7 = 0.983, P < 0.05) and no preferences with the injection procedure itself under saline conditions (15 minute trial: saline-paired environment mean ± SEM = 477.65 ± 37.41; alternate environment mean ± SEM = 424.73 ± 37.69; t1, 7 = 0.462, P < 0.05). Mice were trained either previously drug-naive or opiate-dependent. Immediately prior to the conditioning trial, animals were given an intraperitoneal (i.p.) injection of either 1 or 10 mg/kg morphine and exposed to one of the two conditioning environments for a 15 minute period. On the alternate day, animals were given an injection of saline and exposed to the alternate environment for 15 min. We have observed that a 15 minute conditioning trial produces optimum morphine place preference in mice (data not shown). Animals received one conditioning trial per day and the conditioning procedure was repeated until each subject had received four pairings in each environment for a total conditioning period of 8 days. Both treatment compartment and order of drug presentation were counterbalanced within groups.
Animals who were conditioned opiate-dependent and in withdrawal were treated with 0.1 mg/kg naloxone (s.c.) 5 min prior to the subsequent morphine or saline injection. Although this naloxone dose is sufficient to produce robust somatic signs of withdrawal within 5 min of injection, it is not sufficient to block the acute effects of either 1 or 10 mg/kg of morphine in mice (Dockstader & van der Kooy, unpublished observations). Osmotic pumps were removed from opiate-dependent mice following the last conditioning period. After the final conditioning trials, mice were allowed to rest uninterrupted in their home cage for one week until test day. At this time, animals conditioned opiate-dependent were no longer in withdrawal.
On test day, under drug-free conditions, the animals were given equal access to both boxes simultaneously by removing the shared wall and introducing the animal to the centre of the test area. Time and activity in each environment were recorded over a 10 minute period.
Conditioned place aversion procedure
Mice conditioned in the single-sided procedure received 0.1 mg/kg (s.c.) naloxone injections 5 min prior to being placed in one of the two environments for a 40 minute period. This naloxone dose precipitates strong somatic signs of withdrawal in opiate-dependent animals and produces a conditioned place aversion to the naloxone-paired environment (Bechara et al, 1995). Animals who have received saline injections in one environment show no preference between a saline-paired environment and a novel environment on test day (40 minute trial: saline-paired environment mean ± SEM = 1067.3 ± 128.18; novel environment mean ± SEM = 1325.2 ± 130.44; t1,7 = 0.995, P < 0.05). For this paradigm, there were four conditioning trials, one per day for four consecutive days. Animals did not receive exposure to the alternate environment until the test day which followed one week subsequent to the last conditioning period.
Recording somatic signs of withdrawal
D2R (+/+) and (–/–) mice were made opiate-dependent by the implantation of morphine mini-pumps as previously described. Control D2R (+/+) and (–/–) mice were implanted with saline pumps. Animals were individually placed in a 60 × 30 × 30 cm environment with clear, Plexiglas walls and bedding-covered floor. Both naive and opiate-dependent animals habituated to the environment for 30 min prior to 0.1 mg/kg (s.c.) naloxone injections and were subsequently observed for somatic signs for the following 30 min. Incidence of wet dog shakes, jumping, sniffing, and paw tremor were recorded as actual numbers and rate of occurrence of diarrhoea, tremor, ptosis, and teeth chattering were recorded up to a maximum of 6 occurrences (exactly as measured in Maldonado et al., 1997).
Drugs
The experimental drugs used in the procedures were morphine sulphate (University of Toronto Drug Dispensary, Ontario, Canada) and naloxone hydrochloride (Sigma, Ontario, Canada), both dissolved in a 0.9% saline solution at a concentration of 1 mL/kg. Both experimental groups and saline controls were injected at a volume of 1 mL/kg.
Results
Generation of D2R (–/–) mice
The D2R (–/–) mice used in our experiments were N5 congenic C57BL/6 mice genotyped using Southern blot analyses and generated by a backcrossing strategy outlined in Kelly et al. (1997). Even though low levels of truncated D2 receptor mRNA are expressed, these mice appear to be functional nulls as determined by a variety of pharmacological, endocrinological, and behavioural measures (Kelly et al., 1997; Kelly et al., 1998). Even if some truncated receptor protein was present, a truncated dopamine D2 receptor appears to have no function and actually inhibits ligand binding to the D2R (+/+) receptor (Lee, 2000). Collectively these data suggest that it is extremely unlikely that the D2 knockout mice have endogenous D2 receptor activity of any kind. The D2R (–/–) mice manifested only minor observable abnormalities compared to the D2R (+/+) mice. The two genotypes displayed no noticeable differences in postnatal development but adult male D2R (–/–) mice were on average 15% smaller than age-matched D2R (+/+) mice. There were no deficits in the acquisition or expression of basic motor skills and mice displayed no abnormal posture or tremor. Detailed locomotor activity testing revealed relatively small decreases in rearing activity (Kelly et al., 1998).
