Spatial patterns of invertebrate species richness in a river: the relationship between riffles and microhabitats
*Corresponding author.
Abstract
This study describes the pattern of invertebrate species richness in a river reach with large differences in habitat complexity at two, hierarchically nested, spatial scales. The aim was to determine whether the mass effect was likely to be increasing invertebrate species richness in epilithic microhabitats in this river. The mass effect is the process by which the species richness of a patch is increased when it acts as a ‘sink’ for species generated by ‘source’ patches. Microhabitat patch types in Mountain River, Tasmania, were distinguished on the basis of physical structure and orientation on the river bed. They were nested within two types of riffle with contrasting structural complexity: bedrock and boulder-cobble riffles. It was hypothesized that microhabitats with high species richness would act as source patches, contributing species to other microhabitats (sinks) and thereby increasing their species richness. Microhabitat sampling was carried out in four consecutive seasons and rarefaction was used to estimate riffle-scale species richness. Analysis of variance ( ANOVA) was used to compare the identical microhabitats present in the contrasting riffle types, to detect evidence of the mass effect in either riffle type. The more structurally complex boulder-cobble riffles had higher species richness than did bedrock riffles. Amongst the microhabitats, the spaces beneath the cobbles had the most species. Microhabitats accounted for a higher percentage of the variation in species richness than did differences between riffles of the same type. No evidence was found for the operation of the mass effect in either riffle type. The majority of species found only in boulder-cobble riffles were unique to the beneath-cobble microhabitat and appeared to be unable to colonize other microhabitats, even as transients. In Mountain River, small-scale habitat characteristics appeared to be more important than larger-scale effects in determining microhabitat species richness.
INTRODUCTION
The study of species richness in habitats has been motivated largely by the need to understand the mechanisms creating levels of richness measured in the field. In aquatic habitats, enquiry has mainly centred around the role of the relative amount and complexity of habitat space available in determining species richness (e.g. Douglas & Lake 1994) and on the effects of disturbance on species richness. In terrestrial ecology, the potential role of processes acting at different spatial scales to control species richness has also been studied (e.g. Cornell & Lawton 1992; Straw & Ludlow 1994) to determine which spatial scales have most control over species richness.
From a patch dynamics perspective, habitats can be viewed as mosaics of patches of internally similar habitat, and these small-scale patches (or microhabitats) are nested within larger-scale habitat types, creating a nested hierarchy of spatial scales. The precise definition of habitats and microhabitats depends on the grain and extent of the taxa studied ( Cornell & Lawton 1992). High species richness in a habitat or community may increase microhabitat species richness by contributing species to patches where they are not able to survive permanently, simply because there are so many propagules ( Cornell & Lawton 1992). In this way, the dispersal of juveniles may increase the species richness of local species pools, regardless of whether the dispersers can persist in any particular microhabitat type. This is analogous to the ‘mass effect’ described by Townsend (1989), whereby individuals of species not normally occurring in a habitat are present as transients, and has also been termed ‘source and sink dynamics’ and ‘spillover effects’ ( Kunin 1998). This mechanism of increasing species richness has been studied less often than the effects of habitat space or disturbance but may be a relatively common process that increases the numbers of rare species, in particular.
The only experimentally based test for the operation of the mass effect has been reported by Kunin (1998), studying grassland plant species in a long-term experimental facility. He found evidence for the operation of the mass effect on plant species richness but its impact was weak. The weakness of the effect may reflect the relative similarity of the different experimental patches in terms of some physical conditions (e.g. soil depth and type), particularly structural similarity. Further observations of species richness in patches that differ structurally may reveal a stronger effect. Kunin (1998) also counted only the reproductive species and it may well be that the strongest impact of the mass effect is in contributing non-reproductive individuals to patches.
Underlying this mechanism is the question of spatial control of species richness: is it the microhabitat or a larger spatial scale that controls the total number of species found in a given microhabitat? In a study of insect species richness on terrestrial plants, Straw & Ludlow (1994) found that small-scale factors such as individual plant biomass were strongly correlated with insect species richness and suggested that small-scale processes may be responsible for limiting larger-scale species richness.
