Selective predation on the broad-toothed rat, Mastacomys fuscus (Rodentia: Muridae), by the introduced red fox, Vulpes vulpes (Carnivora: Canidae), in the Snowy Mountains, Australia
Abstract
Abstract Since 1981 there has been debate over whether foxes (Vulpes vulpes Linnaeus) selectively prey on the broad-toothed rat (Mastacomys fuscus Thomas) relative to the bush rat (Rattus fuscipes (Waterhouse)). In the present study, three areas of the argument are examined. (i) In a study of fox diet over 3 years at both alpine and subalpine altitudes, M. fuscus outnumbered R. fuscipes in faecal remains in all seasons, in all years, and at both altitudes. Overall, M. fuscus occurred in scats five times as frequently as did R. fuscipes in the alpine zone and twice as often in the subalpine zone. (ii) Data from population studies of M. fuscus and R. fuscipes showed no evidence that M. fuscus is trap shy; neither the pattern of captures of individuals caught once, twice and so on, nor the proportion of the estimated population of each species captured during trapping sessions was significantly different. (iii) The suitable habitat for M. fuscus within the potential home ranges of foxes contributing to the subalpine fox scat collection constituted approximately 50% of the total area. However, there was no significant difference between the numbers of fox trails encountered in habitat suitable or unsuitable for M. fuscus in 19 paired transects skied in winter, indicating no preferential foraging in either habitat. Selective feeding on M. fuscus was therefore established, but how that choice is exercised was not determined.
Introduction
Selective predation has been documented for a variety of organisms based on the size of prey (Blomberg & Shine 2000; Kloskowski 2000; Ruggerone et al. 2000), differences in sex and reproductive condition in a single prey species (Ruggerone et al. 2000), and the behaviour of different prey species (Feldman & Savitz 1999). Selective predation has been documented for a number of mammals, including foxes. For example, red foxes (Vulpes vulpes Linnaeus) show a clear preference for voles over murine rodents (Macdonald 1977; Jensen & Seqira 1978), and arctic foxes (Alopex lagopus) either prefer lemmings (Lemmus lemmus) over other small mammals, or forage disproportionately in good lemming habitat (Elmhagen et al. 2000). However, since the publication of a paper examining the diet of introduced red foxes and the concurrent local food availability in Australia (Green & Osborne 1981), there has been intermittent criticism of one of the key suggestions of that paper: that foxes exhibited disproportionately higher predation on the less common broad-toothed rat (Mastacomys fuscus) than on the more common bush rat (Rattus fuscipes).
Support for the findings of Green and Osborne (1981) has come from more recent research within the distribution of M. fuscus in New South Wales (Broome 1992; Bubela 1995; Bubela et al. 1998), whereas criticism has come mainly from studies conducted in Victoria (Wallis et al. 1982; Brunner & Wallis 1986; Menkhorst 1995) but see also Wallis (1992). These criticisms were based on the proposition that M. fuscus is more common than trapping suggests. If foxes do not selectively prey on M fuscus, then one of two conditions must be met for remains of M. fuscus to outnumber those of R. fuscipes in fox scats. Either M. fuscus is trap shy (Wallis et al. 1982) and in fact outnumbers R. fuscipes in the wild, or the two prey species differ in habitat use, and foxes prey non-randomly in the habitat preferred by M. fuscus.Menkhorst (1995) discounted the suggestion by Green and Osborne (1981) of selective predation on M. fuscus on two bases, one of which will be dealt with here. Menkhorst assumed that because 21 of 52 new localities recorded for M. fuscus in Victoria between 1972 and 1981 by Wallis et al. (1982) were from predator scats, then this indicated that M. fuscus was more common than trapping suggested. However, the same results could be used to argue a case for selective predation on a prey species in extremely low abundance.
The question of selective predation on M. fuscus is important because in Victoria the species is classified as ‘rare’ (CNR 1993) and in New South Wales as ‘vulnerable’. The population at Barrington Tops (NSW) is now classified as ‘endangered’ and is under severe threat from foxes, which may well have assisted in the reduction in numbers of M. fuscus and its replacement by Rattus lutreolus (Gray) (Green 2000). However, Seebeck (1995) regarded R. fuscipes as ‘widespread and common in suitable habitat’ and stated that it ‘may, in fact, be the most abundant native small mammal in south-eastern Australia.’ The present study addresses three areas of the argument. Is greater predation on M. fuscus than R. fuscipes more general than in the study by Green and Osborne (1981)? Is M. fuscus trap shy? Within the foraging area of foxes, is the occurrence of M. fuscus habitat significantly higher than that of R. fuscipes or do foxes concentrate their foraging within this habitat?