Opiate place conditioning in opiate-naive and opiate-dependent and withdrawn D2 receptor (+/+) and (–/–) mice
The behaviour of D2R (+/+) and D2R(–/–) mice was examined using the place preference paradigm to determine the role of the dopamine D2 receptor in mediating opiate motivation. D2R (+/+) and D2R (–/–) mice were conditioned on a morphine-conditioned place preference paradigm in the opiate-nondeprived state. Previously drug-naive, 4-month-old, D2R (–/–) male and female mice and their (+/+) siblings were exposed to a morphine-paired environment and a saline-paired environment over a series of conditioning trials. The D2R (+/+) mice showed a significant preference for the morphine-paired environment at both the low (1 mg/kg) (t1,11 = 2.53, P < 0.05) and high (10 mg/kg) (t1,7 = 6.88, P < 0.05) doses of morphine (Fig. 1). Similarly, the D2R (–/–) siblings also exhibited a significant preference for the morphine-paired environment at both the low (t1,11 = 3.07, P < 0.05) and high (t1,10 = 4.29, P < 0.05) doses. A three-way anova indicated a significant main effect for the morphine-paired vs the saline-paired environments (F1,39 = 4.73, P < 0.05) but no effect of genotype, dose, nor any interactions. The male to female ratio was approximately the same for each group and no gender effects were observed under any conditions.
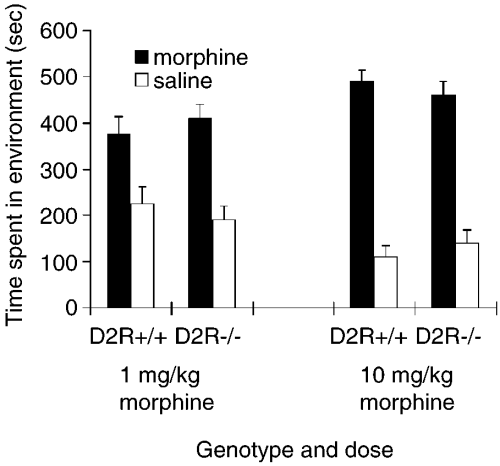
Conditioned place preferences induced by morphine at 1 and 10 mg/kg (i.p.) in previously drug-naive D2R (–/–) mice and D2R (+/+) littermates. Data represent means + SEM of absolute times spent in the previously morphine- and saline-paired compartments during testing (from left to right: n = 12, n = 8, n = 12 and n = 11).
During the deprived assays, mice were opiate-dependent and withdrawal was precipitated with naloxone, an opiate antagonist, just prior to each conditioning trial. Drug-naive control animals were treated with saline or naloxone (0.1 mg/kg). During conditioning trials, the opiate-dependent and withdrawn mice demonstrated strong somatic signs of withdrawal. Analyses of variance indicated that both the D2R genotypes demonstrated significantly more somatic signs of withdrawal when opiate-dependent and treated with naloxone compared to opiate-naive naloxone-treated groups (F1,80 = 45.86, P < 0.05) and that signs were seen equivalently in D2R (+/+) and D2R (–/–) mice (F1,80 = 0.0009, P > 0.05) (Fig. 2). There was no interaction between genotype and condition or genotype and test. Although the data suggested a trend toward increased jumping behaviour in opiate-dependent and withdrawn D2R (–/–) mice compared to D2R (+/+) mice, this trend did not reach statistical significance.
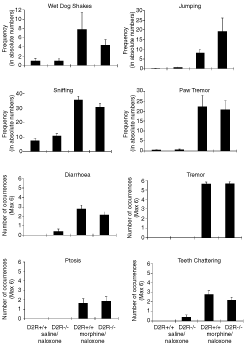
Somatic signs of withdrawal in D2R (–/–) mice and D2R (+/+) littermates. Control groups received saline mini-pumps and either an injection of saline or naloxone (0.1 mg/kg s.c.) prior to observation period. Wet dog shakes, jumping, sniffing, and paw tremor were recorded in actual numbers. The presence of diarrhoea, tremor, ptosis, and teeth chattering during six consecutive, five minute periods were recorded. Data represent means + SEM (n = 8 for all groups).