Few similar studies have been carried out in freshwater ecosystems, with research into the relationship between species richness and habitat structure tending to focus on quantifying the amount and arrangement of habitat space (e.g. Jeffries 1993; Douglas & Lake 1994; Downes et al. 1998 ) rather than the scale of spatial control. Increased habitat complexity has generally been found to increase species richness at whatever spatial scale it exists, both in freshwater and in terrestrial communities. However, the question remains: what influence does microhabitat complexity have on the species richness of ecosystems? The mass effect provides one way in which small-scale increases in species richness could influence larger spatial scales (through the production of dispersers) and, therefore, further investigation of its role is warranted.
It is the purpose of this study to seek evidence of the mass effect in operation on a temperate river bed by careful sampling of epilithic microhabitats (<1 m in linear dimension). If patterns characteristic of the mass effect are detected, it suggests a mechanism by which high microhabitat species richness could have a community-wide effect. However, as Kunin (1998) pointed out for his own study, the scope of these results is limited by the lack of manipulative experimentation on species richness and will simply indicate the likelihood that the mass effect is in operation at the study site. In addition, the spatial scales in this study are relatively small, at least by the standards of terrestrial ecology.
This study focuses on two spatial scales in a river: riffles (= habitat-scale) and microhabitats. The riffles used were composed of several microhabitats that differed in their physical structure and species composition. Therefore, the microhabitats were nested within the riffles and were the small scale studied (<1 m linear dimensions), while the riffle habitats were composed of the microhabitats and were treated as the larger spatial scale (riffles ranged between 5 and 8 m wide and 10 and 25 m long). Rivers have been divided into five spatial scales based on their geomorphology, ranging from whole catchments to microhabitat systems ( Frissell et al. 1986 ). This study uses only the smallest two spatial scales of this classification: riffle/pool systems and microhabitat systems that were defined by Frissell et al. (1986) . Riffles are defined by breaks in the riverbed profile, and microhabitat systems are defined by differences in substratum architecture, water depth and velocity.
Most experimentally based manipulations of habitats to alter species richness have been carried out at the microhabitat scale (as defined here, e.g. Dudgeon 1993; Jeffries 1993), and in most descriptive studies, replication of riffles is the largest spatial scale considered ( Downes et al. 1995 ). It is reasonable, therefore, to study the relationship between riffles and their microhabitats in order to clarify results obtained in previous studies; but it is also desirable that future studies begin to consider the mechanisms operating at larger spatial scales in rivers, that may in turn affect species richness at riffle and microhabitat scales.
Mountain River, where this study was carried out, is ideal for the purpose because it consists of a series of clearly defined riffles of contrasting structural complexity: bedrock and boulder-cobble. In addition, it is bedrock-controlled, has little hyporheos and so is dominated by epilithic habitats, making the riffle types more comparable. Within both riffle types is a mosaic of microhabitats that differ in their physical structure, two of which are common to both riffle types and some of which are unique. The microhabitats sampled in this study were defined a priori based on their different substratum architecture, with the aim of determining the effect of microhabitat substratum complexity on species richness before looking at the effects of spatial scale.
The two microhabitats that are present in both riffle types are bare rock surfaces and bare rock overgrown with the filamentous green alga, Ulothrix zonata Weber et Kutzing. At a scale of 10 cm, the complexity of rock surfaces in both bedrock and boulder-cobble riffles is indistinguishable, measured by fractal dimension (mean D for bedrock = 1.020; mean D for boulder- cobble = 1.029; P > 0.05, not significantly different: see Robson (1995) for further detail). Both riffle types are also overgrown with the same species of macroalga in equal densities ( Robson & Barmuta 1998). Therefore, these microhabitats have very similar substratum architecture, regardless of riffle type, increasing the probability that they experience similar near-bed flow conditions. Other aspects of hydraulic habitat such as depth and mean velocity are influenced by larger-scale characteristics of the riffle and as such may contribute to differences between riffles and riffle types. The remaining microhabitats are unique to each riffle type and differ in terms of the type and complexity of substratum architecture. These differences in physical structure will alter near-bed flow conditions in a predictable way ( Davis & Barmuta 1989) but will also be affected by their larger-scale hydraulic setting.