Methods
Scats
The study area was located in the Snowy Mountains of New South Wales, Australia (Fig. 1). One subalpine transect for collection of fox scats ran mainly through woodland along a four-wheel-drive track from approximately the 1500-m contour on Disappointment Spur, then followed a Snowy Mountains Hydro-electric Authority aqueduct at 1600–1650 m to the junction with the Guthega Power Station–Geehi Dam gravel road (Fig. 1). This constituted a part of the study transect used by Green and Osborne (1981). A second, alpine, transect was on the gravel road between Charlottes Pass and the summit of Mount Kosciuszko (2228 m), with the scat collection area running from the summit, through alpine vegetation, to the treeline (1840 m) approximately 1 km from Charlottes Pass. Scats were collected at the end of each month. Foxes still used the subalpine transect when it was covered by winter snow because it provided an easy route through the trees, so winter scat collection was possible. However, with snow cover in the alpine zone, there was little benefit to foxes in travelling on the sites of summer tracks; the dispersed nature of the foxes' movements, combined with greater frequency of drifting snow than on the subalpine transect, made regular scat collection difficult either on or off the alpine transect. Consequently, few winter scats were collected in the alpine zone. Data for the occurrence of R. fuscipes and M. fuscus in scats were derived from these transects (K. Green, unpubl. data) and published sources (see Table 1) and were compared using a paired Student's t-test. The seasonal occurrences of R. fuscipes and M. fuscus in scats were arcsine transformed and compared for both the alpine and subalpine transects using a paired Student's t-test.
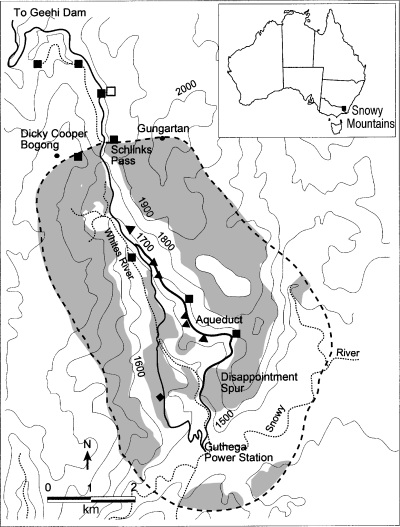
Subalpine fox transect and surrounding study area. Trapping sites are from: (▾) Calaby and Wimbush (1964); (▪) Green and Osborne (1979); (□) Green and Osborne (1981); () Carron (1985); (▴) Sanecki (1999). The dashed line is situated one fox home range length (based on Bubela 1995) from the scat collection transect (solid line on Disappointment Spur and Aqueduct). Broad vegetation classifications within this line are heathland and grassland (shaded) and woodland (unshaded).
| Study | Location | No. scats | M. fuscus | R fuscipes | Ratio |
|---|---|---|---|---|---|
| Green and Osborne (1981) | Snowy Mountains | 1159 | 108 | 88 | 1.22:1 |
| Bubela (1995) | Snowy Mountains | 272 | 12 | 6 | 2.00:1 |
| Broome (1992) | Snowy Mountains | 197 | 55 | 33 | 1.67:1 |
| K. Green, unpubl. data (alpine) | Snowy Mountains | 613 | 195 | 41 | 4.76:1 |
| K. Green, unpubl. data (subalpine) | Snowy Mountains | 1859 | 395 | 180 | 2.19:1 |
| J. Seebeck, pers. comm. | Bellell Creek (V) | 59 | 6 | 7 | 0.86:1 |
| Brunner and Wallis (1986) | Near Powelltown (V) | 576 | 18 | 19 | 0.95:1 |
| Brunner et al. (1977) | Sherbrooke Forest (V) | 1265 | 51 | 164 | 0.31:1 |
| Bertuch (1975) | Sherbrooke Forest (V) | 263 | 8 | 26 | 0.31:1 |
| Wallis et al. (1982) | Sherbrooke Forest (V) | 94 | 4 | 20 | 0.20:1 |
| Brunner et al. (1977) | 600–700 m a.s.l. near Powelltown (V) | 359 | 75 | 65 | 1.15:1 |
Trapping
Data for the occurrence of R. fuscipes and M. fuscus in traps within the study area and within Victoria were derived from published and unpublished sources (see Table 2 for details), and were compared using the paired Student's t-test. For statistical analysis, the data from Happold (1978) were not used because figures were for percentage of total animals caught, which could not be converted to numbers of animals. For studies where a range is given in Table 2, comparison was between the highest occurrence of each species. For subalpine altitudes in New South Wales, only those trapping sites within one average home-range length (MCP) for a red fox (Bubela 1995) of the transects used by Green and Osborne (1981) and K. Green (unpubl. data) for scat collection were considered. See subsequent discussion for further details.