In a separate experiment, mice of both genotypes were conditioned in an opiate-dependent and withdrawn state in both naloxone conditioned place aversion and morphine conditioned place preference paradigms. Mice were conditioned on a single-sided conditioned place aversion assay where one environment was paired with naloxone and the animals were not exposed to the other environment until test day. D2R (+/+) mice subject to naloxone conditioned place aversion in the opiate-dependent and withdrawn state demonstrated a strong aversion to the naloxone-paired environment (t1,9 = 3.67, P < 0.05, whereas D2R (–/–) mice showed no preference for either environment (t1,8 = 0.241, P > 0.05 (Fig. 3a). Opiate-dependent and withdrawn D2R (+/+) mice conditioned on a morphine-conditioned place preference procedure, exhibited a significant preference for the morphine-paired environment at both the low (1 mg/kg) (t1,7 = 4.302, P < 0.05 and high (10 mg/kg) (t1,6 = 3.032, P < 0.05) morphine doses. However, D2R (–/–) mice did not display a preference for either environment at either the low dose (t1,6 = 0.473, P > 0.05] or the high dose (10 mg/kg) of morphine (t1,8 = 0.121, P > 0.05; Fig. 3b). The male to female ratio was approximately the same for each group and no gender effects were observed under any conditions.
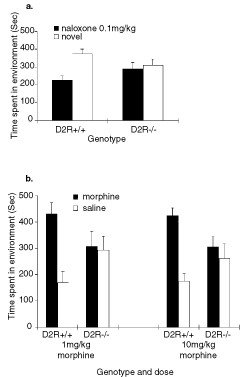
(a) Conditioned place aversions induced in opiate-dependent D2R (–/–) mice (n = 9) and D2R (+/+) siblings (n = 10) by naloxone (0.1 mg/kg s.c.). Data represent means + SEM of absolute times spent in a previously withdrawal-paired environment and a novel environment during testing. (b) Conditioned place preferences induced by morphine at 1 and 10 mg/kg (i.p.) in D2R-mutant mice and wild-type littermates after conditioning in a state of opiate-dependence and withdrawal. Data represent means + SEM of absolute times spent in the previously drug- and saline-paired compartments during testing (from left to right: n = 8, n = 7, n = 7 and n = 9).
Opiate place conditioning in opiate-naive C57BL/6, 129/SvJ and C57BL/6 × 129/SvJ mice
We asked whether isogenic mice from 129 and C57 mouse strains were able to express morphine place conditioning. 129/SvJ and C57BL/6 mouse strains (strain of targeted D2 receptor mutation and parental strain, respectively) were conditioned on the nondeprived version of the morphine-conditioned place preference assay. C57BL/6 mice displayed morphine place preference for the morphine-paired environment (t1,7 = 6.88, P < 0.001), however, 129/SvJ mice showed no preference for either environment (t1,7 = 0.762, P > 0.05; Fig. 4a). Additionally, we conditioned the F1 generation from these two strains of mice (C57BL/6 × 129/SvJ) in an identical morphine-conditioned place preference assay. On test day, as a group, the offspring did not exhibit morphine place preference (t1,28 = 2.035, P > 0.05). However, when this group was divided into two subgroups, those who exhibited morphine place preference and those who did not (Fig. 4b, see figure legend), then half of the offspring exhibited preference for the morphine-paired environment and half of the offspring showed no acquisition of the task. These results represent an intermediate effect between C57BL/6 mice that almost all showed place preference and 129/SvJ mice that almost all failed to show preference when divided into subgroups using the same criteria as used for the F1 mice (Fig. 4).