The visible difference in physical complexity of the microhabitats in Mountain River alters their potential for high species richness. The close connection between highly complex habitat structure and high species richness has been shown for a wide range of ecosystems ( August 1983) and for different levels of complexity on artificial stream stones ( Douglas & Lake 1994; Downes et al. 1998 ). It was therefore expected, a priori, that particular microhabitats would contain more species than their structurally simpler counterparts.
In Mountain River, patterns consistent with the operation of the mass effect would be detected when the microhabitats common to both riffle types have different species richness. Such a difference would result from differences in the surrounding riffle, most probably the contribution of species from other, richer microhabitats acting as sources. The species richness recorded in each of these microhabitats will be discussed in relation to their small-scale complexity as well as in the context of their contribution to total riffle species richness.
METHODS
Study site
Mountain River lies about 35 km southwest of Hobart, Tasmania, originating in alpine heath in the Wellington Range at an altitude of around 1000 m. It is a short, unregulated perennial river with a catchment area of about 40 km2 and a mean annual rainfall of approximately 900 mm. The river is 25 km long (fractal stream length calculated from 1 : 25 000 maps), with a steep profile and a relatively straight channel (fractal dimension = 1.09, minimum scale = 250 m). Its mean annual discharge, measured by a gauging weir at the study site, is 0.7 m3 s–1, and mean monthly discharge is lowest in late summer (January–March, 0.2–0.5 m3 s–1) and highest in late winter and early spring (1.0–1.3 m3 s–1) (Department of Primary Industry and Fisheries, Tasmania, unpublished data, 1995). Water temperatures range from 12 to 23°C in summer and autumn, and from 2 to 8°C in winter and spring (for a more detailed site description see Robson 1995).
The river bed consists of alternating sections of Permian mudstone bedrock platforms and riffles containing boulders and cobbles of dolerite and mudstone. The median particle size in boulder-cobble riffles was 180 mm (interquartile range: 123–217 mm) and the rocks tend to be oblate spheroid with quite flat surfaces (roundness ≈0.7; see Gordon et al. 1992 ).
The 800 m reach sampled in this study was at an altitude of 150 m above sea level, where Mountain River is a third- and fourth-order stream. At this point it occupies a rectangular channel 6–12 m wide and up to 2 m deep, with an average slope of 2.3%. The reach contained one mid-channel cobble bar and several mid-channel rock outcrops (further detail regarding this reach can be found in Robson & Barmuta 1998). During the period of this study, water depths in the riffles varied from 3 to 50 cm.
Sampling
Benthic invertebrates were collected at 3-month intervals, in spring, summer, autumn and winter, from bedrock and boulder-cobble riffles. Collections were made in four consecutive seasons to encompass the seasonal variation in species richness that may occur within microhabitats. This is particularly important in a community largely dominated by insect larvae.
The microhabitats were defined (within riffle types) based on observed differences in physical structure. In bedrock riffles only rock-based (epilithic) microhabitats were present but in boulder-cobble riffles there were several epilithic microhabitats plus the beneath-cobble microhabitat that consisted of detrital material as well as the underlying rock. The beneath-cobble microhabitat differed from the epilithic microhabitats in several important ways. It generally contained more detritus, particularly large items such as leaves and strips of bark. It was darker, with little direct exposure to light, and therefore had a lower algal biomass. It also provided much larger refuges both from current and from predation by fish ( Robson 1996). The epilithic microhabitats, with the exception of the under-surface of the cobbles, differed mainly in structural complexity (and, therefore, near-bed flow conditions) and the type of food source that was most abundant ( Table 1). The under-cobble surface microhabitat was darker than the other epilithic microhabitats and was also sheltered from direct current.