Trappability
An 80-trap grid over 2 ha at Smiggin Holes (6 km south of the subalpine transect used in the present study) was trapped over the period February 1978 to May 1980 (Carron 1985) and between November 1995 and March 1996 (Hill 1996). This trapping grid has been used during a number of studies of M. fuscus (Carron 1985; Happold 1989; Bubela et al. 1991; Bubela & Happold 1993; Bubela 1995; Hill 1996; K. Green, unpubl. data; D. Happold, unpubl. data). Two approaches were taken in order to determine whether R. fuscipes and M. fuscus were equally trappable. The distribution of the number of individual R. fuscipes and M. fuscus caught once, twice and so on was compared for two studies (Carron 1985; Hill 1996). Using mark–recapture data from Carron (1985), estimates were made of the population of R. fuscipes and M. fuscus (Jolly 1965; Seber 1965). The proportion of the estimated population of R. fuscipes and M. fuscus captured by Carron (1985) during trapping sessions was arcsine transformed and compared using Student's t-test, with 14 paired estimates.
Habitat
The home ranges (minimum convex polygon; MCP) of eight foxes, radiotracked by Bubela (1995) from October 1990 to May 1991, between the subalpine and alpine transects of the present study, were examined to determine the area around the subalpine scat transect from which prey might be expected to be taken by foxes using the transect. These eight foxes were four adult females, two adult males and two juvenile males. The average maximal length of the home range (MCP) of foxes radiotracked by Bubela (1995) was 2.25 km. A line was therefore drawn at this distance on both sides of the subalpine transect and out from the upper and lower ends (Fig. 1). This allowed for the largest calculable catchment because the situation of a territory straddling the transect was not considered. The area encompassed 39.5 km2. Based on Bubela (1995), the total area would have provided home ranges for approximately 70 foxes in winter and spring and twice that figure in summer and autumn with the addition of cubs. Aerial photographs were used to delineate woodland and non-wooded habitats. To ground-truth the resulting map, 93 sites located by stratified random sampling were examined for the type of vegetation supported. Additionally, at all sites visited, searches were made for scats of M. fuscus (see Wallis 1992) to determine whether the habitat was, or had been, occupied by M. fuscus. The search began at the most likely site within a 100-m radius of the chosen location and searches were terminated after 10 min of searching if no scats were found. Scats of M. fuscus are easily distinguished from those of sympatric species and, with an individual producing 200–400 scats per day and scats lasting for up to 5 years, searches for scats are a quick method of determining use of the habitat by M. fuscus (Happold 1989). In a study of the broader distribution of M. fuscus, K. Green (unpubl. data) found that of 223 sites searched, scats were found within 5 min in 185 of 189 sites where the presence of M. fuscus was confirmed.
Distribution of foraging effort by foxes
The distribution of foraging effort by foxes between woodland and treeless areas was investigated during winter 2000. Nineteen pairs of transects covering most of the subalpine study area were skied, with one transect of each pair in woodland and the other in adjacent treeless areas. All fox trails that crossed the transect and then disappeared from sight were counted. Trails that criss-crossed the transect were only counted once. The numbers of fox trails encountered for the pairs of transects were compared using Student's paired t-test. Fox trails encountered on the interface between woodland and treeless areas were not counted.