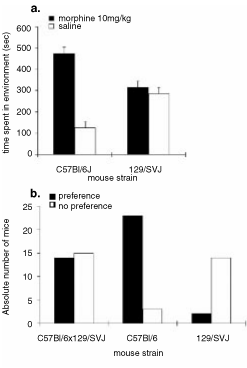
(a) Conditioned place preferences induced by morphine (10 mg/kg i.p.) in previously drug-naive C57BL/6 (n = 8) and 129/SvJ (n = 8) mouse strains. Data represent means + SEM of absolute times spent in the previously drug- and saline-paired compartments during testing. (b) Conditioned place preferences induced by morphine (10 mg/kg i.p.) in previously drug-naive C57BL/6 (n = 26), 129/SvJ (n = 16) and F1 generation C57BL/6 × 129/SvJ (n = 29) mouse strains. Data represent absolute numbers of mice that show significant preferences for the morphine-paired compartment vs the absolute numbers of mice that show no preferences. Preference was determined as a subject spending more than the sum of 300 s (half the test time) plus the time indicated by the standard error, in the morphine-paired environment during testing.
Discussion
The objective of our investigation was to resolve the role of the dopamine D2 receptor in various stages of opiate motivation. Additionally, we endeavoured to determine the phenotype of the 129 mouse strain in opiate place conditioning and compare their behaviour with that of animals with varying contribution of 129 genes. The dual systems model purports that dopamine mediates the motivational effects of stimuli when an animal is in a deprived state but not in a nondeprived state (Nader et al, 1997). Our data clearly demonstrate that the activity of the dopamine D2 receptor is critical in mediating opiate motivation when an animal is opiate-dependent and in withdrawal, but not when an animal is previously opiate-naive. Additionally, we demonstrate that by varying the genetic contribution of C57 and 129 mouse strains one can dramatically alter the phenotype in these paradigms, as the 129 strain shows a great deficit in opiate place conditioning. This is consistent with previous conditioning data examining these strains (Miner, 1997). The behaviour of the C57BL/6 × 129/SvJ generation suggests that this deficit is the result of a single, dominant allele harbored in the 129/SvJ strain whose gene product somehow inhibits morphine place preference.
Opiate motivation requires the integrity of the D2 receptor in opiate-dependent and withdrawn mice
The dopamine D2 receptor appears to be involved in the rewarding effects of many stimuli including electrical brain stimulation (Knapp & Kornetsky, 1994; Nakajima & Patterson, 1997), food motivation (Chausmer & Ettenberg, 1997) and water motivation (Ågmo et al., 1993). The D2 receptor plays a role in opiate motivation, when an animal is opiate-deprived, given that pimozide, a relatively selective D2 receptor antagonist, blocks conditioned place preference for high doses of heroin in the opiate-dependent and withdrawn animal (Nader et al., 1994). Pharmacological data demonstrate that repeated opiate administration is associated with selective modification of dopamine D2 receptor signalling activity(Elwan & Soliman, 1995; Nestby et al., 1995; Turchan et al., 1997; Georges et al., 1999). In the present study, we analysed the behaviour of previously drug-naive and opiate-dependent and withdrawn D2R (–/–) mice and their D2R (+/+) siblings on opiate place conditioning paradigms to examine the role of the dopamine D2 receptor in opiate motivation. Both the D2R (+/+) and mutant D2R (–/–) mice demonstrate a preference for a morphine-paired environment when conditioned in a nondeprived state. Indeed, pretreatment with dopamine antagonists, prior to conditioning in a nondeprived opiate conditioned place preference procedure, does not interfere with the acquisition of opiate place preference (Nader et al., 1994; Nader & van der Kooy, 1997). Interestingly, the nondeprived data directly challenge the conclusions of Maldonado et al. (1997) that previously drug-naive dopamine D2R (–/–) mice do not exhibit opiate reward as measured in a morphine-conditioned place preference paradigm. We suggest that the deficit observed in their D2R (–/–) mice may be an artefact of their breeding strategy such that a 129-derived gene(s) that is linked to the D2 receptor gene may be expressed in the their mutant mice. This general suggestion is elaborated further below. When D2R (+/+) mice were conditioned while opiate-dependent and in withdrawal, they demonstrated a conditioned place aversion to a naloxone-paired environment as well as a conditioned place preference to a morphine-paired environment. To the contrary, mice homozygous for the null mutation of the dopamine D2 receptor showed no acquisition of either motivational task. The ability of D2R (–/–) mice to learn morphine place preference in the nondeprived state, but not in the deprived state, demonstrates that the D2 receptor deficit in the homozygous mutant mice is not due to a learning or sensorimotor impairment, but rather a motivational deficit.