| Microhabitat | Complexity | Likely near-bed flow conditions | Food supply |
|---|---|---|---|
| (D = fractal dimension) | |||
| Bare rock | Very simple | Potentially high shear and velocity | Epilithic diatomsd |
| (D = 1.02–1.029)a | |||
| Filamentous algal patches | Relatively complex | Reduced shear stress and velocity | Epiphytic diatoms and |
| (D = 1.67–1.9)b | Ulothrix zonata e | ||
| Bedrock crevices with | Very complex | Greatly reduced shear stress and velocity | Epiphytic diatoms |
| moss | (D = 1.8–1.9)c | & FPOMf | |
| Undersurface of cobbles | Very simple | Greatly reduced shear stress and velocity | Surface biofilm? |
| (D = 1.02–1.029)a |
- Likely near-bed flow conditions based on principles given by Davis & Barmuta (1989). Data compiled from the following sources: aRobson 1995, bSinsabaugh et al. 1991 and Sarnelle et al. 1993 , cPalmer 1988, dRobson 1996, eRobson & Barmuta 1998; fSuren & Winterbourn 1992.
In Mountain River, the bedrock crevices usually contained a dense growth of aquatic moss, either Fissidens rigidulus J. D. Hooker & Wilson or Tridontium tasmanicum J. D. Hooker, creating a highly complex microhabitat ( Table 1). Moss did not grow in the boulder-cobble riffles or on smooth rock surfaces. The filamentous alga Ulothrix zonata dominated large areas of both the bedrock and the boulder-cobble riffles in Mountain River but was only present in winter and spring. This alga added structural complexity to otherwise very simple rock surfaces.
Three bedrock riffles and three boulder-cobble riffles were selected haphazardly along the study reach. Within each of these, several microhabitats were sampled in each season, depending on availability ( Table 2). Ten samples were taken from each microhabitat at each riffle, with the exception of the bedrock crevices (in autumn, winter only). For these samples, precision calculations showed that five samples were sufficient for an error of less than 10% of the mean and so, to save time, only five samples from each site were processed. Five samples per site were also taken from cobble undersides (winter only) and beneath-cobble (summer, autumn, winter) microhabitats.
| Riffle type | Bedrock | Boulder-cobble | ||||||
|---|---|---|---|---|---|---|---|---|
| Micro-habitat | Bare rock | Crevice | Filamentous | Bare rock | Filamentous | Side | Under- | Beneath |
| algae | algae | surface | ||||||
| Spring | S | S | S | S | S | S | S | NS |
| Summer | S | NS | NA | S | NA | NS | NS | S |
| Autumn | S | S | NA | S | NA | NS | NS | S |
| Winter | S | S | S | S | S | NS | S | S |
- S, sampled; NS, not sampled; NA, not available. Patches of Ulothrix zonata were only present in winter and spring. The spring samples were taken first and analysis of the results showed that cobble sides were not significantly different to cobble tops, so this microhabitat was not sampled again. Bedrock crevices and cobble under-surfaces were not sampled in summer, and the beneath cobble habitat was not sampled during the first season due to logistical difficulties.
A box sampler was used that had a sample area of 100 cm2 and a net mesh size of 65 μm. This small sample area was selected to allow stratified random sampling of the two riffle types. All epilithic habitats were sampled in this way. The rock surface within the sampler was scrubbed for 1 min using a nylon brush, for each sample. In addition, the microhabitat space beneath the cobbles was sampled using a Sürber sampler (mesh size 250 μm). When a rock was upturned to sample its underside with the box sampler, it was done within a Sürber sampler, allowing the material disturbed to be washed into the net. The riverbed area within the Sürber sampler was also disturbed by hand to capture all detritus and associated fauna. This method is not directly comparable with the box samples and has been analysed separately where necessary.
The invertebrate samples were preserved in ethanol and transported to the laboratory, where invertebrates were counted and identified using a microscope. Taxa were identified to species level, except for the oligochaetes, and a complete list can be found in Robson (1995).