Results
Scats
The proportion of M. fuscus and R. fuscipes in 6500 predator scats collected over nine studies ranged from 0.2:1 to 4.8:1, with the higher ratios in the Snowy Mountains and at higher altitudes (Table 1). The difference overall was not significant (P = 0.3628, t = 0.9534, d.f. = 10). In 2453 fox scats collected for 3 years on alpine and subalpine transects in the Snowy Mountains, M. fuscus occurred more commonly than R. fuscipes in all seasons (Table 3). The difference was significant for alpine (P < 0.001, t = 6.6732, d.f. = 8) and subalpine samples (P < 0.001, t = 4.9853, d.f. = 11).
| Year | Season | Alpine | Subalpine | ||
|---|---|---|---|---|---|
| M. fuscus | R. fuscipes | M. fuscus | R. fuscipes | ||
| 1996 | Summer | 18.8 | 2.4 | 14.3 | 8.3 |
| Autumn | 13.3 | 0.7 | 20.3 | 9.8 | |
| Winter | 53.3 | 16.7 | |||
| Spring | 56.3 | 12.5 | 14.3 | 3.8 | |
| 1997 | Summer | 34.7 | 1.4 | 18.2 | 3.7 |
| Autumn | 20.6 | 2.9 | 14.1 | 9.0 | |
| Winter | 52.8 | 25.9 | |||
| Spring | 52.6 | 26.3 | 21.5 | 12.4 | |
| 1998 | Summer | 52.5 | 10.2 | 16.8 | 6.9 |
| Autumn | 38.0 | 13.0 | 23.9 | 10.3 | |
| Winter | 46.2 | 15.4 | |||
| Spring | 57.4 | 12.8 | 25.6 | 13.7 | |
Trapping
In all studies but one, R. fuscipes outnumbered M. fuscus in traps (Table 2). The difference overall was significant (P < 0.05, t = 2.9025, d.f. = 12). The site at Wilkinson Valley to the north-west of Mount Kosciuszko is generally above an altitude of 1900 m a.s.l., and both Caughley (1982) and Hill (1996) trapped more M. fuscus than R. fuscipes at this site, although numbers in the study by Hill (1996) were minimal.
| Location and author | No. trap nights | M. fuscus | R. fuscipes |
|---|---|---|---|
| Subalpine | |||
| Whites River, Schlinks Pass1 | 680 | 7% | 91% |
| Whites River1 | 220 | 5% | 64% |
| Aqueduct, three sites2 | 148 | 0 | 20 |
| Schlinks Hut, 14 sites2 | 1312 | 17 | 264 |
| Schlinks Hut, heath3 | 520 | 0–11.1 | 0–30.9 |
| Schlinks Hut, woodland3 | 520 | 0 | 0–25.0 |
| Horse Camp4 | 960 | 2–5 | 30–51 |
| Aqueduct, 5 sites (February) 5 | 1400 | 1 | 108 |
| Aqueduct, 5 sites (April) 5 | 800 | 0 | 69 |
| Charlotte Pass6 | 300 | 6 | 50 |
| Alpine | |||
| Etheridge Range6 | 210 | 2 | 8 |
| Mount Kosciuszko6 | 150 | 0 | 3 |
| Wilkinson Valley7 | 3000 | 73 | 47 |
| Victoria | |||
| Bunyip Park8 | 2180 | 59 | 186 |
| 600–700 m a.s.l., near Powelltown9 | 3409 | 3 | 56 |
- Data for study 1 (Happold 1978) are percentage abundance of each species, data for study 2 (Green & Osborne 1979) are number per 100 trap nights and for the remainder, data are number of individuals. Where a trapping grid was trapped on a number of occasions, the range of successful trapping is given. 1Happold (1978), 2Green and Osborne (1979), 3Green and Osborne (1981), 4Carron (1985), 5Sanecki (1999) 6L. Broome, K. Green & M. Walter (unpubl. data), 7Caughley (1982), 8Macreadie et al. (1998), 9Brunner and Wallis (1986).