It is possible that, in the opiate-dependent and withdrawn state, the animal prefers the morphine-paired compartment due to the rewarding effects of the alleviation of withdrawal, rather than the euphoric effects of the drug itself (Bechara et al., 1992). Nonetheless, we maintain that the motivational effects of opiates are mediated by the dopamine D2 receptor in the deprived state. Alternate explanations for the differences between our present results and those of Maldonado et al. (1997) include the possibility of subtle laboratory differences in protocols, or subtle differences in the generation and rearing of the mouse strains used. These seem to be unlikely explanations as place conditioning paradigms are robust paradigms that demonstrate motivated behaviour over a plethora of methodological variations (Mucha & Walker, 1987; Bechara et al., 1992; Miner, 1997; Belzung & Barreau, 2000). Moreover, the C57 and 129 strains have exhibited consistency in performance when derived from a number of different sources (Balogh et al., 1999; Crawley et al., 1997; Miner, 1997). It is feasible that the deprived motivational state that the animals experience functions as a stressor which interferes with acquisition of the task. The 129 mouse strains are generally considered as ‘anxious’ strains of mice. It may be that the knockout mice (with their contribution of 129 alleles) are more vulnerable to the stress of opiate withdrawal and it is their anxiety that masks the motivational effects of the drug, rather than the mutation itself. There are two factors that argue against this interpretation. First, as the D2R (+/+) and D2R (–/–) mice in the paradigms are backcrossed siblings, they would both be vulnerable to any stressful effects of the deprived paradigm and therefore both would demonstrate a decrease in preference. Second, and more important, the preference in the deprived D2R (+/+) mice is actually greater than that in the nondeprived D2R (+/+) mice. If stress interfered with conditioning in the deprived paradigm we would expect, at the very least, a decrease in preference of the deprived D2R (+/+) mice relative to the nondeprived D2R (+/+) mice.
In summary, D2 receptor function is not critical in mediating the rewarding effects of opiates in nondeprived animals. Moreover, even if the D2 receptor is critical in mediating nondeprived opiate reward on some mouse genetic backgrounds, certainly it is not critical on all mouse strains and, therefore, the importance of the D2 receptor in such circumstances cannot be generalized. We, therefore, conclude that the D2 receptor is critical in mediating opiate motivation when an animal is in a deprived state, but not when an animal is in a nondeprived state.
Altering the background strain alters the behavioural phenotype
It was clear that the 129 strain contributed distinct phenotypic effects that differed from the C57 strain once we examined the behaviour of both strains in the nondeprived morphine-conditioned place preference paradigm. Whereas, the C57BL/6 strain acquired a preference for the morphine-paired environment, the 129/SvJ strain showed no such preference. To determine the nature of this deficit we bred wild-type C57BL/6 with wild-type 129/SvJ mice and conducted the same assay using the F1 offspring. Overall, the F1 generation did not demonstrate morphine place preference. Upon examining the individual place preference scores of the mice it was discovered that half of the offspring exhibited morphine place preference and half did not. This outcome is what would be expected if the deficit is the result of the expression of a single, dominant 129 allele. We suggest that deficits in opiate place conditioning observed in some mice with a contribution from the 129 genetic background may be due to the segregation of a dominant allele contributed by the 129 strain. If so, the inconsistent D2 data existing in the literature may indicate that this detrimental 129 gene does not segregate randomly amongst the genotypes. It is plausible that the detrimental 129 allele may be linked to the D2 receptor locus. In the data of Maldonado et al. (1997), the D2R (+/+) mice, having both D2 receptor loci represented by the C57 genome, show the predicted morphine-conditioned place preference. In their D2R (–/–) mice, morphine-conditioned place preference may be blocked due to the detrimental 129 allele linked to the D2 receptor gene. In all of the present backcrossed D2R (–/–) mice the 129 allele linked to the null D2 receptor locus has been backcrossed away, allowing the naive morphine place preference to be demonstrated.
Our findings, that a dominant allele contributed by the 129 background can apparently modify the rewarding effects of morphine in animals that are genetically heterogeneous, underscores the important influence that genetic background can have on a given phenotype.
Abbreviations
-
- D2R
-
- dopamine D2 receptor
-
- i.p.
-
- intraperitoneal
-
- s.c.
-
- subcutaneous
-
- (+/+)
-
- wild-type
-
- (+/–)
-
- heterozygote
-
- (–/–)
-
- homozygote/knockout.