Statistical analysis
Species richness data at the riffle scale were analysed using rarefaction ( Simberloff 1978; Magurran 1988). The advantage of the method is that comparisons can be made between habitats where different numbers and types of samples were collected. Using the method of Simberloff (1978), the two riffle types were compared using rarefaction curves and by contrasting expected species richnesses for collections of up to 1000 individuals, for each season sampled. Rarefaction assumes that individuals are dispersed randomly, so large sample sizes are required to avoid overestimating species richness ( Simberloff 1978). Consequently, data from all microhabitats in all three riffles of each type were pooled so that samples contained between 1600 and 7900 individuals. These data are subsequently referred to as species richness, whereas the numbers of species within the samples are referred to as species density because they are expressed as numbers of species per 100 cm2. Rarefaction was not repeated on microhabitat samples as sample sizes were too small.
Analysis of variance ( ANOVA) was used to compare species densities in the microhabitats, which were coded separately for each riffle type (the beneath- cobble microhabitat was excluded). Data were log-transformed where necessary to meet the assumptions of ANOVA; assumptions were checked by plotting residuals and normal probability plots. Tukey’s multiple comparison test was used to determine the significance of pairwise differences between combinations of microhabitat by site. The per cent variation among and between groups was also calculated using formulae from Sokal & Rohlf (1969). The two microhabitats common to both riffle types were of particular interest because they could indicate the operation of the mass effect. Two- or three-way ANOVA (depending on season) was carried out on species density in these microhabitats, with ‘site’ (three riffles of each type, random factor) and riffle type (two types, fixed factor) as factors.
RESULTS
Expected species richnesses determined by rarefaction showed that boulder-cobble riffles always had higher numbers of species than did bedrock riffles (all plots were similar so only winter is presented: Fig. 1). This difference was significant in all seasons except spring ( Fig. 2), when the beneath-cobble microhabitat was not sampled. It appears that the absence of this microhabitat from the sample pool reduced the species richness recorded in the boulder-cobble riffles in spring. Higher numbers of species were also collected from bedrock riffles in spring than in the other seasons.
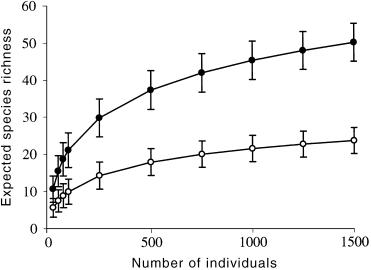
Rarefaction curve for bedrock and boulder-cobble riffles in winter, with approximate 95% confidence limits. ●, Boulder-cobble; ○, bedrock riffles.
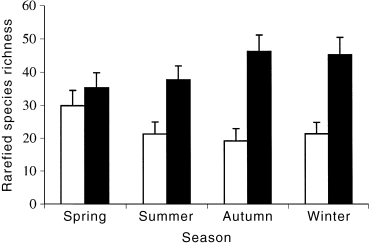
Rarefied species richness per thousand individuals in each riffle type and season, with approximate 95% confidence limits. ▪, Boulder-cobble; □, bedrock riffles.
Many more species were unique to the boulder- cobble riffles than to the bedrock riffles, although many of these were single occurrences. However, 12 taxa were unique to boulder-cobble riffles and collected in more than one season: oligochaetes, planarians, ostracods, Eusthenia costalis Banks (Plecoptera), Tasmanocoenis sp. (Ephemeroptera), two species of chironomid, Hydrobiosella waddama Mosely (Trichoptera), a species of Philorheithridae larvae (Trichoptera), Caenota plicata Mosely (Trichoptera), Costora rotosca Mosely (Trichoptera) and Sclerocyphon aquaticus Lea (Coleoptera). Of these, E. costalis, S. aquaticus, C. plicata and the philorheithrid were the only species that could be regarded as common. Only one species was both unique to bedrock riffles and present in more than one season: the elmid larva Simsonia L12E.