Trappability
The distribution of captures for individual R. fuscipes and M. fuscus(Table 4) was not significantly different in 1978–1980 (G2 = 11.383, P = 0.25, d.f. = 9) nor 1995–1996 (G2 = 4.332, P = 0.6318, d.f. = 6). The proportion of the estimated population of R. fuscipes and M. fuscus captured during trapping sessions (Table 5) was not significantly different (P = 0.102, t = 1.693, d.f. = 26).
| Times individuals captured | Carron (1985) | Hill (1996) | ||
|---|---|---|---|---|
| M. fuscus | R. fuscipes | M. fuscus | R. fuscipes | |
| 1 | 25 | 23 | 27 | 24 |
| 2 | 13 | 13 | 12 | 20 |
| 3 | 12 | 9 | 8 | 12 |
| 4 | 8 | 10 | 12 | 12 |
| 5 | 4 | 14 | 3 | 8 |
| 6 | 7 | 4 | 6 | 5 |
| 7 | 3 | 1 | 1 | 1 |
| 8 | 2 | 2 | ||
| 9 | 5 | 1 | ||
| 10+ | 8 | 8 | ||
| Total | 87 | 84 | 69 | 82 |
| Statistics | G2 = 11.383, P = 0.25, d.f. = 9 | G2 = 4.332, P = 0.63, d.f. = 6 | ||
| Trapping session | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population M. fuscus | 5.4 | 11.9 | 10.6 | 8.2 | 6.7 | 5.4 | 3.8 | 12.4 | 16.3 | 19.7 | 21.6 | 28.0 | 22.6 | 20.0 |
| Percentage captured | 73.7 | 83.7 | 94.2 | 73.6 | 59.5 | 73.7 | 52.6 | 80.4 | 79.6 | 81.2 | 78.8 | 67.9 | 83.9 | 100.0 |
| Population R. fuscipes | 25.5 | 26.0 | 25.7 | 17.4 | 17.3 | 13.0 | 9.9 | 6.4 | 9.0 | 13.0 | 13.7 | 11.2 | 13.1 | 17.2 |
| Percentage captured | 86.4 | 80.9 | 74.0 | 63.4 | 86.7 | 100.0 | 90.8 | 93.2 | 100.0 | 84.6 | 80.5 | 89.6 | 76.6 | 92.9 |
Habitat
Within the catchment area for the prey of foxes sampled on the subalpine scat transect, the area of 39.5 km2 contained 17.6 km2 of woodland, 21.8 km2 of heath and grass and 0.1 km2 of other habitat. Much of the heath and grass in the subalpine area was suitable habitat for M. fuscus. Although evidence of M. fuscus was found within 100 m of most alpine sites, large areas were unsuitable and, based on vegetation mapping by Costin et al. (1979), alpine habitat suitable for M. fuscus (heath and boulders) probably makes up as little as 27% of the alpine zone.
Distribution of foraging effort by foxes
In all, 19 paired transects totalling 28.3 km were skied in the subalpine study area during winter 2000. The numbers of fox tracks in woodland (169) and adjacent treeless areas (118) when expressed as tracks per kilometre skied were not significantly different (P = 0.759, t = 0.309, d.f. = 36).
Discussion
Considering the widespread distribution and common status of R. fuscipes (Seebeck 1995), the lower level of predation on this species by foxes within the Snowy Mountains relative to predation on M. fuscus requires some explanation. However, to account for the lesser predation on R. fuscipes than M. fuscus simply on the basis of trap shyness, one would have to postulate an average of five times as many M. fuscus as R. fuscipes in the alpine zone and twice as many in the subalpine zone (Table 3). At alpine altitudes, the greatest disparity in captures of M. fuscus and R. fuscipes recorded was 1.6:1 (Caughley 1982). At Smiggin Holes, the subalpine trapping grid contains wet and dry heath, with parts of the grid extending into woodland and tussock grassland (Carron 1985). At this site, Hill (1996) found densities of M. fuscus of 21.8 ha−1 with 12.3 ha−1 for R. fuscipes, a ratio of 1.8:1. Carron (1985) recorded an average of 1.4:1 for the same site. In five woodland grids along the subalpine scat collection transect used in the present study, Sanecki (1999) found densities of 23.1 ± 3.4 ha−1 for R. fuscipes and 0.22 ha−1for M. fuscus, a ratio of 105:1.