In all four seasons a greater percentage of variation in species density was associated with differences between the microhabitats than between the sites, although microhabitat frequently interacted with site in the analysis of variance ( Table 3). In spring, bedrock crevices had significantly more species on average than did the other microhabitats ( Fig. 3), with the exception of crevices at site 1, which were not significantly different to bare patches of rock. Patches of filamentous algae also had high species density and were not significantly different to bedrock crevices except at site 3, where species density was lower among filamentous algae. In autumn, the greatest proportion of the variation was due to the very high number of species in bedrock crevices ( Table 3). This was also true in winter, but patches of filamentous algae in boulder-cobble riffles also had a high (and not significantly different) species density. Of all the epilithic microhabitats, bedrock crevices had the highest density of species in the three seasons in which they were sampled ( Fig. 3). The high complexity of the aquatic moss present in that microhabitat appeared to be providing space for a higher diversity of invertebrates. By far the highest number of species were recorded from the only non-epilithic microhabitat: beneath cobbles ( Fig. 3). (But note that because these samples were taken with a larger sampler, they are not directly comparable with the other samples, in terms of density.)
| Source | d.f. | F | P | % Variation |
|---|---|---|---|---|
| Spring | ||||
| Microhabitat | 6 | 4.073 | 0.018 | 42.5 |
| Error (microhabitat × site) | 12 | |||
| Site | 2 | 3.641 | 0.028 | 12.5 |
| Microhabitat × site | 12 | 3.035 | 0.001 | 10.5 |
| Error | 188 | 34.5 | ||
| Summer | ||||
| Microhabitat | 1 | 18.94 | <0.001 | 49.9 |
| Error (microhabitat × site) | 2 | |||
| Site | 2 | 3.029 | 0.057 | 8.1 |
| Microhabitat × site | 2 | 5.459 | 0.007 | 14.5 |
| Error | 54 | 27.5 | ||
| Autumn | ||||
| Microhabitat | 2 | 18.01 | 0.01 | 70.1 |
| Error (microhabitat × site) | 4 | |||
| Site | 2 | 0.2 | 0.819 | 0.5 |
| Microhabitat × site | 4 | 1.5 | 0.209 | 3.9 |
| Error | 81 | 25.5 | ||
| Winter | ||||
| Microhabitat | 5 | 11.47 | <0.001 | 53.0 |
| Site | 2 | 2.89 | 0.059 | 13.3 |
| Error | 117 | 33.7 |
- In winter, a site-microhabitat interaction was not possible as sample sizes of the filamentous algae microhabitat from different sites were unbalanced owing to differences in algal cover between sites.
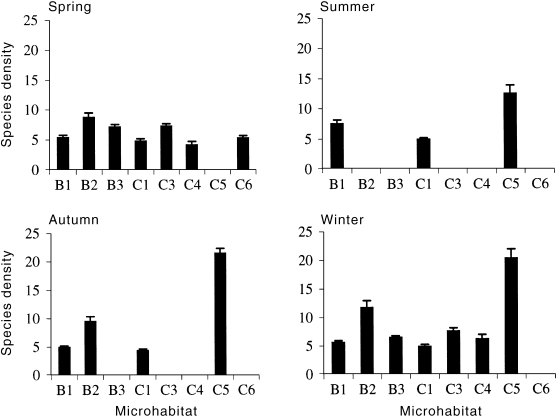
Mean species density in each microhabitat and season (± 1 SE). Microhabitats were: B, bedrock; C, boulder-cobble; B1, bare rock in bedrock riffles; B2, bedrock crevices; B3, patches of filamentous algae on bedrock; C1, bare rock in boulder-cobble; C3, patches of filamentous algae on cobble tops; C4, undersurfaces of cobbles; C5, beneath cobbles; C6, sides of cobbles. Densities are presented per sample, which is per 100 cm2 for the epilithic microhabitats, but not the beneath cobble microhabitat (C5). Note that microhabitat C5 had a larger sample area than the other microhabitats and is therefore not directly comparable in terms of species density.
Of particular interest is the comparison of species density in patches of bare rock and patches of filamentous algae between the two riffle types, as these were the only microhabitats common to both riffle types. Patches of bare rock on the tops of cobbles and on the bedrock platforms had similar densities of species in all seasons: spring P = 0.084; summer P = 0.204; autumn P = 0.156; winter P = 0.403. Patches of filamentous algae were only present in Mountain River in winter and spring, and in neither riffle type were there more species, in either season (P = 0.084, 0.403, respectively). Therefore, there does not appear to have been any effect of riffle-scale species richness on microhabitat species density in either riffle type.
DISCUSSION
There were clearly more taxa in boulder-cobble riffles than in bedrock riffles in Mountain River. This difference appears to be largely attributable to the larger number of taxa found beneath the cobbles than in any other microhabitat. By using rarefaction we were able to establish that this was not simply a function of the larger amounts of space available (and sampled) in this microhabitat; rather, there were more taxa recorded per number of individuals in boulder-cobble riffles.
Many of the taxa recorded in the beneath-cobble microhabitat were unique (around 20% of all the taxa collected), suggesting that the environmental conditions there were necessary for those species. For example, the predatory stonefly E. costalis is much larger than other stream insects in Mountain River, and may require suitably large refuges that can only be provided beneath cobbles. The trichopteran C. plicata is a shredder that makes its portable case from bark and leaves and consumes coarse particulate organic matter. It seems likely that this species is only found beneath cobbles because this is where such large detritus was lodged. Therefore, it is probable that the beneath- cobble microhabitat contributed more species to the boulder-cobble riffles because it provided unique environmental conditions required by many species. This microhabitat could have been acting as a source, contributing species to the other microhabitats that were present in boulder-cobble riffles, if the mass effect was operating.
There were no additional species found on bare rock or in filamentous algal patches in boulder-cobble riffles compared with bedrock riffles, suggesting that the mass effect was not operating in these riffles. One uncertain feature of the mass effect is whether a greater effect will be observed when the difference between two microhabitats is very great. The more similar two microhabitats are, the more species they will have in common and therefore the lower the potential for a noticeable effect ( Kunin 1998). In contrast, very great differences between adjacent microhabitats may prevent species moving across the boundary at all, because they cannot survive in the other microhabitat. Kunin (1998) concluded that the strongest mass effects would be observed where differences between adjacent microhabitats were intermediate. It may be that the differences between the beneath-cobble microhabitat and the bare rock and filamentous algal patches were too great for beneath-cobble species to move into the epilithic microhabitats. Indeed, as most of the species found in epilithic microhabitats were also found in the beneath-cobble microhabitat, it suggests that the unique taxa beneath the cobbles were unable to colonize epilithic microhabitats.
The bedrock crevices were also a complex habitat with a high number of species but with environmental conditions more similar to the other epilithic microhabitats than to the microhabitat beneath cobbles. Perhaps this microhabitat would represent an intermediate difference and contribute species to the other epilithic microhabitats in bedrock riffles? This was clearly not the case as the presence of mossy crevices in bedrock riffles did not increase species richness in patches of bare rock or filamentous algae in that riffle type. Indeed, the differences between the microhabitats were relatively clear and consistent and appear to be independent of species richnesses in adjacent microhabitats. This suggests that many species in Mountain River were highly selective of microhabitats and, despite the potential mixing effects of drift and juvenile dispersal, were not acting as a community-level species pool where individuals colonize patches on the probabilistic basis of local abundance.