Given the low occurrence of M. fuscus along the subalpine transect, the high occurrence in scats was probably due to foxes foraging away from this transect. In the area contained within one home-range length of a fox from the subalpine transect, 17.6 km2 or 44.6% was woodland (Fig. 1). If foxes were to forage randomly throughout this area of woodland and heath then, given the patterns of distribution of the two rodents in woodland and, taking the highest recorded disparity in densities in heath between them of 1.8 :1 from Hill (1996), there would still be a 1.4 times greater chance of encountering R. fuscipes than M. fuscus (for R. fuscipes 0.446 × 23.1 + 0.554 × 12.3, for M. fuscus 0.446 × 0.22 + 0.554 × 21.8). The greater predation by foxes on M. fuscus rather than on R. fuscipes must, therefore, have an explanation other than the accidental choice of a transect where the habitat for the former species predominates.
There are no data to show any significant difference in the behavioural responses of M. fuscus and R. fuscipes to traps in the present study area (Tables 4,5). However, to confirm that the relative trappability of two species reflects their abundance requires a second independent method of recording individuals. Macreadie et al. (1998) suggested that evidence from hair tubes showed that M. fuscus was more difficult to trap than R. fuscipes. However, unless genetic or other techniques are used, hair samples cannot measure the number of individuals. Wallis et al. (1982) suggested that using prebaiting and removal of R. fuscipes would improve the trapping results for M. fuscus. Wallis et al. did, in fact, improve the ratio of animals caught from 0.03:1 to 0.5:1, albeit with a small sample size (303 trap nights). However, at a time of high predation by foxes on the trapping grid used by Hill (1996), when foxes took animals directly from traps, many individually marked M. fuscus and R. fuscipes disappeared from the grid. These were ‘quickly replaced by adults of both species’ (Hill 1996). This suggests that if trapping grids are located in preferred habitat, then there is constant pressure from all species to occupy that habitat, and removal of animals of any species may result in immigration by others. This also occurs with Antechinus swainsonii (Waterhouse) in preferred habitat in the Snowy Mountains during the autumn when the species suffers a high mortality prior to the formation of the winter snowpack (Green 2001).
If the higher numbers of M. fuscus relative to R. fuscipes in fox scats generally are neither due to a higher number of M. fuscus nor to a greater amount of its preferred habitat within the home range of the foxes contributing to the sample, then the explanation must lie in the behaviour, either of the foxes or M. fuscus. In fact, the present study found no concentration of foraging effort by foxes in winter in areas where M. fuscus is most common and Bubela (1995) found only a slight bias towards non-wooded habitat in summer (at a time when foxes were feeding mainly on grasshoppers). By contrast, Halpin and Bisonette (1988) found that red foxes in winter snow in eastern Maine concentrated their foraging in open areas inhabited by voles rather than in hardwood forest inhabited by deer mice and shrews.
Two factors may predispose M. fuscus to greater predation than R. fuscipes. First, M. fuscus nests communally in winter beneath the snow, but above the ground (Green & Osborne 1994). Capturing more than one prey at a nest would be energetically more favourable for the fox, especially if digging is required, and might lead to a greater occurrence in scats (particularly if excess prey are cached and eaten later). However, this does not explain the greater abundance of M. fuscus in scats in seasons without snow (Table 3). Second, M. fuscus is slower and less aggressive than R. fuscipes (K. Green, pers. obs.) and may therefore be an easier prey to handle.
Menkhorst (1995) stated that at Bellell Creek near Lake Mountain, trapping results for R. fuscipes and M. fuscus were similar and they occurred in similar frequency in predator scats. He continued, ‘thus discounting selective predation on Broad-toothed Rats’. In fact, the area trapped at Bellell Creek was only approximately 1 ha, surrounded by forest area unsuitable for M. fuscus (J. Seebeck, pers. comm., 2001). It is inconceivable that the foxes only foraged in this 1 ha, and to extrapolate the trapping results from this one grid over the entire foraging area of the foxes involved is invalid, and therefore the conclusion of Menkhorst (1995) is without foundation. Brunner and Wallis (1986) quoted data from fox scats and trapping data, and asked ‘does the high percentage of Mastacomys in predator scats indicate a high population density of a difficult to trap mammal, or does it indicate strong preference by foxes for Mastacomys which occurs in low numbers?’ The results of the present paper suggest the latter.
Acknowledgements
I thank David Happold, John Seebeck and Will Osborne for their discussions and for making critical comment on the manuscript. John Seebeck, Christine Hill, David Happold and Linda Broome provided access to unpublished material. Barbara Triggs identified approximately half of the hair samples.