Microhabitat selection was based on differences in structural complexity and orientation on the river bed. The pattern of invertebrate species richness across these microhabitats suggests that benthic microhabitats in rivers that differ in structural complexity will differ in species richness and, roughly, that species richness will increase with increasing complexity, as has been found in many other studies and habitats (e.g. Menge et al. 1985 ; Denno & Roderick 1991; Douglas & Lake 1994; Downes et al. 1995 ). As a first attempt to identify the patterns that may be created by the mass effect in a river, this study suggests that ‘sink’ patches may not be found within riffles. It is possible that species dispersing from the beneath-cobble and bedrock crevice microhabitats (sources) are being deposited in other parts of the river, such as pools. This may be due to passive transport processes or to some form of active selection by species. With the exception of a few species, little is known about the patch colonization processes of stream invertebrates, in particular the events that end dispersal ( Downes & Keough 1998). It is therefore difficult to explain why patches in riffles do not act as sinks when there appear to be ample source patches nearby. Further research into colonization processes in streams will assist in understanding the distribution of species among microhabitats, and experiments manipulating species richness would allow mechanisms such as the mass effect to be evaluated directly.
This study, then, supports the findings of Straw & Ludlow (1994) for terrestrial insects, that small-scale habitat characteristics appear to be more important than larger-scale effects in determining microhabitat species richness. This result may have been overestimated by the method of stratified sampling used in this study, which imposed microhabitat boundaries onto the riverbed on the basis of visually differentiable patches. A transect based approach, such as that used by Kunin (1998), which focused on detecting edge effects across microhabitat boundaries, may have been more able to detect the mass effect. However, the results so far do suggest that the mass effect may indeed be relatively weak ( Kunin 1998; and this paper), but further studies are warranted before this process of increasing species richness is discounted. Perhaps researchers are looking at too small a spatial scale. The mass effect may be more important, for example, across boundaries between upland and lowland sections of rivers than within riffles. At the moment, however, it seems that small-scale species richness is controlled mainly by microhabitat characteristics.
Indeed, in Mountain River, microhabitat differences controlled more of the variation in species density than did differences between riffles of the same type. These differences were also relatively predictable throughout the year and between sites and had relatively low variance (suggesting that the samples were relatively homogeneous internally). The microhabitats studied in Mountain River therefore appear to be perceived as different by the fauna as well as being observably different to the authors.
The study of patterns of variation in biotic and physical parameters in rivers at different spatial scales is a relatively recent area of investigation but significant riffle-to-riffle variation in invertebrate density has already been demonstrated ( Downes et al. 1993 ). In contrast, variation in epilithon biomass and flow conditions within riffles have been shown to exceed differences between riffles ( Downes et al. 1995 ), suggesting a high level of microhabitat differentiation within riffles. Indeed, significant variation in invertebrate densities adjacent to upstream and downstream sides of river boulders, associated with different microflow patterns around those boulders ( Bouckaert & Davis 1998), show that microhabitat structure may have very fine-scale effects on within-riffle variation. In a study of four nested spatial scales in a river (individual stones, groups of stones, riffles and pairs of riffles), differences between the species richness of invertebrates on individual stones encompassed 92% of the total variation in species richness ( Downes et al. 1993 ). While these individual stones encompass several of the microhabitats distinguished in the present study (and in the study of Bouckaert & Davis 1998), this high level of variability at such a small spatial scale also suggests the importance of microhabitat variability in maintaining species richness in streams.
Overall, the accumulating evidence suggests that variation in species richness among structurally different microhabitats on river beds may exceed variation at larger spatial scales and may therefore be of significant importance in maintaining species richness in rivers. However, few studies have included spatial scales larger than relatively short reaches (e.g. this study: approximately 800 m; and Downes et al. (1993) : approximately 1500 m), so it remains possible that there are larger scales within rivers at which species richness varies markedly and which may have influence at smaller scales. Future studies need to integrate and compare a wider range of spatial scales within rivers to fully understand the control of species richness in rivers.
Acknowledgements
We would like to thank the University of Tasmania for supporting this research and Murdoch University for supporting the preparation of this paper.
David Oldmeadow (University of Tasmania) helped with sample collection and Leon Barmuta (University of Tasmania) assisted with the analysis. Dr David Fuller (Department of Primary Industry and Fisheries, Tasmania) provided discharge records. We are especially grateful to the adjacent landholders for access across their properties.




